Abstract
EcoCyc (EcoCyc.org) is a freely accessible, comprehensive database that collects and summarizes experimental data for Escherichia coli K-12, the best-studied bacterial model organism. New experimental discoveries about gene products, their function and regulation, new metabolic pathways, enzymes and cofactors are regularly added to EcoCyc. New SmartTable tools allow users to browse collections of related EcoCyc content. SmartTables can also serve as repositories for user- or curator-generated lists. EcoCyc now supports running and modifying E. coli metabolic models directly on the EcoCyc website.
INTRODUCTION
Over the course of many decades, thousands of researchers have contributed to the experimental study of Escherichia coli. Considering its moderate-size genome that contains roughly 4500 genes, it is easy to assume that we already know most of what there is to know about E. coli—its genes, gene products and their functions, as well as its cellular metabolism and interactions with the environment. In fact, however, many facets of E. coli biology are still unknown; for example, there are still a number of essential genes of unknown function (e.g. (1)). Basic E. coli research continues to generate surprising insights, and new experimental methods enable researchers to fill holes in our knowledge and solve long-standing mysteries. Because E. coli serves as a model bacterium for many less well-studied organisms, new discoveries should have broad impact. One of the most significant uses of a model organism database such as EcoCyc is to collect and disseminate both longstanding knowledge as well as recent research advances in an easily accessible format. EcoCyc continues to serve in this role for the E. coli research community and, through its integration within the BioCyc collection of thousands of organism-specific databases, provides a simple way to find and compare orthologous genes and metabolic pathways across a wide spectrum of organisms.
Since our last publication on EcoCyc in the 2013 NAR Database Issue (2), many improvements to EcoCyc, the Pathway Tools software, the BioCyc family of websites and the display and analysis tools available there have been described elsewhere (3–6). Here, we focus on recent updates to the EcoCyc web site and on additions to the database that reflect new knowledge about E. coli biology.
UPDATES TO EcoCyc
Manual curation within EcoCyc continues to adopt a two-pronged approach. Priority is given to adding new functions for gene products when they are reported in the literature. Considerable effort also goes into updating older entries in the database; typically this is undertaken by reviewing all the proteins belonging to a specific family, metabolic pathway or regulon. Occasionally, large datasets containing, for example, gene essentiality or protein localization information are added computationally. An overview of the current data content of EcoCyc is shown in Table 1.
Table 1. EcoCyc content and E. coli gene product functions.
| Data type | Number (Release 20.1) |
|---|---|
| Genes | 4505 |
| Gene products covered by a mini-review | 3884 |
| Gene products with GO terms with EXP evidence | 3350 |
| Enzymes | 1567 |
| Metabolic reactions | 1913 |
| Compounds | 2699 |
| Transporters | 282 |
| Transport reactions | 485 |
| Transported substrates | 338 |
| Transcription factors | 204 |
| Regulatory interactions | 6399 |
| Transcription initiation | 3457 |
| Transcription attenuation | 23 |
| Regulation of translation | 212 |
| Enzyme modulation | 2675 |
| Other | 32 |
| Literature citations | 31 999 |
Update of the sequence version in EcoCyc from GenBank U00096.2 to U00096.3
As of May 2016 (release 20.0), EcoCyc uses the U00096.3 version of the E. coli K-12 MG1655 genome sequence. The GenBank sequence record had been updated from version U00096.2 to U00096.3 in 2013, because the U00096.2 sequence did not precisely correspond to a specific isolate of the MG1655 strain (7). Importantly for next-generation sequencing studies, U00096.2 differed from the genome of the strain deposited as the sequenced MG1655 strain at the American Type Culture Collection (ATCC 700926) and the Yale Coli Genetic Stock Center (CGSC7740). Sequence differences between common E. coli strains have been described by Freddolino et al. (8). Most significantly, the final sequenced strain differs from other MG1655 strains by carrying mutations that inactivate the transcriptional regulators encoded by crl and glpR and the galactitol transporter component encoded by gatC. Due to an IS1 element insertion and other indels, the nucleotide coordinates of genes and other features differ between U00096.2 and U00096.3. To ease the transition for researchers with datasets that use the prior genome coordinates, the EcoCyc web site is offering a coordinate mapping service that translates data files containing the old genome coordinates to new coordinates. The ‘Map Sequence Coordinates’ function can be found under the Genome menu on the EcoCyc homepage. However, researchers should keep in mind that the genome of any given laboratory stock of MG1655 will also differ from the published genome sequence, and that some differences will be physiologically significant.
Updates to the data content in EcoCyc
The transporter systems in EcoCyc
Since our last publication in the 2013 NAR database issue (2), 14 new transport proteins or complexes have been characterized and curated accordingly (Table 2). We have also reviewed and updated the curation of 48 proteins, both membrane and cystosolic, which belong to the functional superfamily of the phosphoenolpyruvate (PEP)-dependent, sugar transporting phosphotransferase systems (PTSsugar). This work extends our representation of the range of substrates, both physiological and non-physiological, that E. coli can transport across the membrane. For example, methyl α-d-glucoside was added as a non-physiological substrate of the glucose PTS, and d-sorbitol was added as a physiologically relevant substrate of the galactitol PTS. Our coverage of the literature was improved through the addition of a further 144 citations.
