Abstract
Analysis of signaling pathways and their crosstalk is a cornerstone of systems biology. Thousands of papers have been published on these topics. Surprisingly, there is no database that carefully and explicitly documents crosstalk between specific pairs of signaling pathways. We have developed XTalkDB (http://www.xtalkdb.org) to fill this very important gap. XTalkDB contains curated information for 650 pairs of pathways from over 1600 publications. In addition, the database reports the molecular components (e.g. proteins, hormones, microRNAs) that mediate crosstalk between a pair of pathways and the species and tissue in which the crosstalk was observed. The XTalkDB website provides an easy-to-use interface for scientists to browse crosstalk information by querying one or more pathways or molecules of interest.
INTRODUCTION
Cells assess and respond to their environment via signaling pathways. A signaling pathway typically contains a well-defined set of regulatory and physical interactions among receptors, intermediate signaling proteins, transcription factors and target genes. The perception of an external signal by receptors causes the activities of intermediate proteins to change and ultimately results in the regulation of transcription factors, which modulate the expression of target genes. Stimulation of one pathway's receptors may also result in regulatory downstream effects on the target genes of a second, biochemically distinct pathway (Figure 1). We refer to this phenomenon as ‘pathway crosstalk.’ Crosstalk is widely studied in biology and in medicine. Crosstalk between pairs of signaling pathways has been identified in healthy organismal development (1). A number of developmental processes rely on crosstalk among the well studied and phylogenetically conserved Notch, Wnt, and Hedgehog pathways (2–4). Crosstalk has also been shown to govern inflammatory responses to pathogenic stress and tissue damage (5,6). Aberrant regulation of crosstalk has been linked to cancer (7) and neurodegeneration (8). In the context of a specific disease, crosstalk studies attempt to elucidate the role of a pathway or pathway pair in that pathology, often to identify therapeutic targets (9–11). Crosstalk is challenging to explore and understand since it depends on the organism, the tissue, the external signal and the experimental context. A database that explicitly documents experimentally confirmed instances of pathway crosstalk will be a valuable resource. Existing signaling pathway databases usually document information on the proteins and interactions within each pathway (see ‘Comparison to Other Databases’ under ‘DISCUSSION’). We have developed XTalkDB (http://www.xtalkdb.org) to fill this very important gap.
Figure 1.
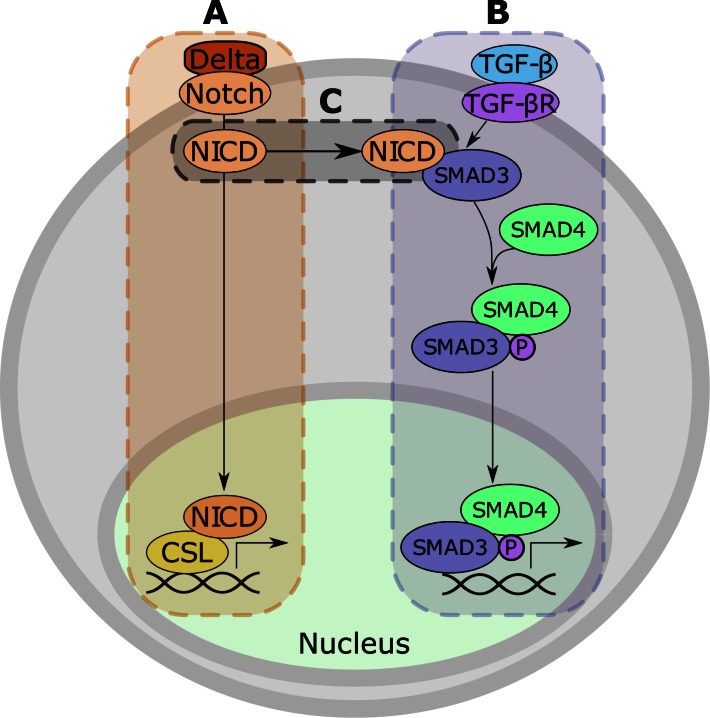
An example of activating crosstalk. (A) The Notch signaling pathway. When the Delta ligand binds to Notch, the Notch intracellular domain (NICD) is cleaved and translocates to the nucleus, where it binds to CSL. The NICD:CSL complex controls the transcription of developmental genes. (B) The TGF-β signaling pathway. The binding of TGF-β to its receptor results in the heterodimerization of SMAD3 and SMAD4 and the subsequent phosphorylation and translocation of the complex to the nucleus. Here, this complex regulates the transcription of genes involved in differentiation. (C) Crosstalk from the Notch signaling pathway to the TGF-β signaling pathway occurs when NICD binds to SMAD3 to further promote complex formation between SMAD3 and SMAD4. After crosstalk, the transcriptional complex includes NICD (not shown in the figure).
