Abstract
Introduction
We assessed changes in metal-on-metal hip arthroplasties (MoMHAs) after repeat ultrasound examination.
Methods
This retrospective, single-centre cohort study involved all patients undergoing two ultrasound examinations of the same MoMHA. Between 2010 and 2014, 96 ultrasound examinations were performed in 48 MoMHAs (mean time between scans = 1.1 years). A radiologist assigned each scan to one of four grades and measured volumes of any solid/cystic masses. Changes in grade and lesion volume between scans were analysed.
Results
Change in grade between scans was significant (p=0.012); 27% (n=13) of MoMHAs increased in grade, 67% (n=32) had no grade change, and 6% (n=3) decreased in grade. The mean increase in lesion volume was 24.2cm3 by the second scan, and was significant (p=0.023). Evidence of progression in findings was observed in 54% (26/48) of MoMHAs. Of patients with normal scans initially, 44% (8/18) developed abnormalities. No factors (including blood metal ion concentrations and cup position) were associated significantly with progression of ultrasound findings.
Conclusions
Repeat ultrasound in MoMHA patients demonstrated that findings frequently progress in the short-term. Therefore, regular surveillance of MoMHA patients is important, with ultrasound representing an effective investigation for identifying the development and progression of lesions.
Keywords: Adverse reaction to metal debris, follow-up, hip arthroplasty, metal-on-metal, ultrasound
More than 1 million individuals worldwide have received large-diameter metal-on-metal hip arthroplasties (MoMHAs).1 In recent years, a high prevalence of short-term failure due to adverse reactions to metal debris (ARMD) has been observed with several MoMHA designs.2–5 Patients with ARMD often require revision surgery. However, lesions can be destructive and cause considerable bone loss and muscle damage, so short-term outcomes after revision are, in general, poor.6,7
To expedite early identification of patients who develop ARMD, several regulatory authorities have published guidance regarding regular follow-up of MoMHA patients.8–10 In general, this follow-up guidance was conservative because ARMD was poorly understood when it was formulated. Indeed, little is known about the natural history of ARMD lesions associated with MoMHAs (eg peri-prosthetic effusions and pseudotumours). Most studies published on serial imaging of MoMHA patients have involved repeat magnetic resonance imaging (MRI) because it provides excellent visualisation of peri-prosthetic soft-tissues, and can be used to identify ARMD.11–13 However, compared with ultrasound, MRI is: expensive (at our centre, MRI of the hip costs £116 compared with £66 for ultrasound of the hip); time-consuming; subject to degradation of image quality due to metal artifacts from the prosthesis; contraindicated in some patients.
Ultrasound has been observed to be a sensitive screening method for ARMD lesions associated with MoMHAs.14 A recent study comparing ultrasound with MRI for the detection of ARMD in 40 MoMHA patients concluded that a negative ultrasound carried out by an experienced radiologist excluded ARMD lesions because ultrasound had a sensitivity of 100%.15 Almousa and colleagues used repeat ultrasound examination at short-term follow-up to determine the natural history of 20 asymptomatic patients with ARMD.16 They observed that most lesions increased in size, with occasional remission of small masses.16 Their findings suggested that ultrasound may have a role in the surveillance of MoMHA patients.
We wished to determine the: (i) proportion of MoMHA patients with progression of initial ultrasound findings; (ii) proportion of MoMHA patients with normal ultrasound findings who developed abnormalities subsequently; (iii) factors associated with progression of ultrasound findings.
Methods
Study design, follow-up protocol, and patient demographics
This retrospective cohort study was undertaken at a specialist arthroplasty centre. Since 1997, 4,816 primary MoMHAs (3,792 hip resurfacing (HR) and 1,024 total hip arthroplasties (THAs)) have been implanted at this centre.
