Abstract
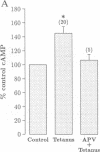
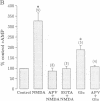
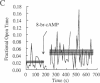
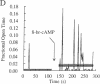
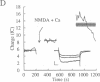
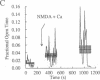
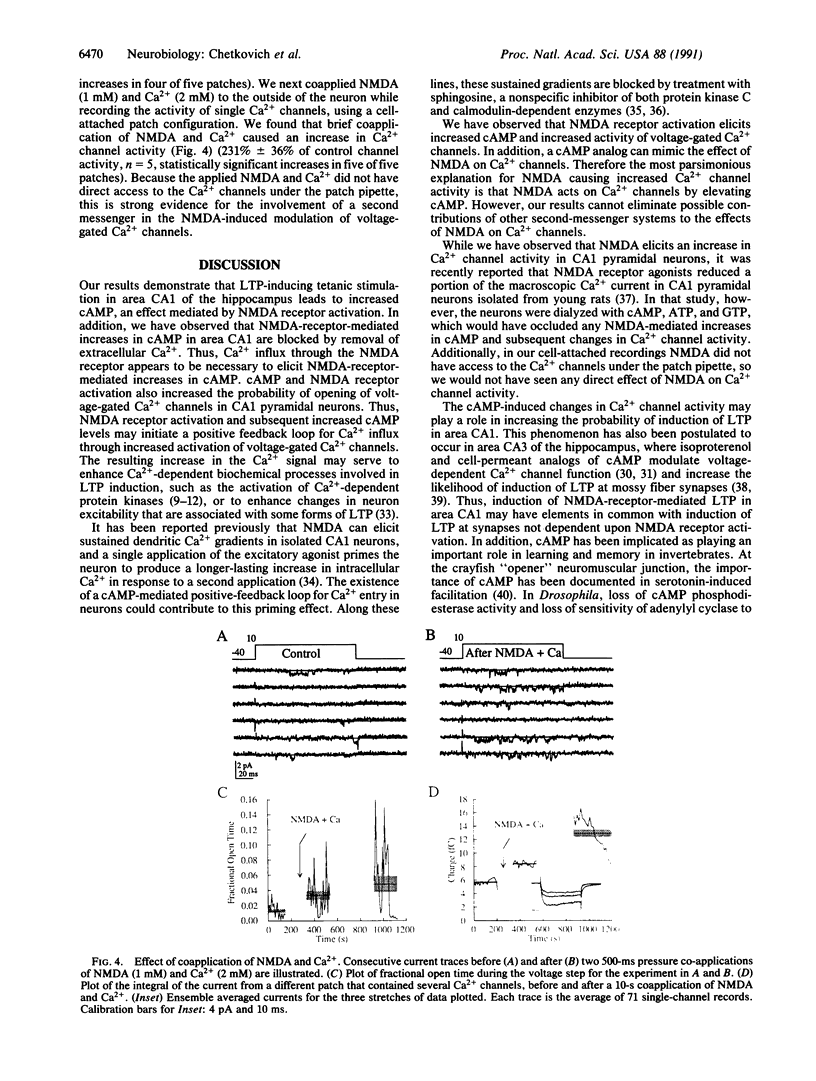
Tetanic stimulation of the Schaffer collateral inputs into area CA1 of the hippocampus causes N-methyl-D-aspartate (NMDA) receptor activation, an effect that contributes to the induction of long-term potentiation (LTP) in this region. The present studies demonstrate that LTP-inducing tetanic stimulation in rat hippocampal area CA1 elicited increased levels of cAMP. The elevation of cAMP was blocked by the NMDA receptor antagonist DL-2-amino-5-phosphonovaleric acid (APV). Bath application of NMDA also resulted in an increase in cAMP in CA1, an effect that was blocked by both APV and removal of extracellular Ca2+. These findings suggest that activation of NMDA receptors elicits a Ca(2+)-dependent increase in cAMP, and taken together with the data from tetanic stimulation, suggest that NMDA-receptor-mediated increases in cAMP could play a role in the induction of LTP in area CA1. One role for cAMP may be to increase Ca2+ influx through voltage-gated Ca2+ channels, as it was observed that application of either 8-bromo-cAMP or NMDA increased the fractional open time of high-threshold Ca2+ channels in CA1 pyramidal cells. Our results raise the possibility that a positive-feedback loop for Ca2+ influx in area CA1 exists. In this model, NMDA receptor-mediated Ca2+ influx leads to an enhancement of further Ca2+ influx via intermediate steps of increased cAMP and subsequent increased voltage-gated Ca2+ channel activity.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baba A., Nishiuchi Y., Uemura A., Tatsuno T., Iwata H. Inhibition by forskolin of excitatory amino acid-induced accumulation of cyclic AMP in guinea pig hippocampal slices. J Neurochem. 1988 Jul;51(1):237–242. doi: 10.1111/j.1471-4159.1988.tb04861.x. [DOI] [PubMed] [Google Scholar]
- Barzilai A., Kennedy T. E., Sweatt J. D., Kandel E. R. 5-HT modulates protein synthesis and the expression of specific proteins during long-term facilitation in Aplysia sensory neurons. Neuron. 1989 Jun;2(6):1577–1586. doi: 10.1016/0896-6273(89)90046-9. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chavez-Noriega L. E., Halliwell J. V., Bliss T. V. A decrease in firing threshold observed after induction of the EPSP-spike (E-S) component of long-term potentiation in rat hippocampal slices. Exp Brain Res. 1990;79(3):633–641. doi: 10.1007/BF00229331. [DOI] [PubMed] [Google Scholar]
- Chernevskaya N. I., Obukhov A. G., Krishtal O. A. NMDA receptor agonists selectively block N-type calcium channels in hippocampal neurons. Nature. 1991 Jan 31;349(6308):418–420. doi: 10.1038/349418a0. [DOI] [PubMed] [Google Scholar]