Table 2. New membrane transporters characterized in E. coli K-12 and curated in EcoCyc.
| Gene name (old gene name) | Protein Function | Reference |
|---|---|---|
| dauA (ychM) | C4 dicarboxylate transporter | (23) |
| ghxP (yjcD) | Guanine/hypoxanthine transporter | (24) |
| ghxQ (ygfQ) | Guanine/hypoxanthine transporter | (24) |
| adeQ (yicO) | Adenine transporter | (24) |
| satP (yaaH) | Succinate/acetate:H+ symporter | (25) |
| yihO | Putative sulfoquinovose transporter | (17) |
| cysZ | Sulfate:H+ symporter | (26) |
| yijE | Cystine exporter | (27) |
| tcyJ tcyL tcyN (fliY yecS yecC) | Cystine/cysteine ABC transporter | (28,29) |
| nimT (yeaN) | Drug efflux transporter | (30) |
| lysO (ybjE) | l-Lysine exporter | (31) |
| yjeH | l-Methionine/branched chain amino acid exporter | (32) |
| osmF yehY yehX yehW | Glycine betaine ABC transporter | (33) |
Update of transporter classification
We have reviewed and updated transporter classes within the Pathway Tools ontology. The class ‘a transporter’ within EcoCyc now contains 6 child classes (Figure 1) plus numerous sub-classes, named according to IUBMB recommendations as based on the Transporter Classification Database (9). All 577 transport proteins in EcoCyc are classified within this ontology, making it straightforward for a user to accurately determine the number and identity of proteins within a particular class. For example, searching EcoCyc with the term ‘a primary active transporter’ will return the transporter class of the same name containing the two child classes of ‘P-type ATPases’ (four proteins) and ‘an ATP binding cassette transporter’ (52 substrate binding proteins, 64 ATP binding proteins and 86 integral membrane subunits).
Figure 1.
Transporter classes in EcoCyc.
Update of electron transport pathways and respiratory enzymes
We have completed a long-term project to update the curation of electron transport (ET) pathways and respiratory enzymes in EcoCyc. An initiative to represent ET pathways in EcoCyc was first described in 2009 (10) along with the subsequent addition of 11 ET pathways. We have now added a further 15 pathways (Table 3), bringing the total number to 26. All pathways contain a fully referenced text summary, including (when known) information on energetics, isoenzyme involvement and the identity of membrane quinone(s). The curation of all 23 respiratory enzymes involved in ET pathways has also been updated. Particular attention has been given to ensuring that the correct cofactors of each respiratory enzyme are identified (when known). In addition, a new, recently-described cofactor, the 4Fe-3S iron–sulfur cluster of hydrogenase I (11), was added to the database as part of this project. Supplementary Table S1 summarizes the respiratory enzymes and their associated cofactors as currently represented in EcoCyc. Just over 200 references were added to the database, the majority of these dating from the last quarter of the 20th century, a period of intense research activity in E. coli bioenergetics.
Table 3. Electron transport pathways added to EcoCyc.
| Pathway name | Enzymes involved | Quinone species used |
|---|---|---|
| NADH to cytochrome bo oxidase electron transfer II | NADH:ubiquinone oxidoreductase II; cytochrome bo oxidase | Ubiquinone |
| NADH to cytochrome bd oxidase electron transfer II | NADH:ubiquinone oxidoreductase II; cytochrome bd-1 oxidase | Ubiquinone |
| Nitrate reduction VIIIb (dissimilatory) | NADH:ubiquinone oxidoreductase II; nitrate reductase A | Ubiquinone |
| Pyruvate to cytochrome bo oxidase electron transfer | Pyruvate oxidase; cytochrome bo oxidase | Ubiquinone |
| Pyruvate to cytochrome bd oxidase electron transfer | Pyruvate oxidase; cytochrome bd-I oxidase | Ubiquinone |
| Glycerol-3-phosphate to cytochrome bo oxidase electron transfer | Aerobic glycerol-3-phosphate dehydrogenase; cytochrome bo oxidase | Ubiquinone |
| Glycerol-3-phosphate to fumarate electron transfer | Anaerobic glycerol-3-phosphate dehydrogenase; fumarate reductase | Menaquinone |
| Nitrate reduction IX (dissimilatory) | Anaerobic glycerol-3-phosphate dehydrogenase; nitrate reductase A; nitrate reductase Z | Menaquinone |
| Nitrate reduction X (dissimilatory, periplasmic) | Aerobic glycerol-3-phosphate dehydrogenase; periplasmic nitrate reductase | Ubiquinone |
| d-Lactate to cytochrome bo oxidase electron transfer | d-Lactate dehydrogenase; cytochrome bo oxidase | Ubiquinone |
| Proline to cytochrome bo oxidase electron transfer | Proline dehydrogenase; cytochrome bo oxidase | Ubiquinone |
| Formate to nitrite electron transfer | Formate dehydrogenase; formate dehydrogenase N; formate-dependent nitrite reductase | Menaquinone |
| Hydrogen to dimethyl sulfoxide electron transfer | Hydrogenase I; hydrogenase II; dimethyl sulfoxide reductase | Menaquinone |
| Hydrogen to fumarate electron transfer | Hydrogenase II; fumarate reductase | Menaquinone |
| Hydrogen to trimethylamine N-oxide electron transfer | Hydrogenase I; hydrogenase II; trimethylamine N-oxide reductase | Menaquinone |
New transcription factors and updated information related to transcriptional regulation in EcoCyc
A total of 14 new transcription factors (TFs) regulating a variety of different biological processes have been identified in the experimental literature and have been added to the database since fall 2012. The functions of these TFs are summarized in Table 4. In addition to the new TFs, there has been an increase in other database objects like transcription units, regulatory interactions and transcription factor binding sites (TFBSs), promoters and terminators (Table 5).