In 2010, the UK Medical and Healthcare Products Regulatory Agency (MHRA) sent out an alert regarding ARMD associated with MoMHAs.17 Our institution contacted all MoMHA patients. In addition to clinical examination, radiographs of the pelvis (anteroposterior view), and completion of the Oxford Hip Score (OHS) questionnaire,18 all symptomatic MoMHA patients underwent blood metal ion sampling and ultrasound of the hip. All asymptomatic MoM THA patients and asymptomatic HR patients with ARMD risk factors (small-sized femoral components; malpositioned acetabular components; radiological evidence suggestive of implant failure; bilateral MoM hip bearings) underwent a similar clinical assessment (including blood metal ion sampling). If blood metal ion concentrations were raised above the MHRA upper-limit of 7µg/l,8 metal artifact reduction sequence (MARS) MRI was performed. Ultrasound was carried out in these asymptomatic patients if MRI was contraindicated.
A second ultrasound scan was performed if the patient (i) was initially asymptomatic and then developed symptoms; (ii) had an abnormal first ultrasound scan (regardless of symptoms) and did not undergo revision surgery (because the surgeon wished to monitor the patient, or because the patient declined further surgery); (iii) was asymptomatic with normal levels of metal ions in the blood and a normal ultrasound examination initially, but repeat blood sampling revealed increasing concentrations of cobalt and/or chromium.
The present study comprised all patients who underwent two ultrasound examinations of the same MoMHA between 2010 and 2014. The final cohort consisted of 48 MoMHAs in 40 patients, with 96 images available for analysis (Table 1). Mean time between ultrasound imaging was 1.1 (range, 0.2–3.3) years.
Table 1.
Summary of the study cohort
| Total cohort for analyses(48 hips in 40 patients) | ||
|---|---|---|
| Sex | Female Male |
26 (54%) 22 (46%) |
| Age at first ultrasound scan | Mean (range) in years | 61.9 (39.0–82.2) |
| Time between primary hip arthroplasty and first ultrasound scan | Mean (range) in years | 5.0 (0.5–11.7) |
| Time interval between repeat ultrasound scans | Mean (range) in years | 1.1 (0.2–3.3) |
| Indication for repeat ultrasound examination | Under surveillance and/or patient refused revision Asymptomatic initially, then developed symptoms Remains asymptomatic but concentration of metal ions in blood increasing |
34 (71%) 8 (17%) 6 (13%) |
| Implant type and design |
Total hip arthroplasty > Synergy/modular head (Smith & Nephew, Warwick, UK) > Corail-Pinnacle (DePuy International Limited, Leeds, UK) > Caparo Amoda (Comis Orthopaedics, Birmingham, UK) > Other Hip resurfacing > Birmingham Hip Resurfacing (Smith & Nephew, Warwick, UK) > Other |
27 (56%) 13 8 2 4 21 (44%) 19 2 |
| Initial inclination of the acetabular component | Mean (range) in degrees | 44.9 (25.3–66.5) |
| Number of patients with unilateral or bilateral metal-on-metal hips | Unilateral Bilateral* |
22 (46%) 26 (54%) |
| Blood metal ion concentration | Median (interquartile range) in µg/l | Cobalt = 7.6 (2.9–10.9) Chromium = 4.8 (2.1–6.3) |
| Oxford hip score | Median (interquartile range) > % scale > 0 to 48 scale |
43.8 (25.0–60.4) 27.0 (19.0–36.0) |
| Hips with pseudotumours revised after repeat ultrasound scan | Adverse reaction to metal debris | 15 of 26 (58%) |
Of 26 patients with bilateral metal-on-metal hip bearings, 8 patients required repeat ultrasound imaging of both hips (therefore, these 16 hips have been included in the study). The remaining 18 patients with bilateral metal-on-metal hip bearings did not have repeated imaging of their contralateral metal-on-metal hip bearing (therefore, these contralateral hips were not eligible for study inclusion).