- Collingridge G. L., Kehl S. J., McLennan H. Excitatory amino acids in synaptic transmission in the Schaffer collateral-commissural pathway of the rat hippocampus. J Physiol. 1983 Jan;334:33–46. doi: 10.1113/jphysiol.1983.sp014478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor J. A., Wadman W. J., Hockberger P. E., Wong R. K. Sustained dendritic gradients of Ca2+ induced by excitatory amino acids in CA1 hippocampal neurons. Science. 1988 Apr 29;240(4852):649–653. doi: 10.1126/science.2452481. [DOI] [PubMed] [Google Scholar]
- Dixon D., Atwood H. L. Conjoint action of phosphatidylinositol and adenylate cyclase systems in serotonin-induced facilitation at the crayfish neuromuscular junction. J Neurophysiol. 1989 Dec;62(6):1251–1259. doi: 10.1152/jn.1989.62.6.1251. [DOI] [PubMed] [Google Scholar]
- Dudai Y. Neurogenetic dissection of learning and short-term memory in Drosophila. Annu Rev Neurosci. 1988;11:537–563. doi: 10.1146/annurev.ne.11.030188.002541. [DOI] [PubMed] [Google Scholar]
- Edmonds B., Klein M., Dale N., Kandel E. R. Contributions of two types of calcium channels to synaptic transmission and plasticity. Science. 1990 Nov 23;250(4984):1142–1147. doi: 10.1126/science.2174573. [DOI] [PubMed] [Google Scholar]
- Eliot L. S., Dudai Y., Kandel E. R., Abrams T. W. Ca2+/calmodulin sensitivity may be common to all forms of neural adenylate cyclase. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9564–9568. doi: 10.1073/pnas.86.23.9564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskin A., Garcia K. S., Byrne J. H. Information storage in the nervous system of Aplysia: specific proteins affected by serotonin and cAMP. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2458–2462. doi: 10.1073/pnas.86.7.2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher R., Johnston D. Differential modulation of single voltage-gated calcium channels by cholinergic and adrenergic agonists in adult hippocampal neurons. J Neurophysiol. 1990 Oct;64(4):1291–1302. doi: 10.1152/jn.1990.64.4.1291. [DOI] [PubMed] [Google Scholar]
- Gray R., Johnston D. Noradrenaline and beta-adrenoceptor agonists increase activity of voltage-dependent calcium channels in hippocampal neurons. Nature. 1987 Jun 18;327(6123):620–622. doi: 10.1038/327620a0. [DOI] [PubMed] [Google Scholar]
- Grover L. M., Teyler T. J. Two components of long-term potentiation induced by different patterns of afferent activation. Nature. 1990 Oct 4;347(6292):477–479. doi: 10.1038/347477a0. [DOI] [PubMed] [Google Scholar]
- Harris E. W., Ganong A. H., Cotman C. W. Long-term potentiation in the hippocampus involves activation of N-methyl-D-aspartate receptors. Brain Res. 1984 Dec 3;323(1):132–137. doi: 10.1016/0006-8993(84)90275-0. [DOI] [PubMed] [Google Scholar]