Table 4. New transcription factors characterized in E. coli K-12 and curated in EcoCyc.
| Gene name (old gene name) | Processes regulated by the transcription factor | Reference |
|---|---|---|
| nimR (yeaM) | Resistance to 2-nitroimidazole | (30) |
| sutR (ydcN) | Sulfur metabolism | (34) |
| ydcI | Stress response | (35) |
| mraZ (yabB) | Cell division, cell wall | (36) |
| rclR (ykgD) | Survival under reactive chlorine stress | (37) |
| pgrR (ycjZ) | Peptidoglycan degradation | (38) |
| rcdA (ybjK) | Stress response, biofilm formation | (39) |
| ydfH | rspAB operon expression | (40) |
| yjjQ | Flagellar synthesis, capsule formation | (41) |
| yebK | Adaptation to growth in cellobiose minimal medium | (42) |
| ypdB | Carbon control network, nutrient scavenging | (43) |
| yedW | H2O2 sensing | (44) |
| cecR (ybiH) | Sensitivity to cefoperazone and chloramphenicol | (15) |
| decR (ybaO) | Cysteine detoxification | (16) |
Table 5. Data related to transcriptional regulation.
| Data type | Total | New |
|---|---|---|
| Transcription Unit | 3553 | 95 |
| Promoter | 3841 | 73 |
| Terminator | 283 | 31 |
| Transcription Factor | 205 | 14 |
| Transcription Factor Binding Site | 2836 | 199 |
| Regulatory Interaction | 3374 | 183 |
Table 6 summarizes updates to existing TFs within EcoCyc. For several TFs, active or inactive protein conformations have been identified. For example, it was shown that only the homotetrameric conformation of the quorum-sensing regulator LsrR is active, while the autoinducer-bound conformation (LsrR-AI-2) is inactive. Binding of AI-2 to LsrR may disrupt the tetrameric assembly of LsrR, resulting in its dissociation from DNA (12).
Table 6.
| Type of update | TFs |
|---|---|
| Updated summaries | AcrR, AraC, ArcA, BaeR, BasR (PmrA), BluR, BolA, CadC, ChbR, CpxR, CRP, CspA, CueR, DecR (YbaO), DksA, ExuR, FadR, FeaR, Fur, H-NS, HipB, HU, HypT, IHF, IscR, LacI, LeuO, LsrR, MalT, MarA, MarR, MarRAB, MazE, McbR, MlrA, MqsR, MraZ, NarL, NemR, NikR, NorR, NrdR, OmpR, PhoB, PspF, RbsR, RcnR, RcsA, RcsB, RcsB- BglJ, Rob, RpoD, RpoE, RpoH, RpoS, RstA, RutR, SdiA, SoxR, SoxS, TyrR, UxuR, YehT, YpdB, Zur |
| New conformations | LsrR (homotetramer), LsrR-AI-2, MetJ-MTA, MetJ-adenine, IscR-2Fe-2S |
| Relocalization of TFBSs | PuuR |
The newly discovered iron-sulfur cluster-bound conformation of IscR (IscR-[2Fe-2S]) was shown to regulate the expression of genes involved the iron-sulfur cluster assembly pathway through negative feedback that depends on the cellular Fe-S cluster demand (13). IscR-[2Fe-2S] negatively regulates the expression of iscRSUA, genes of the Isc Fe–S cluster biosynthesis pathway, and activates expression of the sufABCDSE operon, which encodes the Suf Fe-S cluster biosynthesis pathway. Coordinated regulation of these two pathways maintains differential control of Fe–S cluster biogenesis and ensures viability under a variety of growth conditions (14). Both apo-IscR and the IscR-[2Fe–2S] holoprotein conformations were able to activate the Suf pathway (14).