RESULTS
The XTalkDB data model
XTalkDB uses a biologically grounded principle to determine if there is crosstalk from one pathway (say, A) to another (say, B): an experiment demonstrates that perturbing at least one protein in pathway A causes a response in or downstream of pathway B. We manually curated all entries of pathway crosstalk in XTalkDB. For every pair of pathways, XTalkDB records detailed information describing the experimental conditions under which crosstalk was discovered:
the PubMed id of the paper that reported the crosstalk,
the direction, e.g. from the Notch to the TGF-β pathway,
type of regulation, i.e. activation or inhibition of B,
if the crosstalk is mediated by a transcriptional event, i.e. pathway A regulates the expression of a gene whose protein product is a member of pathway B,
the molecules that mediate the crosstalk, and
the tissue type (mapped to the Brenda Tissue Ontology) (12) and the organism.
We discuss these features further in ‘Curation procedure.’ Table S1 in Supplementary Section 1 includes detailed descriptions of all features in XTalkDB.
We motivate these features using the Notch and TGF-β pathways as examples. Crosstalk is asymmetric, i.e. crosstalk from one pathway to another is not indicative of crosstalk in the opposite direction. For example, the crosstalk from the Notch to the TGF-β pathway (13) involves different mechanisms and proteins than the crosstalk from the TGF-β to the Notch pathway (14). In the first case, the crosstalk is mediated by the interaction between the Notch intracellular domain (NICD) and SMAD3. This interaction promotes complex formation between SMAD3 and SMAD4 (Figure 1) (13). This crosstalk was identified in the Gallus gallus embryo. In the latter case, TGF-β signaling induces the expression of Jagged1, a ligand for the Notch pathway (14), i.e. the crosstalk is mediated by a transcriptional event. The corresponding experiment was conducted in human bone marrow cells. In both cases, the first pathway activates the second. XTalkDB includes several examples where the crosstalk is inhibitory or there is crosstalk in one direction but not in the other.
Curation procedure
For each pathway pair, we used the following procedure to manually curate and identify publications in the literature that may support their crosstalk.
Select two pathways to be queried (referred to as ‘A’ and ‘B’). For every such selection, there are two ordered pairs: (A, B) and (B, A). Note that the crosstalk from A → B is different from crosstalk from B → A.
We searched PubMed for publications that may provide evidence for crosstalk between two pathways using a structured query. We formatted these queries as follows: ‘A AND B AND crosstalk’ or ‘A AND B AND pathway’ Occasionally, we modified these queries by using synonyms for pathway names or by including proteins within the pathways. For example, we used the query ‘GLP-1 Adipocytokine Signaling’ instead of ‘Insulin Signaling AND Adipocytokine AND pathway.’ The downloadable files provided on the XTalkDB website specify the precise queries for each pathway pair.
If none of the queries returned any results, we recorded each of the pathway pairs with a dummy PubMed id.
We processed the search results in order of relevance.
If there were at most 10 publications returned, we read each abstract. Otherwise, we selected paper titles that we judged were likely to report crosstalk.
- Note that some publications may support crosstalk from pathway A to pathway B and other papers in the other direction. It is also possible for a publication to support crosstalk in both directions. We describe how we curated a paper for evidence of crosstalk from pathway A to pathway B. We used an analogous procedure for crosstalk in the other direction. In effect, we evaluated each publication twice.
- We searched the abstract for a sentence that supported crosstalk. We recorded that there was no crosstalk if the abstract had enough evidence to support this conclusion. For example, an abstract may have mentioned both pathways (among others) but did not mention them together or the paper was included in the results because it reported intercellular crosstalk.
- If we found a sentence in the abstract that supports crosstalk or there was not enough information in the abstract to conclude that there is no crosstalk, we then searched the full text of the paper (when available) for sentences that contained the names of one or both pathways. We then read each of these sentences within its context as well as the ‘Discussion’ or ‘Conclusion’ section of the paper to judge their support of crosstalk.
- We also examined the publication for sentences that provided further information on crosstalk: the molecules that mediate the crosstalk, whether pathway A activates or inhibits pathway B and the species and tissue type in which the experiment reported in the publication was performed.
- For each pair of pathways shown to crosstalk in a publication, we recorded the following information in the database: whether the crosstalk was transcriptional, the regulation type (activating or inhibiting), molecular component A, molecular component B, species, tissue, condition and the sentence supporting the crosstalk between the two pathways. Supplementary Table S1 contains all the attributes recorded in XTalkDB and their descriptions.
