Abstract
Assessing the movement patterns and key habitat features of breeding humpback whales is a prerequisite for the conservation management of this philopatric species. To investigate the interactions between humpback whale movements and environmental conditions off Madagascar, we deployed 25 satellite tags in the northeast and southwest coast of Madagascar. For each recorded position, we collated estimates of environmental variables and computed two behavioural metrics: behavioural state of ‘transiting’ (consistent/directional) versus ‘localized’ (variable/non-directional), and active swimming speed (i.e. speed relative to the current). On coastal habitats (i.e. bathymetry < 200 m and in adjacent areas), females showed localized behaviour in deep waters (191 ± 20 m) and at large distances (14 ± 0.6 km) from shore, suggesting that their breeding habitat extends beyond the shallowest waters available close to the coastline. Males' active swimming speed decreased in shallow waters, but environmental parameters did not influence their likelihood to exhibit localized movements, which was probably dominated by social factors instead. In oceanic habitats, both males and females showed localized behaviours in shallow waters and favoured high chlorophyll-a concentrations. Active swimming speed accounts for a large proportion of observed movement speed; however, breeding humpback whales probably exploit prevailing ocean currents to maximize displacement. This study provides evidence that coastal areas, generally subject to strong human pressure, remain the core habitat of humpback whales off Madagascar. Our results expand the knowledge of humpback whale habitat use in oceanic habitat and response to variability of environmental factors such as oceanic current and chlorophyll level.
Keywords: humpback whales, satellite telemetry, Madagascar, movement patterns, environmental parameters, habitat use
1. Introduction
Most baleen whales are highly mobile, and their distribution and abundance are influenced by the marine environment at different spatial and temporal scales [1]. Migratory species, such as the humpback whale (Megaptera novaeangliae), have a broad spatial range and therefore can exploit a variety of habitats (e.g. feeding versus breeding grounds in the case of migratory baleen whales). Distribution and habitat preferences over the annual cycle are partially dependent on environmental parameters, including oceanographic and climatological characteristics [2]. While the most frequently invoked factor to explain movement and habitat patterns in animals is resource availability [3], other factors such as predation risk or social interaction with conspecifics can affect both temporal distribution and habitat selection [4–6]. Habitat heterogeneity, biological requirements and social behaviour of a given species interact to influence patterns of distribution and habitat use.
The migratory cycle of humpback whales alternates seasonally between a summer feeding period in productive high latitudes and a winter breeding period in low latitudes [7,8]. Humpback whales typically fast during the breeding period, because productivity in wintering habitat is generally low. One explanation for why humpback whales migrate is to reduce the predation threat on their breeding grounds [9]. Other hypotheses have been suggested such as energetic advantages (e.g. warm water temperatures) which could benefit calf growth and reproductive success, but this is still debated[10–12]. The aggregation of humpback whales in localized breeding areas is a consequence of their mating system which involves intrasexual competition among males for females [8,13,14]. Furthermore, the widespread distribution of females is assumed to result from the absence of predation and prey [8]. In this context, social organization, breeding status or environmental variables seem to shape the selection of habitat [15,16].
Studies on different humpback whale breeding areas around the world show that animals occur in high densities in a variety of habitats, including continental coasts, coastal and oceanic islands, reef systems or over shoals (e.g. [16–20]). Humpback whales are frequently found in warm (21.1–28°C), shallow (15–60 m depth), near-shore or shelf waters [7,12,21–23]. In particular, it is believed that females with calves tend to prefer shallow waters in protected areas (20 m depth or less) [16,24]. Social groups, as mother–calf pairs, could also show a flexible response and adopt alternative behavioural strategies according to abiotic features of regions and the potential human disturbance [25]. Typically, humpback whales are found within close proximity to the coast during the breeding period. However, they are also found in deep waters [26,27] and in shallow waters extended offshore during winter [28,29], where movement patterns and habitat use are poorly known. To date, only one study has investigated the movements of individual humpback whales in a breeding ground and established direct links with environmental parameters [30].
The range of the southwestern Indian Ocean humpback whale population, referred to as Breeding Stock C by the International Whaling Commission (IWC), extends from southeastern Africa to the Mascarenes Islands [31,32]. Within this region, Madagascar Island, defined as the subregion C3, is an important breeding ground for humpback whales [33–40]. The population abundance of humpback whales around Madagascar in 2015 was estimated at 8854 (95% CI of 6906–16 106) whales [39,41]. Humpback whales are widely distributed around Madagascar [33,34,36,42,43], but higher concentrations are reported in certain areas including the northeast (Ile Sainte Marie, Antongil bay) [15,39,44,45], and the southern region from Toliara to Fort Dauphin [39,46].