Genomic SELEX screening usually results in the discovery of many target sites for transcriptional regulators. Surprisingly, only one target was found for each of the two newly discovered regulators, CecR and DecR, These regulators were found to be associated with novel roles in the control of sensitivity to cefoperazone and chloramphenicol (CecR) (15) and cysteine detoxification systems (DecR) (16).
In EcoCyc, evidence codes are attached to many types of data and generally contain a supporting literature citation. Filling gaps in our representation of transcriptional regulation, we have added missing references to the published experimental evidence to a set of 225 promoter objects.
Predicted transporters and transcription factors
To encourage further research on E. coli gene products that have no known function to date, the EcoCyc project has generated a set of public SmartTables that contain (i) inner membrane proteins with minimal or no characterization, (ii) predicted, but uncharacterized inner membrane transporters and (iii) predicted transcription factors. These tables will be updated on an ongoing basis and are available under the ‘SmartTables > Public SmartTables’ menu or by using the following links:
http://ecocyc.org/group?id=biocyc13-4655-3682893892 (uncharacterized inner membrane proteins)
http://ecocyc.org/group?id=biocyc17-4655-3682299327 (predicted inner membrane transporters)
http://ecocyc.org/group?id=biocyc17-1553-3682788185 (predicted transcriptional regulators)
Static versions of these tables are available as Supplementary Tables S2–S4.
E. coli metabolism
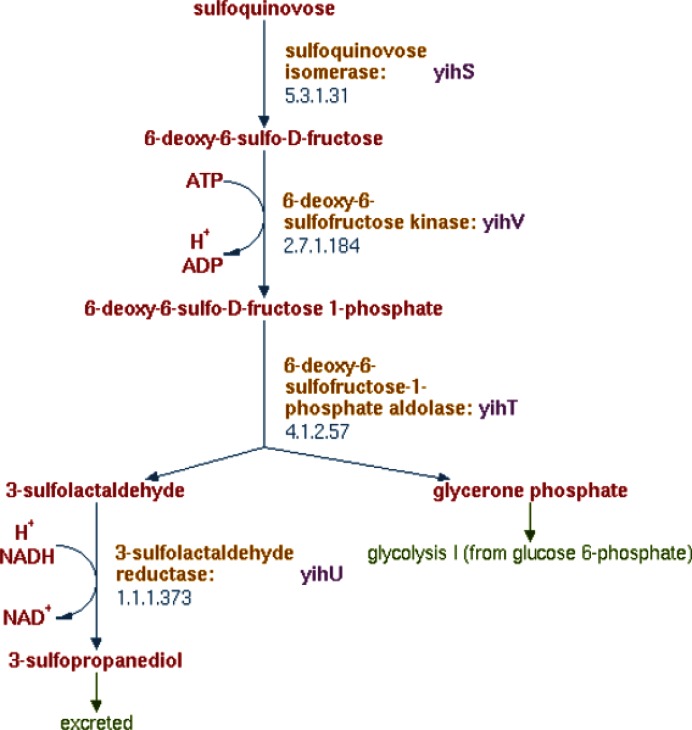
Although the basic metabolic capabilities of E. coli have been well studied, surprising new discoveries continue to be made. A recent example is E. coli's ability to utilize the six-carbon sulfur sugar sulfoquinovose as the sole source of carbon and energy for growth (17). Sulfoquinovose is a major component of organo-sulfur compounds in nature (18). It is synthesized by higher plants, mosses, ferns, algae and most photosynthetic bacteria and serves as the polar headgroup of the sulfolipid in photosynthetic membranes (19,20). Sulfoquinovose is structurally similar to glucose, and degradation of this sugar follows a pathway that is highly similar to glycolysis (Figure 2). The sulfur-containing three-carbon degradation product sulfopropanediol is excreted and can be utilized as both a carbon and sulfur source by other organisms (21). However, open questions remain. Although proteins with suggestive predicted functions or mutant phenotypes are encoded in the genomic vicinity of the sulfoquinovose-degrading enzymes, neither the importer for sulfoquinovose nor the exporter for sulfopropanediol has been firmly established, and no regulatory mechanisms are yet known.
Figure 2.
Sulfoquinovose degradation pathway.
New discoveries in E. coli K-12 also further our understanding of the physiology of microbial cell envelopes as exemplified by the recent characterization of a periplasmic methionine sulfoxide reducing system (22). This system, comprised of a periplasmic methionine sulfoxide reductase (MsrP) and an inner membrane, heme-binding, quinol dehydrogenase (MsrQ), functions to protect periplasmic proteins from oxidative damage and is conserved throughout Gram-negative bacteria (22). The use of membrane quinols as a source of reducing power in the cell envelope is a novel finding and represents a notable advance in our understanding of how bacteria repair damaged proteins.