- Hopkins W. F., Johnston D. Frequency-dependent noradrenergic modulation of long-term potentiation in the hippocampus. Science. 1984 Oct 19;226(4672):350–352. doi: 10.1126/science.6091272. [DOI] [PubMed] [Google Scholar]
- Hopkins W. F., Johnston D. Noradrenergic enhancement of long-term potentiation at mossy fiber synapses in the hippocampus. J Neurophysiol. 1988 Feb;59(2):667–687. doi: 10.1152/jn.1988.59.2.667. [DOI] [PubMed] [Google Scholar]
- Jahr C. E., Stevens C. F. Glutamate activates multiple single channel conductances in hippocampal neurons. Nature. 1987 Feb 5;325(6104):522–525. doi: 10.1038/325522a0. [DOI] [PubMed] [Google Scholar]
- Jefferson A. B., Schulman H. Sphingosine inhibits calmodulin-dependent enzymes. J Biol Chem. 1988 Oct 25;263(30):15241–15244. [PubMed] [Google Scholar]
- Johnson J. A., Clark R. B. Multiple non-specific effects of sphingosine on adenylate cyclase and cyclic AMP accumulation in S49 lymphoma cells preclude its use as a specific inhibitor of protein kinase C. Biochem J. 1990 Jun 1;268(2):507–511. doi: 10.1042/bj2680507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel E. R., Schwartz J. H. Molecular biology of learning: modulation of transmitter release. Science. 1982 Oct 29;218(4571):433–443. doi: 10.1126/science.6289442. [DOI] [PubMed] [Google Scholar]
- Kennedy M. B. Regulation of synaptic transmission in the central nervous system: long-term potentiation. Cell. 1989 Dec 1;59(5):777–787. doi: 10.1016/0092-8674(89)90601-6. [DOI] [PubMed] [Google Scholar]
- Klein M., Camardo J., Kandel E. R. Serotonin modulates a specific potassium current in the sensory neurons that show presynaptic facilitation in Aplysia. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5713–5717. doi: 10.1073/pnas.79.18.5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingstone M. S., Sziber P. P., Quinn W. G. Loss of calcium/calmodulin responsiveness in adenylate cyclase of rutabaga, a Drosophila learning mutant. Cell. 1984 May;37(1):205–215. doi: 10.1016/0092-8674(84)90316-7. [DOI] [PubMed] [Google Scholar]
- Lynch G., Larson J., Kelso S., Barrionuevo G., Schottler F. Intracellular injections of EGTA block induction of hippocampal long-term potentiation. Nature. 1983 Oct 20;305(5936):719–721. doi: 10.1038/305719a0. [DOI] [PubMed] [Google Scholar]
- MacDermott A. B., Mayer M. L., Westbrook G. L., Smith S. J., Barker J. L. NMDA-receptor activation increases cytoplasmic calcium concentration in cultured spinal cord neurones. 1986 May 29-Jun 4Nature. 321(6069):519–522. doi: 10.1038/321519a0. [DOI] [PubMed] [Google Scholar]
- Malenka R. C., Kauer J. A., Perkel D. J., Mauk M. D., Kelly P. T., Nicoll R. A., Waxham M. N. An essential role for postsynaptic calmodulin and protein kinase activity in long-term potentiation. Nature. 1989 Aug 17;340(6234):554–557. doi: 10.1038/340554a0. [DOI] [PubMed] [Google Scholar]