Ultrasound examination
All 96 ultrasound scans of the hip were performed by one of six fellowship-trained musculoskeletal radiologists (MRs), with most being undertaken by the senior author SLJJ (n=75; 78%). After obtaining verbal consent, examinations were carried out using an ACUSON S2000 ultrasound system employing a 9.4MHz transducer (Siemens Medical Solutions, Malvern, PA, USA). All ultrasound examinations were done using a systematic approach to assess the anterior, medial, lateral and posterior hip regions. This approach is recommended by the European Society of Skeletal Radiology, and is used widely for examination of the hip joint.19 The routine ultrasound protocol after hip arthroplasty used at our centre has been described.20
All 96 ultrasound images and complete reports were stored in the electronic picture-archiving and communication system at our centre. In May 2014, these images and reports were studied by two MRs (SJ and SLJJ) with consensus review. Both MRs were blinded to all clinical information. Each ultrasound image was assigned to one of four grades: (i) ‘normal’, (ii) ‘bursa’ (psoas bursa, trochanteric bursa/thickening), (iii) ‘pathological effusion’, and (iv) ‘pseudotumour’. A small amount of intra-articular fluid was considered to be normal, but if the depth of fluid was >15mm at the anterior joint line, this finding was classified as a ‘pathological effusion’. Simple fluid collections in the anatomical psoas or trochanteric bursa were classified as such, but complex bursal collections with evidence of communication with the hip joint were classified as ‘pseudotumours’. A pseudotumour was defined as a cystic, solid, or mixed mass with evidence of communication with, but extending beyond the confines of, the anatomical hip joint. If lesions were present, the volume (product of the maximum recorded dimension in each of three orthogonal planes in centimetres), consistency (solid, cystic, or mixed) and location were recorded for each lesion.
Blood metal ion sampling
Whole blood was collected from patients for analyses of metal ions, which was done in an MHRA-approved laboratory. Concentrations of cobalt and chromium were measured in all samples using an inductively-coupled plasma mass spectrometer (7500cx; Agilent Technologies, Santa Clara, CA, USA). The limit of detection and reporting limit were 0.06 µg/l and 0.6 µg/l, respectively.
Data collection
Remaining data for the present study were obtained from a prospectively maintained hospital database (MySQL database; Oracle Corporation, Redwood Shores, CA, USA), medical records, and radiographs of the pelvis. The database contains details on patient demographics, type of surgery undertaken, concentrations of metal ions in the blood, and OHSs. At our centre, the OHS is expressed as a percentage (0% = healthy joint; 100% = worst possible joint),21 but has also been provided on the more frequently used scale of 0 to 48 points (0 = worst possible joint; 48 = healthy joint).22 Medical records were reviewed to confirm intraoperative findings if patients had undergone revision surgery subsequently. The inclination of the acetabular component was measured in each radiograph of the pelvis (anteroposterior view) using the teardrop line for reference.23
Outcomes of interest
Outcomes of interest were the proportion of MoMHAs with: (i) progression of initial ultrasound findings; (ii) ultrasound examinations that were normal initially but which developed abnormalities. ‘Progression of ultrasound findings’ was defined as MoMHAs with any of the three following criteria observed after repeat examination: (i) increase in scan grade; (ii) increase in lesion volume by ≥20% but no increase in scan grade; (iii), change in pseudotumour consistency from liquid to solid. An increase in lesion volume by ≥20% was considered to be clinically significant given that: (i) no consensus is available in the literature; and (ii) this value accounts for inter-observer and intra-observer errors associated with ultrasound measurements. Progression of pseudotumours to a solid consistency has been associated with adverse outcomes,6,24 so this change was deemed to be clinically significant. MoMHAs not meeting these criteria after repeat ultrasound examination were considered to have no evidence of progression.
Statistical analyses
Statistical analyses were performed using the R library.25 Depending on data distribution, the median and interquartile range (IQR) or the mean and range were used. For paired analyses, change in volume between ultrasound scans was assessed using a paired t-test, and change in grade between scans was assessed using a Wilcoxon signed-rank test. To assess factors associated with progression of imaging findings, statistical tests were chosen to reflect the exposure variable and data distribution. These tests included unpaired t-tests (age; inclination of the acetabular component; time from primary MoMHA to first scan; time between repeat scans; initial lesion volume), the Wilcoxon rank-sum test (OHS; concentrations of cobalt and chromium), and the chi-squared test with Yates’ correction (sex; primary hip type; bilateral MoMHAs; grade of first ultrasound scan). p<0.05 was considered significant. Inter-observer and intra-observer reliability between the radiologist carrying out the initial ultrasound and the retrospective review undertaken for the present study was not assessed because ultrasound is a dynamic investigation with the retrospective review influenced largely by the initial ultrasound report and particular images saved.