Updates to the EcoCyc website and Pathway Tools software
Updates to the genome browser
We have added a new zoom level to the BioCyc genome browser. It is now possible to zoom to the sequence level, which will show details such as transcription start sites, transcription factor binding sites, other regulatory sites such as attenuators and interaction sites for small regulatory RNAs, as well as gene and protein sequences. This zoom level thus enables inspection of the relative location of sites within the sequence. Figure 3 shows the new zoom level, comparing it to two previously available zoom levels.
Figure 3.
Zoom levels of the genome browser, with the previously available ‘genes’ and ‘sites’ levels compared to the new ‘sequence’ level.
Updates to SmartTables
The SmartTables tools for manipulating sets of genes, chemical compounds, and other objects within EcoCyc and other BioCyc databases have been expanded in several respects. A new set of special SmartTables (see menu SmartTables→Special SmartTables) allows the user to explore and manipulate various sets of entities within EcoCyc, such as all chemical compounds, all metabolic pathways, all promoters, all terminators, all riboswitches, all non-coding RNAs, and all proteins or protein subtypes such as all transporters and all transcription factors.
Execution of the E. coli metabolic model via the EcoCyc Website
Because the metabolic model associated with EcoCyc is derived directly from EcoCyc using the MetaFlux module of Pathway Tools, its data content has continued to evolve as we update the set of metabolic reactions and transporters within EcoCyc. Previously, it was only possible to execute the EcoCyc metabolic model using the downloadable Pathway Tools software. To make it more accessible to users, the model can now be executed directly on the EcoCyc web site. Web-based metabolic models are also available for two other gut microbiome organisms in the BioCyc database collection, Bacteroides thetaiotaomicron and Eubacterium rectale.
In basic terms, a metabolic model consists of a set of active reactions plus the conditions of growth of the organism; the models stored within the EcoCyc website contain both. The active reactions correspond to those reactions that are active at a given time based on cellular regulation, and can be either the full set or a subset of the reactions stored within EcoCyc. For each modeled growth situation, the conditions of growth consist of the nutrients available to the growing E. coli cell, the set of biomass metabolites (the end products of the metabolic network) produced by the cell, and the metabolic end-products that are secreted by the cell (the secretions). The process of running a metabolic model through the EcoCyc website consists of the following steps.
First, choose EcoCyc as your current organism by clicking Select Organism Database in the upper-right corner of the screen.
Second, click Run Metabolic Model under the Metabolism menu.
Third, log in to your EcoCyc (BioCyc) account if you are not already logged in.
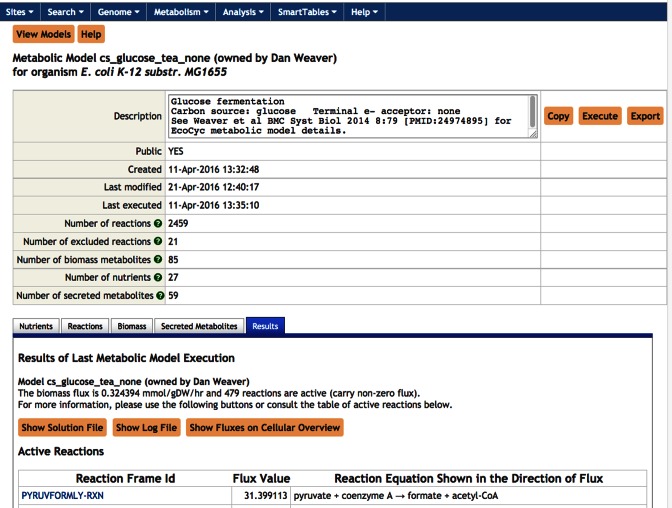
At this point you can either select an existing model to run, or you can create a new model if none of the existing models cover exactly the situation you wish to model. Usually, it is easiest to create a new model by copying and editing an existing model. You can select an existing model to run from either the list of models that other people have made public, or from a list of models that you may have saved in the past. For example, select the public Glucose Fermentation model to select a model that anaerobically ferments glucose. Once you select a model you can inspect the nutrients, reactions, biomass metabolites, and secretions that it contains by selecting a tab of the corresponding name toward the bottom of the model-summary page (see Figure 4). To actually run the model, click Execute within the Results tab. The result of running a model is a list of steady-state reaction flux values for those metabolic reactions that carry non-zero flux, which are presented in a table. For example, Figure 4 shows that the two highest fluxes in the entire metabolic network during glucose fermentation are through two reactions in glycolysis. Those fluxes can be painted on the EcoCyc metabolic map diagram by clicking the button Show Fluxes on Cellular Overview. Additional information is available from the solution file and the log file (which can be accessed via buttons in the Reactions tab), such as the uptake fluxes of each nutrient. Imagine you want to run a model that ferments galactose instead of glucose. To do so, click the Copy button at the top of the model-selection page, enter the Nutrients tab, and then replace β-d-glucopyranose with β-d-galactose. The Nutrients tab allows you to place upper and lower bounds on the uptake rates of different nutrients. Since models typically attempt to optimize the cellular growth rate, an upper bound must be provided for some nutrient, otherwise the model would attempt to produce infinite growth, which would stymie the mathematical solver software. Gene knock-outs can be simulated by specifying reactions to remove from the model from within the Reactions tab. Detailed instructions for running a metabolic model through the EcoCyc website are available by selecting the ‘Getting Started Guide’ link (http://ecocyc.org/PToolsWebsiteHowto.shtml#metabolicmodels).