- If the publication did not support crosstalk, we recorded that this publication had no evidence for crosstalk from pathway A to pathway B.
Additional notes on the curation procedure.
Through this procedure, we recorded up to 20 attributes for each pair of pathways and each publication (Supplementary Section 1).
If the full text of a paper was not available, we recorded as much information (on support for crosstalk or lack thereof) from the abstract.
On occasion, a publication returned by our search query was a review article. In such a case, we recorded the information documenting crosstalk available in the review article instead of the original source article that reported the crosstalk. We opted for this strategy since we wanted to document which paper and sentence led to the entry in XTalkDB.
Through this curation procedure, we recorded (pathway A, pathway B, PubMed id) triples where the publication did not provide support for crosstalk from pathway A to pathway B. These triples are part of the file downloads on the XTalkDB website. However, the web interface does not include them in the search results.
It was not possible to confirm that a pathway pair does not crosstalk. What we could conclude from this process was that none of the publications that we have curated contained evidence for crosstalk between that pair of pathways.
Publications that we curated typically monitored a pathway by measuring the expression of a target (reporter) gene or the activity of a representative protein. We recorded that pathway A activated (respectively, inhibited) pathway B if a publication reported that the stimulation of pathway A activated (respectively, inhibited) a representative gene or protein for B.
The molecules that mediate the crosstalk between a pair of pathways may not directly interact. Hence, these molecules do not always provide information on the mechanism underlying the crosstalk.
Pathway selection
We selected pathway names from the KEGG database (15) since it has one of the most comprehensive collections of pathways. KEGG divides pathways into seven categories: metabolism, genetic information processing, environmental information processing, cellular processes, organismal systems, human diseases and drug development. We selected the following categories related to intracellular signaling: environmental information processing (signal transduction and signaling molecules and interaction), cellular processes (cell growth and death and cell community) and organismal systems (immune system and endocrine system). Since the definition of crosstalk relies on the transduction of a signal from one pathway to another, we limited ourselves to those pathways that have at least one receptor and one transcription factor.
Statistics on XTalkDB
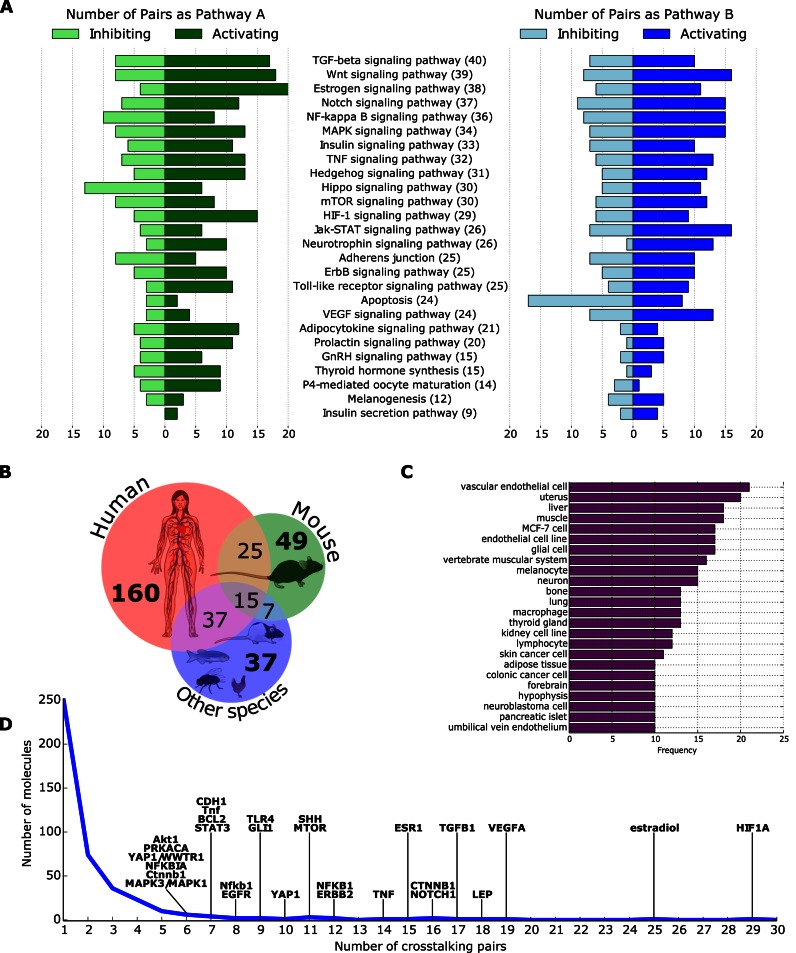
XTalkDB contains information for 650 ordered pairs of pathways based on the curation of over 1600 publications. Of these 650 pairs, XTalkDB contains literature evidence supporting crosstalk for 345 pairs of pathways (53%). For the remaining pathway pairs, the curated publications do not contain any evidence for crosstalk. All pathways in XTalkDB participate in at least one instance of crosstalk (Figure 2A). As many as 14 pathways have crosstalk to or from at least half the pathways. This subset includes phylogenetically-conserved pathways such as Hedgehog, Notch and Wnt and widely-studied pathways such as TGF-β, NF-κB and MAPK. In contrast, the pathways that are less involved in crosstalk may be either poorly studied, tissue-specific (e.g. Prolactin signaling and oocyte maturation) and/or responsible for the production of specific molecules (e.g. Thyroid synthesis, Insulin secretion and Melanogenesis). Interestingly, as