Results
Change between repeat ultrasound scans
Changes that occurred between repeat ultrasound scans are summarised (Table 2) and illustrated (Fig 1). Twenty-seven percent (n=13) of MoMHAs had an increase in grade between scans, 67% (n=32) had no change in grade, and 6% (n=3) had a reduction in grade. Change in grade between scans was significant (p=0.012). Mean change in lesion volume between repeat scans was an increase of 24.2cm3 by the second scan (range, –84.0cm3 to 280cm3). This volume increase was significant (p=0.023).
Table 2.
Change between ultrasound scans repeated in 48 metal-on-metal hip arthroplasties
| Grade of initial scan | Location of lesions and consistency of pseudotumours | Volume (cm3)* | Grade of repeat scan | Location of lesions and consistency of pseudotumours | Volume (cm3)* |
|---|---|---|---|---|---|
|
1 18 hips |
Not applicable |
1 12 hips |
Not applicable | ||
|
2 8 hips |
5 psoas bursa 3 trochanteric bursa |
Mean = 47.7 |
2 4 hips |
4 psoas bursa | Mean =71.9 |
|
3 3 hips |
3 anterior |
3 6 hips |
3 anterior 2 anterior + lateral 1 lateral |
||
|
4 19 hips |
Location 8 Anterior (± lateral) 5 Posterior (± lateral) 3 Lateral only 2 Anterolateral + posterolateral 1 Medial only Consistency 10 Mixed 8 Cystic 1 Solid |
Range = 0–588 |
4 26 hips |
Location 13 Posterior (± lateral) 8 Anterior (± lateral) 5 Anterolateral + posterolateral Consistency 17 Mixed 7 Cystic 2 Solid |
Range = 0–588 |
The volumes provided are across all four ultrasound grades.
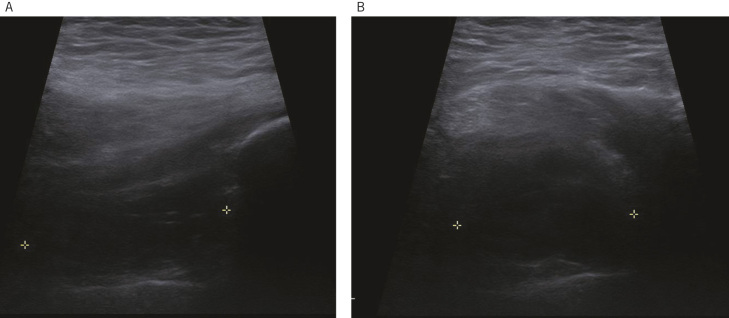
Figure 1.
Repeat hip ultrasound scan in a 66 year-old male with a hip resurfacing showing a 20cm3 cystic pseudotumour 3 months after a normal initial scan. (a) Longitudinal image of the lateral aspect of the hip shows a hypoechoic fluid collection (craniocaudal length = 4cm) deep to the gluteus medius extending down to the lateral joint line. Minor internal septation is observed. (b) Transverse image of the lateral aspect of the hip demonstrating a collection of 3.4cm.
Evidence of progression in findings between scans was observed in 54% (n=26) of MoMHAs. Of these cases, 13 hips had an increase in scan grade, 11 hips had a clinically significant increase in lesion volume with no increase in scan grade, and two hips progressed from liquid to solid pseudotumours. The remaining 46% (n=22) of MoMHAs had no evidence of progression between repeat scans. Further analyses demonstrated that none of the studied factors were associated significantly with progression of ultrasound findings (Table 3).
Table 3.