Figure 4.
Running metabolic models on the EcoCyc web site.
ECOCYC AVAILABILITY
EcoCyc is freely and openly available to all. See http://ecocyc.org/download.shtml for download information. New versions of the downloadable EcoCyc data files and of the EcoCyc website are released three times per year. Access to the website is free; users are required to register for a free account after viewing more than 30 pages in a given month.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
FUNDING
National Institute of General Medical Sciences of the National Institutes of Health under Award Numbers U24GM077678 to P.D.K. and R01GM110597 to J.C.-V. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Funding for open access charge: National Institute of General Medical Sciences of the National Institutes of Health.
Conflict of interest statement. None declared.
REFERENCES
- 1.McClung D.J., Calixto A., Mosera M.N., Kumar R., Neidle E.L., Elliott K.T. Novel heterologous bacterial system reveals enhanced susceptibility to DNA damage mediated by yqgF, a nearly ubiquitous and often essential gene. Microbiology. 2016;162:1808–1821. doi: 10.1099/mic.0.000355. [DOI] [PubMed] [Google Scholar]
- 2.Keseler I.M., Mackie A., Peralta-Gil M., Santos-Zavaleta A., Gama-Castro S., Bonavides-Martinez C., Fulcher C., Huerta A.M., Kothari A., Krummenacker M., et al. EcoCyc: fusing model organism databases with systems biology. Nucleic Acids Res. 2013;41:D605–D612. doi: 10.1093/nar/gks1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karp P.D., Weaver D., Paley S., Fulcher C., Kubo A., Kothari A., Krummenacker M., Subhraveti P., Weerasinghe D., Gama-Castro S., et al. The EcoCyc database. EcoSal Plus. 2014;2014 doi: 10.1128/ecosalplus.ESP-0009-2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weaver D.S., Keseler I.M., Mackie A., Paulsen I.T., Karp P.D. A genome-scale metabolic flux model of Escherichia coli K-12 derived from the EcoCyc database. BMC Syst. Biol. 2014;8:79. doi: 10.1186/1752-0509-8-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karp P.D., Latendresse M., Paley S.M., Krummenacker M., Ong Q.D., Billington R., Kothari A., Weaver D., Lee T., Subhraveti P., et al. Pathway Tools version 19.0 update: software for pathway/genome informatics and systems biology. Brief Bioinform. 2015;17:877–890. doi: 10.1093/bib/bbv079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caspi R., Billington R., Ferrer L., Foerster H., Fulcher C.A., Keseler I.M., Kothari A., Krummenacker M., Latendresse M., Mueller L.A., et al. The MetaCyc database of metabolic pathways and enzymes and the BioCyc collection of pathway/genome databases. Nucleic Acids Res. 2016;44:D471–480. doi: 10.1093/nar/gkv1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Plunkett G., 3rd, Richardson A.J., Rudd K.E. 2013. Unpublished.
- 8.Freddolino P.L., Amini S., Tavazoie S. Newly identified genetic variations in common Escherichia coli MG1655 stock cultures. J. Bacteriol. 2012;194:303–306. doi: 10.1128/jb.06087-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saier M.H., Jr., Reddy V.S., Tsu B.V., Ahmed M.S., Li C., Moreno-Hagelsieb G. The transporter classification database (TCDB): recent advances. Nucleic Acids Res. 2016;44:D372–D379. doi: 10.1093/nar/gkv1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keseler I.M., Bonavides-Martinez C., Collado-Vides J., Gama-Castro S., Gunsalus R.P., Johnson D.A., Krummenacker M., Nolan L.M., Paley S., Paulsen I.T., et al. EcoCyc: a comprehensive view of Escherichia coli biology. Nucleic Acids Res. 2009;37:D464–470. doi: 10.1093/nar/gkn751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pandelia M.E., Bykov D., Izsak R., Infossi P., Giudici-Orticoni M.T., Bill E., Neese F., Lubitz W. Electronic structure of the unique [4Fe-3S] cluster in O2-tolerant hydrogenases characterized by 57Fe Mossbauer and EPR spectroscopy. Proc. Natl. Acad. Sci. U.S.A. 2013;110:483–488. doi: 10.1073/pnas.1202575110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu M., Tao Y., Liu X., Zang J. Structural basis for phosphorylated autoinducer-2 modulation of the oligomerization state of the global transcription regulator LsrR from Escherichia coli. J. Biol. Chem. 2013;288:15878–15887. doi: 10.1074/jbc.M112.417634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giel J.L., Nesbit A.D., Mettert E.L., Fleischhacker A.S., Wanta B.T., Kiley P.J. Regulation of iron-sulphur cluster homeostasis through transcriptional control of the Isc pathway by [2Fe-2S]-IscR in Escherichia coli. Mol. Microbiol. 