many as 17 upstream pathways inhibit the Apoptosis pathway, perhaps due to the prevalence of cancer-related publications on crosstalk. Crosstalk was activating for 74% and inhibiting for 41% pairs. Note that a pathway can both activate and inhibit another pathway. Crosstalk was transcriptional for 48% of the pairs. The curated publications in XTalkDB span 17 species, with 237 and 96 pathway pairs cataloged in human and mouse, respectively (Figure 2B). The experiments recorded in XTalkDB were performed in a wide range of tissues. As many as 24 tissues supported at least 10 crosstalking pairs each, with vascular endothelial cells, uterus, liver and muscle being the most common (Figure 2C). The database contains information on over 440 molecules, each of which can mediate the crosstalk between one or more pairs of pathways (Figure 2D). A vast majority of these molecules (376, 85%) act as mediators for three or fewer pathway pairs. A smaller number (30) mediate crosstalk between six or more pathway pairs. These molecules include ligands (e.g. SHH, TGFB1, TNF), receptors (e.g. ESR1, EGFR, ERBB2, TLR4), intermediate signaling molecules (e.g. Akt1, PRKACA) and transcription factors and regulators (e.g. HIF-1α, β-catenin and the estrogen receptor).
Figure 2.
Statistics summarizing data in XTalkDB. (A) The number of crosstalking pairs in which each pathway participates, either as A (left) or B (right), with activating and inhibiting crosstalk counted separately. The number in parentheses is the total number of crosstalking pairs that include the pathway. Pathways appear in descending order of this number. ‘P4’ is an abbreviation of ‘Progesterone.’ (B) The distribution of the species in XTalkDB. Icons taken from mindthegraph.com under a Creative Commons Attribution Share-Alike 4.0 License. (C) The frequency of Brenda Tissue Ontology terms in XTalkDB. The chart displays only the most specific tissues in the ontology that annotate 10 or more pairs of crosstalking pathways. (D) The distribution of the number of crosstalking pathway pairs mediated by a molecule. We identify the molecules that mediate crosstalk between at least six pairs of pathways. Some of these molecules have similar names (e.g. NFKB1 and Nfkb1) because they belong to different organisms.
The XTalkDB website
The XTalkDB website allows scientists to browse and query information on crosstalk either by pathways or by molecules.
Search by pathways
The main interface to XTalkDB on the website is a search box where the user can select one or more pathways. The result is a list of ordered pairs of crosstalking pathways, depending on the number of pathways selected:
zero pathways: all crosstalking pairs in the database.
one pathway A: all crosstalking pairs (A, X) or (X, A), where X is another pathway in XTalkDB.
two pathways A and B: at most two pairs (A, B) and (B, A), depending on whether XTalkDB records any relevant crosstalk information.
three or more pathways, e.g. A, B and C: the union of the searches for all ordered pairs of pathways in the query. In this case, there are six possible pairs.
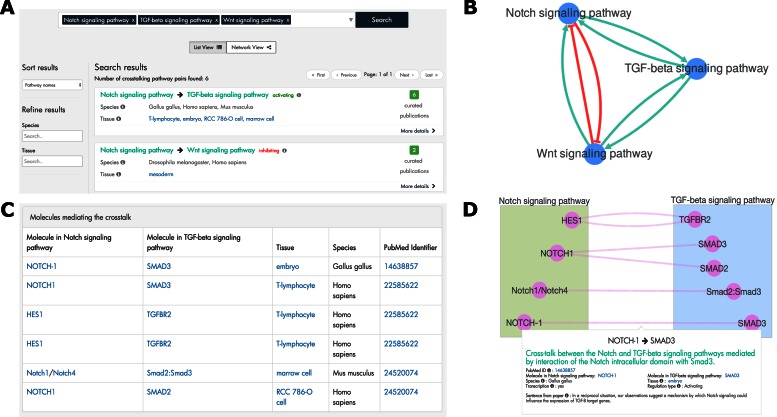
For each resulting pair, the ‘List View’ (Figure 3A) displays the two pathways that crosstalk and provides a summary of the curated evidence. A user may filter the resulting pathway pairs based on the species and/or tissue. The ‘Network View’ (Figure 3B) provides an alternative visualization of the search results. Here each node represents a pathway and each directed edge indicates that XTalkDB contains at least one publication supporting the crosstalk from one pathway to the other. A user may click on an edge between pathways to view a summary of the evidence for their crosstalk.