Factors associated with progression of ultrasound findings between repeat imaging
| Factor | Hips with progression (n=26) | Hips without progression (n=22) | P |
|---|---|---|---|
| Sex | 13 (50%) Female 13 (50%) Male |
13 (59%) Female 9 (41%) Male |
0.735 |
| Age at first scan | Mean, 63.0 years (range, 39.0–82.2 years) |
Mean, 60.5 years (range, 39.0–78.8 years) |
0.433 |
| Primary hip type | 16 (62%) THA 10 (38%) HR |
11 (50%) THA 11 (50%) HR |
0.609 |
| Other hip replacements | 13 (50%) Bilateral 13 (50%) Unilateral |
13 (59%) Bilateral 9 (41%) Unilateral |
0.735 |
| Inclination of acetabular component | Mean, 44.2° (range, 25.3°–61.4°) |
Mean, 45.6 ° (range, 31.5°–66.5°) |
0.585 |
| Time to first scan | Mean, 4.6 years (range, 0.7–11.7 years) |
Mean, 5.5 years (range, 0.5–11.1 years) |
0.252 |
| Time between scans | Mean, 1.2 years (range, 0.2–3.3 years) |
Mean, 0.89 years (range, 0.2–1.7 years) |
0.101 |
| Oxford Hip Score | Median, 43.8% (IQR 26.0–60.4%) OR Median, 27.0/48 (IQR, 19.0/48–35.5/48) |
Median, 43.8% (IQR 17.7–65.6%) OR Median, 27.0/48 (IQR, 16.5/48–39.5/48) |
0.909 |
| Blood cobalt concentration | Median, 7.5µg/l (IQR, 3.0–10.6µg/l) |
Median, 7.6µg/l (IQR, 2.7–11.2 µg/l) |
0.764 |
| Blood chromium concentration | Median, 4.5µg/l (IQR, 2.0–6.3µg/l) |
Median, 4.9µg/l (IQR, 3.1–6.7µg/l) |
0.413 |
| Initial scan volume | Mean, 43.3cm3 (0–316.0cm3) |
Mean, 52.9cm3 (0–588.0cm3) |
0.772 |
| Grade of initial scan | 8 (31%) Grade 1 5 (19%) Grade 2 2 (8%) Grade 3 11 (42%) Grade 4 |
10 (45%) Grade 1 3 (14%) Grade 2 1 (5%) Grade 3 8 (36%) Grade 4 |
0.752 |
THA = total hip arthroplasty; HR = hip resurfacing; IQR = interquartile range
Of the 18 MoMHAs with normal initial scans (grade 1), 44% (n=8) developed abnormalities (two to grade 2, one to grade 3, and five to grade 4), with the remaining 56% (n=10) of hips having a normal second scan. The five new pseudotumours (two cystic and three mixed lesions) in this subgroup developed within a mean of 1.7 years (range, 0.3–3.3 years) from the initial scan with a mean lesion volume of 131cm3 (range, 14–280cm3) (Fig 1).
Outcomes after repeat ultrasound
Revision arthroplasty has been performed in 15 of the 26 hips with pseudotumours on repeat ultrasound scan (58%). The remaining 11 hips have not undergone revision surgery because the surgeon wishes to keep them under regular surveillance or because the patient does not wish to undergo further surgery.
Discussion
Evidence of progression of initial ultrasound findings was observed in 54% of MoMHAs, with significant increases in the volume and grade of lesions by the second examination. The observation that 44% of patients with normal initial ultrasound findings developed abnormalities during short-term follow-up is concerning. None of the factors examined in our study were associated with progression of ultrasound findings.
Only one study has repeated ultrasound examinations at short-term follow-up in asymptomatic patients with ARMD.16 That study involved a considerably smaller cohort of MoMHAs (13 THA and 4 HR) compared with our study (27 THA and 21 HR), but the authors also observed that ARMD lesions frequently increased in size (six of nine pseudotumours in MoMHA patients increased, with three considered to be ‘clinically important increases’).16 Such findings suggest that repeat ultrasound examination of MoMHAs is effective for monitoring ARMD lesions. We and other authors13,16,26 have observed regression of a small number of ARMD lesions during serial cross-sectional imaging, suggesting that surveillance of some ARMD lesions is appropriate in preference to revision surgery.