- Malenka R. C., Kauer J. A., Zucker R. S., Nicoll R. A. Postsynaptic calcium is sufficient for potentiation of hippocampal synaptic transmission. Science. 1988 Oct 7;242(4875):81–84. doi: 10.1126/science.2845577. [DOI] [PubMed] [Google Scholar]
- Malenka R. C., Madison D. V., Nicoll R. A. Potentiation of synaptic transmission in the hippocampus by phorbol esters. Nature. 1986 May 8;321(6066):175–177. doi: 10.1038/321175a0. [DOI] [PubMed] [Google Scholar]
- Malinow R., Madison D. V., Tsien R. W. Persistent protein kinase activity underlying long-term potentiation. Nature. 1988 Oct 27;335(6193):820–824. doi: 10.1038/335820a0. [DOI] [PubMed] [Google Scholar]
- Malinow R., Schulman H., Tsien R. W. Inhibition of postsynaptic PKC or CaMKII blocks induction but not expression of LTP. Science. 1989 Aug 25;245(4920):862–866. doi: 10.1126/science.2549638. [DOI] [PubMed] [Google Scholar]
- Nowycky M. C., Fox A. P., Tsien R. W. Three types of neuronal calcium channel with different calcium agonist sensitivity. Nature. 1985 Aug 1;316(6027):440–443. doi: 10.1038/316440a0. [DOI] [PubMed] [Google Scholar]
- Schacher S., Castellucci V. F., Kandel E. R. cAMP evokes long-term facilitation in Aplysia sensory neurons that requires new protein synthesis. Science. 1988 Jun 17;240(4859):1667–1669. doi: 10.1126/science.2454509. [DOI] [PubMed] [Google Scholar]
- Scholz K. P., Byrne J. H. Intracellular injection of cAMP induces a long-term reduction of neuronal K+ currents. Science. 1988 Jun 17;240(4859):1664–1666. doi: 10.1126/science.2837826. [DOI] [PubMed] [Google Scholar]
- Scholz K. P., Byrne J. H. Long-term sensitization in Aplysia: biophysical correlates in tail sensory neurons. Science. 1987 Feb 6;235(4789):685–687. doi: 10.1126/science.2433766. [DOI] [PubMed] [Google Scholar]
- Smigel M. D. Purification of the catalyst of adenylate cyclase. J Biol Chem. 1986 Feb 5;261(4):1976–1982. [PubMed] [Google Scholar]
- Sweatt J. D., Kandel E. R. Persistent and transcriptionally-dependent increase in protein phosphorylation in long-term facilitation of Aplysia sensory neurons. Nature. 1989 May 4;339(6219):51–54. doi: 10.1038/339051a0. [DOI] [PubMed] [Google Scholar]
- Tsien R. W., Lipscombe D., Madison D. V., Bley K. R., Fox A. P. Multiple types of neuronal calcium channels and their selective modulation. Trends Neurosci. 1988 Oct;11(10):431–438. doi: 10.1016/0166-2236(88)90194-4. [DOI] [PubMed] [Google Scholar]
- Whittingham T. S., Lust W. D., Christakis D. A., Passonneau J. V. Metabolic stability of hippocampal slice preparations during prolonged incubation. J Neurochem. 1984 Sep;43(3):689–696. doi: 10.1111/j.1471-4159.1984.tb12788.x. [DOI] [PubMed] [Google Scholar]