Figure 3.
Screen shots from the XTalkDB website. (A) The List View of the search results for Notch, TGF-β and Wnt signaling pathways. (B) The Network View of the same search results. Green edges with arrowheads represent activating crosstalk and red edges with blunt heads denote inhibiting crosstalk. (C) Molecules mediating the crosstalk from Notch to TGF-β signaling pathway. (D) The network of these molecular interactions. The green and blue rectangles contain the molecules in the Notch and TGF-β signaling pathway, respectively.
For each pair of pathways in the search results, the user can click the ‘More details’ button to visit a webpage specific to that pair. For each curated publication that supports crosstalk between the two pathways, this page lists the information recorded in XTalkDB from that paper. In addition, this page lists any recorded molecules mediating the crosstalk (Figure 3C). A network view of these molecular interactions is also available (Figure 3D).
Search by molecules
A user may also search XTalkDB for crosstalking pathway pairs by the mediating molecules. The search box provides a dropdown of a list of molecules contained in the database. The user may select one or more molecules. The results will depend on the number of molecules selected:
one molecule: XTalkDB will return records for all pathway pairs for which the query molecule mediates the crosstalk.
two or more molecules: XTalkDB will return records for all pathway pairs for which at least one of the query molecules mediates the crosstalk.
Other features
The user can become acquainted with the main features of the XTalkDB website by clicking the ‘Take a tour’ button. The website provides links to download the database in tab-delimited, comma-separated or JSON formats. See Supplementary Section 1 for a description of these files. The user may also download data specific to a particular pathway. The user can access up-to-date documentation and statistics on the website. XTalkDB is available under a Creative Commons Attribution-ShareAlike 4.0 International License (CC BY-SA 4.0).
Implementation
XTalkDB is a NoSQL database that uses MongoDB as the database management system. The web interface is implemented in JavaScript and runs on a NodeJS web server hosted on Amazon Web Services. The frontend interface uses Bootstrap framework for web design and CytoscapeJS (16) for network visualization.
DISCUSSION
Comparison to other databases
Many databases exist for storing physical, regulatory or causal interactions between pairs of proteins and complexes without annotating them to any specific pathway, e.g. (17–20). Other databases document pathways and the proteins and interactions within each pathway (21,22). As far as we know, these resources do not record crosstalk.
KEGG (15), a database of pathways, uses a ‘maplink’ to represent an interaction between a protein in one pathway and a protein in the linked pathway. Such interactions may not necessarily imply crosstalk between pathways. Moreover, the KEGG database does not explicitly document literature support for each interaction. Instead, it only provides a list of publications from which curators have created the pathway. SignaLink (23) is a database that annotates seven pathways and potential crosstalk between them. These authors state that crosstalk occurs between two pathways (say, A and B) if a protein in pathway A interacts with a protein in pathway B. PathwAX (24) is a web server that computes the extent of crosstalk between a query list of proteins and all KEGG pathways. It is based on a similar principle as SignaLink: crosstalk depends on the number of links between the query and a pathway. In contrast, the definition of crosstalk used by XTalkDB is more stringent and biologically grounded since the presence of such an interaction is not a guarantee that stimulation of A will lead to a response in or downstream of B.
Applications of XTalkDB
The crosstalk data stored in XTalkDB is a rich resource for several types of further analysis. Scientists can study tissue-specific crosstalk, e.g. instances discovered in breast-cancer cell lines, or crosstalk that has been validated in multiple organisms. It is also possible to explore the multiple roles played by mediating molecules. As a specific use case, we asked whether one pathway could activate or inhibit another using the same mediating protein. We also checked if two pathways could activate/inhibit each other using the same proteins. XTalkDB contains numerous examples of such pathway pairs: (i) 17 pairs (A, B) where A activates B and A inhibits B using the same protein, (ii) 26 pairs where A activates B and B activates A using the same protein and (iii) 18 pairs where A activates B and B inhibits A using the same protein. We list these pathway pairs on the statistics page on the website. As an example of the first category, the NF-κB pathway can activate the hypoxia-inducible factor 1 (HIF-1) pathway through the action of Inhibitor of nuclear factor-κB α (NFKBIA), which derepresses HIF-1 during moderate hypoxia (25). The NF-κB signaling pathway can also inhibit the HIF-1 pathway: NF-κB-mediated inflammatory signaling blocks the transactivation of HIF-1 (26).