Work from Almousa et al16 did not include patients with initially normal cross-sectional imaging, but we observed progression in 8 of 18 such patients. Of the eight MoMHAs with evidence of progression after initially normal scans, five developed pseudotumours. Studies reporting on serial MRI in MoMHA patients have observed minimal changes within 1 year in HR patients,12 with changes in initially normal scans or progression of abnormalities occurring over several years in MoM THA patients.11 Such contrasting observations between two types of cross-sectional imaging may reflect the fact that ultrasound can be used to detect smaller lesions close to the implant, which MRI may fail to identify due to metal artifacts despite suppression methods.27 However, other factors (eg, specific implants used, component position) could also explain the different findings observed between imaging methods.
Determination of which factors influence the progression of ultrasound findings in MoMHA patients would provide useful information regarding which patient subgroups need closer follow-up or consideration for revision surgery. We could not identify any factors associated with progression of ultrasound findings. A recent serial MRI study of MoMHA patients also could not identify factors (including time between scans and concentrations of metal ions in the blood) associated with progression between scans.26 Our study may not have been powered sufficiently to identify such factors, but represents the largest reported cohort of MoMHA patients undergoing repeat ultrasound examination, and included a patient group that pose management dilemmas to surgeons. Therefore, the present study provides further important evidence regarding the natural history of ARMD associated with MoMHAs. Larger longitudinal studies with extended follow-up are needed to identify which factors are associated with the development and progression of ARMD lesions. Such studies will provide more refined and evidence-based guidance for the follow-up of MoMHA patients, and the threshold for revision surgery.
Surveillance of some ARMD lesions is acceptable given the uncertain natural history of such lesions. This is especially true in asymptomatic patients with smaller lesions and no evidence of destruction of bone or soft tissue. Regulatory authorities have not stated a preference between ultrasound or MRI for MoMHA follow-up,8–10 but few studies have compared these imaging methods directly.15,27,28 Both modalities have advantages and disadvantages,27 but two larger and better-designed comparative studies by Garbuz et al (n=40)15 and Nishii et al (n=64)28 demonstrated ultrasound to be an effective screening tool for the identification of ARMD. Garbuz et al concluded that a negative ultrasound undertaken by an experienced radiologist excludes ARMD lesions because ultrasound had a sensitivity of 100%.15 Given some of the advantages of ultrasound over MRI (lower cost; shorter examination time; better compliance from patients; not affected by metal artifact), it appears that ultrasound is a more suitable screening and surveillance method for MoMHA patients if undertaken by an experienced MR. We acknowledge that MRI has a role in MoMHA follow-up if there is insufficient expertise to carry out ultrasound, and also for planning revision surgery.24,29 Furthermore, MRI may be used to identify early bone involvement, which may be occult initially on radiography.
Our study had limitations. First, there was an obvious selection bias for study inclusion, similar to that noted for other serial imaging studies.11–13,16 As per recommendations,8 symptomatic patients with abnormal investigations were revised, whereas asymptomatic patients with well-functioning HRs were not investigated routinely for ARMD. The second limitation was the retrospective study design. However, we considered it important to use data from routine clinical care to determine the natural history of ARMD rather than subject similar numbers of patients to repeat imaging in a prospective study unnecessarily. Third, comparison of serial measurements on ultrasound examinations may have been subject to error. Ultrasound examination is dynamic, with measurements made of maximal dimensions rather than according to anatomical landmarks, hence measurements may not be truly comparable. Therefore, images were compared at the time of review, with volume measurements and a grading system used to mitigate against this shortfall. In addition, all scans were undertaken by an experienced MR, which is recommended for obtaining findings comparable with MRI.15 Fourth, we did not assess inter-observer and intra-observer reliability. However, given that retrospective ultrasound reviews were assessed by an experienced MR, intra-observer reliability was very likely to be acceptable. Finally, scans were repeated at short-term follow-up given that patients were recalled in 2010. However, the time intervals between scans are similar to those of other serial imaging studies.12,13,16
Conclusions
Repeat ultrasound examination undertaken in a group of MoMHA patients demonstrated that, in the short-term, findings frequently progress between scans. Such progression included effusions and pseudotumours increasing in volume, and scans that were normal initially developing abnormalities. Therefore, regular surveillance of MoMHA patients is important, with ultrasound representing an effective investigation for identifying the development and progression of ARMD lesions. Further studies are needed to determine the natural history of ARMD at extended follow-up, and to identify which patient subgroup(s) are at greatest risk of disease progression. This strategy will assist in risk stratifying patients for clinical surveillance.