2013;87:478–492. doi: 10.1111/mmi.12052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mettert E.L., Kiley P.J. Coordinate regulation of the Suf and Isc Fe-S cluster biogenesis pathways by IscR is essential for viability of Escherichia coli. J. Bacteriol. 2014;196:4315–4323. doi: 10.1128/JB.01975-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamanaka Y., Shimada T., Yamamoto K., Ishihama A. Transcription factor CecR (YbiH) regulates a set of genes affecting the sensitivity of Escherichia coli against cefoperazone and chloramphenicol. Microbiology. 2016;162:1253–1264. doi: 10.1099/mic.0.000292. [DOI] [PubMed] [Google Scholar]
- 16.Shimada T., Tanaka K., Ishihama A. Transcription factor DecR (YbaO) controls detoxification of L-cysteine in Escherichia coli. Microbiology. 2016;162:1698–1707. doi: 10.1099/mic.0.000337. [DOI] [PubMed] [Google Scholar]
- 17.Denger K., Weiss M., Felux A.K., Schneider A., Mayer C., Spiteller D., Huhn T., Cook A.M., Schleheck D. Sulphoglycolysis in Escherichia coli K-12 closes a gap in the biogeochemical sulphur cycle. Nature. 2014;507:114–117. doi: 10.1038/nature12947. [DOI] [PubMed] [Google Scholar]
- 18.Harwood J.L., Nicholls R.G. The plant sulpholipid– a major component of the sulphur cycle. Biochem. Soc. Trans. 1979;7:440–447. doi: 10.1042/bst0070440. [DOI] [PubMed] [Google Scholar]
- 19.Benning C. Questions remaining in sulfolipid biosynthesis: a historical perspective. Photosynth Res. 2007;92:199–203. doi: 10.1007/s11120-007-9144-6. [DOI] [PubMed] [Google Scholar]
- 20.Benning C. Biosynthesis and Function of the Sulfolipid Sulfoquinovosyl Diacylglycerol. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998;49:53–75. doi: 10.1146/annurev.arplant.49.1.53. [DOI] [PubMed] [Google Scholar]
- 21.Denger K., Huhn T., Hollemeyer K., Schleheck D., Cook A.M. Sulfoquinovose degraded by pure cultures of bacteria with release of C3-organosulfonates: complete degradation in two-member communities. FEMS Microbiol. Lett. 2012;328:39–45. doi: 10.1111/j.1574-6968.2011.02477.x. [DOI] [PubMed] [Google Scholar]
- 22.Gennaris A., Ezraty B., Henry C., Agrebi R., Vergnes A., Oheix E., Bos J., Leverrier P., Espinosa L., Szewczyk J., et al. Repairing oxidized proteins in the bacterial envelope using respiratory chain electrons. Nature. 2015;528:409–412. doi: 10.1038/nature15764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karinou E., Compton E.L., Morel M., Javelle A. The Escherichia coli SLC26 homologue YchM (DauA) is a C(4)-dicarboxylic acid transporter. Mol. Microbiol. 2013;87:623–640. doi: 10.1111/mmi.12120. [DOI] [PubMed] [Google Scholar]
- 24.Papakostas K., Botou M., Frillingos S. Functional identification of the hypoxanthine/guanine transporters YjcD and YgfQ and the adenine transporters PurP and YicO of Escherichia coli K-12. J. Biol. Chem. 2013;288:36827–36840. doi: 10.1074/jbc.M113.523340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sa-Pessoa J., Paiva S., Ribas D., Silva I.J., Viegas S.C., Arraiano C.M., Casal M. SATP (YaaH), a succinate-acetate transporter protein in Escherichia coli. Biochem. J. 2013;454:585–595. doi: 10.1042/BJ20130412. [DOI] [PubMed] [Google Scholar]
- 26.Zhang L., Jiang W., Nan J., Almqvist J., Huang Y. The Escherichia coli CysZ is a pH dependent sulfate transporter that can be inhibited by sulfite. Biochim. Biophys. Acta. 2014;1838:1809–1816. doi: 10.1016/j.bbamem.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 27.Yamamoto K., Nonaka G., Ozawa T., Takumi K., Ishihama A. Induction of the Escherichia coli yijE gene expression by cystine. Biosci. Biotechnol. Biochem. 2015;79:218–222. doi: 10.1080/09168451.2014.972328. [DOI] [PubMed] [Google Scholar]
- 28.Deutch C.E., Spahija I., Wagner C.E. Susceptibility of Escherichia coli to the toxic L-proline analogue L-selenaproline is dependent on two L-cystine transport systems. J. Appl. Microbiol. 2014;117:1487–1499. doi: 10.1111/jam.12623. [DOI] [PubMed] [Google Scholar]