Computational methods can identify crosstalk automatically (24,27–29). We previously used an early version of XTalkDB as a gold standard for the evaluation of three such methods (29). Therein we discovered that the commonly-held conjecture that crosstalk occurs when two pathways share proteins is a poor predictor: it achieved an area under the ROC curve (AUC) equal to that of the random classifier. Moreover, a method that relied on interactions that connect proteins in one pathway to another (28) performed only a little better; note that SignaLink 2.0 and PathwAX use this concept to define crosstalk. A novel path-based technique that we developed had superior AUC. The inclusion of both positive (pathway pairs with crosstalk supported in the literature) and negative (pairs for whom we could not find evidence for crosstalk) examples in XTalkDB was critical to the success of the evaluation. We expect that XTalkDB will continue to serve as a powerful resource for evaluating the accuracy of such computational methods.
Aberrant signaling has been observed in cancers, developmental disorders, and neurodegenerative diseases (7–11). Additionally, unexpected drug side effects have been associated with non-canonical signaling events (30,31). XTalkDB provides protein mediators of crosstalk that may be used to better understand these processes, especially in combination with algorithms that systematically integrate protein interaction and signaling networks with experimental data on drugs and their effects (30,31).
Future directions
We are planning to expand XTalkDB in several ways. First, we will provide XTalkDB for download in additional computable formats. We are examining approaches such as BioPAX (32) and the Biological Expression Language (BEL) (33). BEL is a promising option since it is designed to represent scientific findings by capturing causal and correlative relationships at the levels of molecular events and cellular processes.
Second, we will store additional types of information about each crosstalking pair of pathways in order to provide a more comprehensive description of these events. It is well known that signal transduction pathways rely on post-translational modifications (PTMs) to determine protein activities. Therefore, we will document PTMs that participate and mediate the crosstalk between pathways.
Third, we will increase the number of pathways we cover, e.g. by including pathways from the following KEGG sub-categories: ‘Cellular Process’ (e.g. cell cycle and autophagy), ‘Immune System’ (e.g. NOD-like receptor signaling pathway, and ‘Nervous System’ (e.g. retrograde endocannabinoid signaling). Including these categories will increase the scope of XTalkDB beyond signaling pathways and diversify its repertoire of crosstalk events.
However, the manual curation process underlying XTalkDB has its limitations (Supplementary Section 2), especially as we seek to expand the coverage of the database: the number of pairs to be curated grows quadratically with the number of pathways. Hence, our final direction will be to use text mining as a means to augment and accelerate manual curation. Specifically, we plan to develop a natural-language processing (NLP) framework that will rate publications based on their likelihood to document crosstalk. A curator can then focus on highly ranked publications, thereby reducing the time spent on ‘false positive’ publications (i.e. papers returned by the PubMed query that do not yield evidence of crosstalk). XTalkDB contains the specific sentence documenting the crosstalk in each supporting publication. In several cases, XTalkDB also includes sentences that are suggesting of crosstalk but are ultimately misleading. These data promise to be very useful for training NLP systems for automatically curating the literature for instances of crosstalk.
CONCLUSION
Crosstalk is a widely studied phenomenon that has been observed in both healthy and disease states, across multiple tissues and in several species. We were surprised to find that almost half the pathway pairs in XTalkDB have literature evidence supporting their crosstalk. This high degree of crosstalk emphasizes the complex nature of cellular signaling. This database may spur research on relationships between signaling pathways and how they are defined. As far as we are aware, XTalkDB is a unique resource that is not replicated by any other database. We expect XTalkDB to be a valuable resource to the biomedical community.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
FUNDING
Environmental Protection Agency [EPA-RD-83499801 to T.M.M.]; National Science Foundation [DBI-1062380 to T.M.M.]; National Institute of General Medical Sciences of the National Institutes of Health [GM095955 to T.M.M.]; National Research Service Award [F32-ES024062 to A.N.T.]; National Institutes of Health Traineeship [GM072767-09 to S.A.S.].
Conflict of interest statement. None declared.