Acknowledgments
The authors would like to thank The Royal College of Surgeons of England and the Arthritis Research Trust for providing one of the authors with funding in the form of a Surgical Research Fellowship.
References
- 1.Lombardi AV Jr, Barrack RL, Berend KR et al. The Hip Society: algorithmic approach to diagnosis and management of metal-on-metal arthroplasty. J Bone Joint Surg (Br) 2012; : 14–18. [DOI] [PubMed] [Google Scholar]
- 2.Langton DJ, Jameson SS, Joyce TJ, Hallab NJ, Natu S, Nargol AV. Early failure of metal-on-metal bearings in hip resurfacing and larger-diameter total hip replacement: A consequence of excess wear. J Bone Joint Surg (Br) 2010; : 38–46. [DOI] [PubMed] [Google Scholar]
- 3.Langton DJ, Jameson SS, Joyce TJ et al. Accelerating failure rate of the ASR total hip replacement. J Bone Joint Surg (Br) 2011; : 1,011–1,016. [DOI] [PubMed] [Google Scholar]
- 4.Smith AJ, Dieppe P, Vernon K, Porter M, Blom AW. Failure rates of stemmed metal-on-metal hip replacements: analysis of data from the National Joint Registry of England and Wales. Lancet 2012; : 1,199–1,204. [DOI] [PubMed] [Google Scholar]
- 5.Smith AJ, Dieppe P, Howard PW, Blom AW. National Joint Registry for England and Wales. Failure rates of metal-on-metal hip resurfacings: analysis of data from the National Joint Registry for England and Wales. Lancet 2012; : 1,759–1,766. [DOI] [PubMed] [Google Scholar]
- 6.Grammatopoulos G, Pandit H, Kwon YM et al. Hip resurfacings revised for inflammatory pseudotumour have a poor outcome. J Bone Joint Surg (Br) 2009; : 1,019–1,024. [DOI] [PubMed] [Google Scholar]
- 7.Matharu GS, Pynsent PB, Dunlop DJ. Revision of metal-on-metal hip replacements and resurfacings for adverse reaction to metal debris: a systematic review of outcomes. Hip Int 2014; : 311–320. [DOI] [PubMed] [Google Scholar]
- 8.Medical and Healthcare Products Regulatory Agency (MHRA) (2012) Medical device alert: all metal-on-metal (MoM) hip replacements. MDA/2012/036.www.mhra.gov.uk/ Accessed 6December2015.
- 9.European Federation of National Associations of Orthopaedics and Traumatology (2012) Consensus statement: Current evidence on the management of metal-on-metal bearings. www.efort.org/communications/pdf/2012_05_10_MoM_Consensus_statement.pdf Accessed 6December2015.