- 29.Chonoles Imlay K.R., Korshunov S., Imlay J.A. Physiological roles and adverse effects of the two cystine importers of Escherichia coli. J. Bacteriol. 2015;197:3629–3644. doi: 10.1128/JB.00277-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ogasawara H., Ohe S., Ishihama A. Role of transcription factor NimR (YeaM) in sensitivity control of Escherichia coli to 2-nitroimidazole. FEMS Microbiol. Lett. 2015;362:1–8. doi: 10.1093/femsle/fnu013. [DOI] [PubMed] [Google Scholar]
- 31.Pathania A., Sardesai A.A. Distinct paths for basic amino acid export in Escherichia coli: YbjE (LysO) mediates export of L-lysine. J. Bacteriol. 2015;197:2036–2047. doi: 10.1128/JB.02505-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu Q., Liang Y., Zhang Y., Shang X., Liu S., Wen J., Wen T. YjeH is a novel exporter of l-methionine and branched-chain amino acids in Escherichia coli. Appl. Environ. Microbiol. 2015;81:7753–7766. doi: 10.1128/AEM.02242-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lang S., Cressatti M., Mendoza K.E., Coumoundouros C.N., Plater S.M., Culham D.E., Kimber M.S., Wood J.M. YehZYXW of Escherichia coli Is a Low-Affinity, Non-Osmoregulatory Betaine-Specific ABC Transporter. Biochemistry. 2015;54:5735–5747. doi: 10.1021/acs.biochem.5b00274. [DOI] [PubMed] [Google Scholar]
- 34.Yamamoto K., Nakano M., Ishihama A. Regulatory role of transcription factor SutR (YdcN) in sulfur utilization in Escherichia coli. Microbiology. 2015;161:99–111. doi: 10.1099/mic.0.083550-0. [DOI] [PubMed] [Google Scholar]
- 35.Solomon L., Shah A., Hannagan S., Wilson J.W. Bacterial genus-specific tolerance for YdcI expression. Curr. Microbiol. 2014;69:640–648. doi: 10.1007/s00284-014-0631-7. [DOI] [PubMed] [Google Scholar]
- 36.Eraso J.M., Markillie L.M., Mitchell H.D., Taylor R.C., Orr G., Margolin W. The highly conserved MraZ protein is a transcriptional regulator in Escherichia coli. J. Bacteriol. 2014;196:2053–2066. doi: 10.1128/JB.01370-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parker B.W., Schwessinger E.A., Jakob U., Gray M.J. The RclR protein is a reactive chlorine-specific transcription factor in Escherichia coli. J. Biol. Chem. 2013;288:32574–32584. doi: 10.1074/jbc.M113.503516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shimada T., Yamazaki K., Ishihama A. Novel regulator PgrR for switch control of peptidoglycan recycling in Escherichia coli. Genes Cells. 2013;18:123–134. doi: 10.1111/gtc.12026. [DOI] [PubMed] [Google Scholar]
- 39.Shimada T., Katayama Y., Kawakita S., Ogasawara H., Nakano M., Yamamoto K., Ishihama A. A novel regulator RcdA of the csgD gene encoding the master regulator of biofilm formation in Escherichia coli. Microbiologyopen. 2012;1:381–394. doi: 10.1002/mbo3.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sakihama Y., Mizoguchi H., Oshima T., Ogasawara N. YdfH identified as a repressor of rspA by the use of reduced genome Escherichia coli MGF-01. Biosci. Biotechnol. Biochem. 2012;76:1688–1693. doi: 10.1271/bbb.120273. [DOI] [PubMed] [Google Scholar]
- 41.Wiebe H., Gurlebeck D., Gross J., Dreck K., Pannen D., Ewers C., Wieler L.H., Schnetz K. YjjQ Represses Transcription of flhDC and Additional Loci in Escherichia coli. J. Bacteriol. 2015;197:2713–2720. doi: 10.1128/JB.00263-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Parisutham V., Lee S.K. Novel functions and regulation of cryptic cellobiose operons in Escherichia coli. PLoS One. 2015;10:e0131928. doi: 10.1371/journal.pone.0131928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fried L., Behr S., Jung K. Identification of a target gene and activating stimulus for the YpdA/YpdB histidine kinase/response regulator system in Escherichia coli. J. Bacteriol. 2013;195:807–815. doi: 10.1128/JB.02051-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Urano H., Umezawa Y., Yamamoto K., Ishihama A., Ogasawara H. Cooperative regulation of the common target genes between H(2)O(2)-sensing YedVW and Cu(2)(+)-sensing CusSR in Escherichia coli. Microbiology. 2015;161:729–738. doi: 10.1099/mic.0.000026. [DOI] [PubMed] [Google Scholar]