REFERENCES
- 1.Krejčí A., Bernard F., Housden B.E., Collins S., Bray S.J. Direct response to Notch activation: signaling crosstalk and incoherent logic. Sci. Signal. 2009;2:ra1. doi: 10.1126/scisignal.2000140. [DOI] [PubMed] [Google Scholar]
- 2.Aulehla A., Wehrle C., Brand-Saberi B., Kemler R., Gossler A., Kanzler B., Herrmann B.G. Wnt3a plays a major role in the segmentation clock controlling somitogenesis. Dev. Cell. 2003;4:395–406. doi: 10.1016/s1534-5807(03)00055-8. [DOI] [PubMed] [Google Scholar]
- 3.Collu G.M., Hidalgo-Sastre A., Acar A., Bayston L., Gildea C., Leverentz M.K., Mills C.G., Owens T.W., Meurette O., Dorey K., et al. Dishevelled limits Notch signalling through inhibition of CSL. Development. 2012;139:4405–4415. doi: 10.1242/dev.081885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bertrand F.E., Angus C.W., Partis W.J., Sigounas G. Developmental pathways in colon cancer: crosstalk between WNT, BMP, Hedgehog and Notch. Cell Cycle. 2012;11:4344–4351. doi: 10.4161/cc.22134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banoth B., Chatterjee B., Vijayaragavan B., Prasad M., Roy P., Basak S. Stimulus-selective crosstalk via the NF-κB signaling system reinforces innate immune response to alleviate gut infection. Elife. 2015;23:e05648. doi: 10.7554/eLife.05648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eisele P.S., Handschin C. Functional crosstalk of PGC-1 coactivators and inflammation in skeletal muscle pathophysiology. Semin. Immunopathol. 2014;36:27–53. doi: 10.1007/s00281-013-0406-4. [DOI] [PubMed] [Google Scholar]
- 7.Jiang J., Hui C. Hedgehog signaling in development and cancer. Dev. Cell. 2008;15:801–812. doi: 10.1016/j.devcel.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alberi L., Hoey S.E., Brai E., Scotti A.L., Marathe S. Notch signaling in the brain: in good and bad times. Ageing Res. Rev. 2013;12:801–814. doi: 10.1016/j.arr.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 9.Samatar A.A., Poulikakos P.I. Targeting RAS-ERK signalling in cancer: promises and challenges. Nat. Rev. Drug Discov. 2014;13:928–942. doi: 10.1038/nrd4281. [DOI] [PubMed] [Google Scholar]
- 10.Rovida E., Stecca B. Mitogen-activated protein kinases and Hedgehog-GLI signaling in cancer: A crosstalk providing therapeutic opportunities? Semin. Cancer Biol. 2015;35:154–167. doi: 10.1016/j.semcancer.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 11.Guardavaccaro D., Clevers H. Wnt/β-catenin and MAPK signaling: allies and enemies in different battlefields. Sci. Signal. 2012;5:pe15. doi: 10.1126/scisignal.2002921. [DOI] [PubMed] [Google Scholar]
- 12.Gremse M., Chang A., Schomburg I., Grote A., Scheer M., Ebeling C., Schomburg D. The BRENDA Tissue Ontology (BTO): the first all-integrating ontology of all organisms for enzyme sources. Nucleic Acids Res. 2011;39:D507–D513. doi: 10.1093/nar/gkq968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blokzijl A., Dahlqvist C., Reissmann E., Falk A., Moliner A., Lendahl U., Ibáñez C.F. Cross-talk between the Notch and TGF-beta signaling pathways mediated by interaction of the Notch intracellular domain with Smad3. J. Cell Biol. 2003;163:723–728. doi: 10.1083/jcb.200305112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ohnuki H., Jiang K., Wang D., Salvucci O., Kwak H., Sanchez-Martin D., Maric D., Tosato G. Tumor-infiltrating myeloid cells activate Dll4/Notch/TGF-β signaling to drive malignant progression. Cancer Res. 2014;74:2038–2049. doi: 10.1158/0008-5472.CAN-13-3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kanehisa M., Sato Y., Kawashima M., Furumichi M., Tanabe M. KEGG as a reference resource for gene and protein annotation. Nucleic Acids Res. 2016;44:D457–D462. doi: 10.1093/nar/gkv1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Franz M., Lopes C.T., Huck G., Dong Y., Sumer O., Bader G.D. Cytoscape.js: a graph theory library for visualisation and analysis. Bioinformatics. 