- 10.US Food and Drug Administration (2013) Medical devices. Metal-on-metal hip implants. Information for orthopedic surgeons. www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/ImplantsandProsthetics/MetalonMetalHipImplants/ucm241667.htm Accessed 6December2015. [Google Scholar]
- 11.Ebreo D, Bell PJ, Arshad H, Donell ST, Toms A, Nolan JF. Serial magnetic resonance imaging of metal-on-metal total hip replacements. Follow-up of a cohort of 28 mm Ultima TPS THRs. Bone Joint J 2013; : 1,035–1,039. [DOI] [PubMed] [Google Scholar]
- 12.van der Weegen W, Brakel K, Horn RJ et al. Asymptomatic pseudotumours after metal-on-metal hip resurfacing show little change within one year. Bone Joint J 2013; : 1,626–1,631. [DOI] [PubMed] [Google Scholar]
- 13.Hasegawa M, Miyamoto N, Miyazaki S, Wakabayashi H, Sudo A. Longitudinal magnetic resonance imaging of pseudotumors following metal-on-metal total hip arthroplasty. J Arthroplasty 2014; : 2,236–2,238. [DOI] [PubMed] [Google Scholar]
- 14.Nishii T, Sakai T, Takao M, Yoshikawa H, Sugano N. Ultrasound screening of periarticular soft tissue abnormality around metal-on-metal bearings. J Arthroplasty 2012; : 895–900. [DOI] [PubMed] [Google Scholar]
- 15.Garbuz DS, Hargreaves BA, Duncan CP, Masri BA, Wilson DR, Forster BB. The John Charnley Award: diagnostic accuracy of MRI versus ultrasound for detecting pseudotumors in asymptomatic metal-on-metal THA. Clin Orthop Relat Res 2014; : 417–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Almousa SA, Greidanus NV, Masri BA, Duncan CP, Garbuz DS. The natural history of inflammatory pseudotumors in asymptomatic patients after metal-on-metal hip arthroplasty. Clin Orthop Relat Res 2013; : 3,814–3,821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Medical and Healthcare products Regulatory Agency (MHRA) (2010) Medical device alert: ASR™hip replacement implant manufactured by DePuy International Ltd. MDA/2010/069. www.mhra.gov.uk/ Accessed 6December2015.
- 18.Dawson J, Fitzpatrick R, Carr A, Murray D. Questionnaire on the perceptions of patients about total hip replacement. J Bone Joint Surg (Br) 1996; : 185–190. [PubMed] [Google Scholar]
- 19.Beggs I, Bianchi S, Bueno A et al. European Society of Musculoskeletal Radiology. Musculoskeletal Ultrasound Technical Guidelines IV. Hip . www.essr.org/html/img/pool/hip.pdf Accessed 6December2015.
- 20.Douis H, Dunlop DJ, Pearson AM, O‘Hara JN, James SL. The role of ultrasound in the assessment of post-operative complications following hip arthroplasty. Skeletal Radiol 2012; : 1,035–1,046. [DOI] [PubMed] [Google Scholar]
- 21.Pynsent PB, Adams DJ, Disney SP. The Oxford hip and knee outcome questionnaires for arthroplasty. J Bone Joint Surg (Br) 2005; : 241–248. [DOI] [PubMed] [Google Scholar]
- 22.Murray DW, Fitzpatrick R, Rogers K et al. The use of the Oxford hip and knee scores. J Bone Joint Surg (Br) 2007; : 1,010–1,014. [DOI] [PubMed] [Google Scholar]
- 23.Pandit H, Glyn-Jones S, McLardy-Smith P et al. Pseudotumours associated with metal-on-metal hip resurfacings. J Bone Joint Surg (Br) 2008; : 847–851. [DOI] [PubMed] [Google Scholar]
- 24.Liddle AD, Satchithananda K, Henckel J et al. Revision of metal-on-metal hip arthroplasty in a tertiary center: a prospective study of 39 hips with between 1 and 4 years of follow-up. Acta Orthop 2013; : 237–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.R Development Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing 2011. Accessed 6December2015. [Google Scholar]
- 26.Reito A, Elo P, Puolakka T, Pajamäki J, Nieminen J, Eskelinen A. Repeated magnetic resonance imaging in 154 hips with large-diameter metal-on-metal hip replacement. Acta Orthop 2014; : 570–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Siddiqui IA, Sabah SA, Satchithananda K et al. A comparison of the diagnostic accuracy of MARS MRI and ultrasound of the painful metal-on-metal hip arthroplasty. Acta Orthop 2014; : 375–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nishii T, Sakai T, Takao M, Yoshikawa H, Sugano N. Is ultrasound screening reliable for adverse local tissue reaction after hip arthroplasty? J Arthroplasty 2014; : 2,239–2,244. [DOI] [PubMed] [Google Scholar]
- 29.Lainiala O, Elo P, Reito A, Pajamäki J, Puolakka T, Eskelinen A. Comparison of extracapsular pseudotumors seen in magnetic resonance imaging and in revision surgery of 167 failed metal-on-metal hip replacements. Acta Orthop 2014; : 474–479. [DOI] [PMC free article] [PubMed] [Google Scholar]