2015;32:309–311. doi: 10.1093/bioinformatics/btv557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chatr-Aryamontri A., Breitkreutz B.J., Oughtred R., Boucher L., Heinicke S., Chen D., Stark C., Breitkreutz A., Kolas N., O'Donnell L., et al. The BioGRID interaction database: 2015 update. Nucleic Acids Res. 2015;43:D470–D478. doi: 10.1093/nar/gku1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Licata L., Briganti L., Peluso D., Perfetto L., Iannuccelli M., Galeota E., Sacco F., Palma A., Nardozza A.P.P., Santonico E., et al. MINT, the molecular interaction database: 2012 update. Nucleic Acids Res. 2012;40:D857–D861. doi: 10.1093/nar/gkr930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Szklarczyk D., Franceschini A., Wyder S., Forslund K., Heller D., Huerta-Cepas J., Simonovic M., Roth A., Santos A., Tsafou K.P., et al. STRING v10: protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015;43:D447–D452. doi: 10.1093/nar/gku1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perfetto L., Briganti L., Calderone A., Perpetuini A.C., Iannuccelli M., Langone F., Licata L., Marinkovic M., Mattioni A., Pavlidou T., et al. SIGNOR: a database of causal relationships between biological entities. Nucleic Acids Res. 2016;44:D548–D554. doi: 10.1093/nar/gkv1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fabregat A., Sidiropoulos K., Garapati P., Gillespie M., Hausmann K., Haw R., Jassal B., Jupe S., Korninger F., McKay S., et al. The reactome pathway knowledgebase. Nucleic Acids Res. 2016;44:D481–D487. doi: 10.1093/nar/gkv1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kutmon M., Riutta A., Nunes N., Hanspers K., Willighagen E.L., Bohler A., Melius J., Waagmeester A., Sinha S.R., Miller R., et al. WikiPathways: capturing the full diversity of pathway knowledge. Nucleic Acids Res. 2016;44:D488–D494. doi: 10.1093/nar/gkv1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fazekas D., Koltai M., Türei D., Módos D., Pálfy M., Dúl Z., Zsákai L., Szalay-Bekő M., Lenti K., Farkas I.J., et al. SignaLink 2—a signaling pathway resource with multi-layered regulatory networks. BMC Syst. Biol. 2013;7:7. doi: 10.1186/1752-0509-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ogris C., Helleday T., Sonnhammer E.L. PathwAX: a web server for network crosstalk based pathway annotation. Nucleic Acids Res. 2016;44:W105–W109. doi: 10.1093/nar/gkw356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shin D.H., Li S.H., Yang S.W., Lee B.L., Lee M.K., Park J.W. Inhibitor of nuclear factor-kappaB alpha derepresses hypoxia-inducible factor-1 during moderate hypoxia by sequestering factor inhibiting hypoxia-inducible factor from hypoxia-inducible factor 1alpha. FEBS J. 2009;276:3470–3480. doi: 10.1111/j.1742-4658.2009.07069.x. [DOI] [PubMed] [Google Scholar]
- 26.Mendonça D.B., Mendonça G., Aragāo F.J., Cooper L.F. NFκB suppresses HIF-α response by competing for P300 binding. Biochem. Biophys. Res. Commun. 2011;404:997–1003. doi: 10.1016/j.bbrc.2010.12.098. [DOI] [PubMed] [Google Scholar]
- 27.Li Y., Agarwal P., Rajagopalan D. A global pathway crosstalk network. Bioinformatics. 2008;24:1442–1447. doi: 10.1093/bioinformatics/btn200. [DOI] [PubMed] [Google Scholar]
- 28.Dotan-Cohen D., Letovsky S., Melkman A.A., Kasif S. Biological process linkage networks. PLoS One. 2009;4:e5313. doi: 10.1371/journal.pone.0005313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tegge A.N., Sharp N., Murali T.M. XTalk: a path-based approach to identifying crosstalk between signaling pathways. Bioinformatics. 2016;32:242–251. doi: 10.1093/bioinformatics/btv549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuhn M., Campillos M., Letunic I., Jensen L.J., Bork P. A side effect resource to capture phenotypic effects of drugs. Mole. Syst. Biol. 2010;6:343. doi: 10.1038/msb.2009.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guney E., Menche J., Vidal M., Barabasi A.L. Network-based in silico drug efficacy screening. Nat. Commun. 2016;7:10331. doi: 10.1038/ncomms10331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Demir E., Cary M.P., Paley S., Fukuda K., Lemer C., Vastrik I., Wu G., D'Eustachio P., Schaefer C., Luciano J., et al. The BioPAX community standard for pathway data sharing. Nat. Biotechnol. 2010;28:935–942. doi: 10.1038/nbt.1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Slater T. Recent advances in modeling languages for pathway maps and computable biological networks. Drug Discov. Today. 2014;19:193–198. doi: 10.1016/j.drudis.2013.12.011. [DOI] [PubMed] [Google Scholar]