While the western coast of Madagascar is characterized by a wide continental shelf and an extensive barrier reef (90 km at its widest, approx. 16° S; [47]), the east coast features a narrow continental shelf of 25–50 km width and an abrupt break in the slope below 1000 m [48]. The northeastern coast is where the continental shelf is the widest, with Antongil Bay and Ile Sainte Marie characterized by extensive shallow waters [15]. The southern tip of Madagascar is also characterized by a wide continental shelf extending 50 km from land and is referred to as the Madagascar Plateau [49]. Sightings of humpback whales have been recorded further south than the southern tip of Madagascar around the seamount of Walters Shoals located 645 km south of Madagascar and at depths less than 20 m [31,50].
The strongest geostrophic currents (0.2–1 m.s) are observed on the east coast of Madagascar, up to the south of Ile Sainte Marie and Antongil Bay region (electronic supplementary material, figure S1, reconstructed from the dataset of SWIO12 climatological model (see environmental parameters)). The South Equatorial Current (SEC), which flows westward at around 20° S, splits into two branches as it approaches the eastern Madagascar coast: one branch flows northward to the northern tip of Madagascar; and the other flows southward along Madagascar, referred to as the East Madagascar Current (EMC) [51,52] (electronic supplementary material, figure S1). The ocean circulation of the west coast is poorly known, and is defined by slower currents and strong mesoscale features (i.e. eddies) southwest of Madagascar [53,54]. Whether and how these contrasting oceanographic characteristics affect humpback whale habitat utilization around Madagascar during the breeding period has not been investigated to this point (electronic supplementary material, figure S1).
The biology of humpback whales is one of the best known among large whales, but our knowledge of individual movement patterns and habitat use in offshore habitats (i.e. far away from the shoreline along continental shelves and open-ocean habitats) during the breeding season remains limited and probably biased due to a lack of data in regions that are logistically challenging to sample [20,26,55]. Recent technological improvements in cetacean satellite telemetry have increased information on seasonal distribution ranges, movements into remote areas, as well as migration route and stock structure [26,27,56–58]. It is also now possible to relate the individual whale's horizontal movements to their physical environment at appropriate spatial and temporal scales, and to better understand how environmental parameters influence whales' distribution.
In this study, we used data from Argos satellite tags attached to humpback whales off Ile Sainte Marie (northeast Madagascar) and Anakao (southwest Madagascar) during three breeding seasons to investigate movement patterns and habitat utilization of humpback whales in relation to sex and reproductive status. We then investigated how key environmental variables along the whale tracks influenced humpback whale movements.
Identified as important variables in previous published studies investigating the relationship between humpback whale distribution and the environment, six environmental variables were selected including: (1) sea surface temperature (SST), (2) seabed depth (BAT), (3) sea floor slope (SL), (4) distance from shore (DIST), (5) ocean current speed (SSC) and direction, and (6) surface chlorophyll-a concentration (CHL). Because the basin scale, the distribution of humpback whale breeding grounds, is defined by a relatively narrow SST range, we examined whether SST may influence whale movements and habitat preferences at a regional scale. Humpback whales are frequently seen on continental shelves, in shallow waters and reef areas during the breeding season, suggesting that BAT and SL could be important drivers of their distribution. SSC has also been investigated as it is likely to influence the whale's heading and active swimming speed. Finally, CHL is used as a proxy for the marine ecosystem productivity, in order to investigate if whales could feed opportunistically in favourable areas in tropical waters (e.g. thermal front, seamount). Although it is clear that humpback whales fast during winter, some occasional feeding events have been documented in winter populations (e.g. [59–61]).
2. Material and methods
2.1. Tag deployment
Twenty-five humpback whales were equipped with Wildlife Computer SPOT 5 satellite transmitters during the breeding season over the years 2012–2014 (table 1; hereafter we will refer to individual whales using their numerical ID as shown in that table). Whales were tagged in two distinct regions of Madagascar: Ile Sainte Marie (16°50′ S, 49°55′ E) in 2012 and 2014, and off Anakao (23°40′ S, 43°39′ E) in 2013. They were selected for tagging based on sex, reproductive class and behavioural subclass. The latter category includes: single individual (SOLO), one of two individuals in a pair (Pair), an animal in a competitive group with an undetermined role (CG), a principal escort (PE), a secondary escort (SE), a challenger (CH), a nuclear animal (NA), a female with a calf of the year (Mom) and escort to a mother (Esc) (see details in [62]). The sex of each tagged individual was inferred in the field based upon the presence of a calf (i.e. assuming that adults in cow–calf pairs were females), the role in the group of whales (see [62]), when possible, and except for one whale was subsequently confirmed through genetic analysis from biopsy samples [63]. Whale locations were obtained through the Argos data collection system [64]. Further details about tag anchoring, deployment and whale selection can be found in [62]. In 2012, we used both an 8 m long carbon fibre pole, and modified pneumatic tag deliverer (ARTS) to deploy tags on whales, and in subsequent years, we only used the ARTS [26,27,57]. Tags deployed in 2012 were duty-cycled to transmit 6 h on, 6 h off for the first three months after deployment, and then every other day until the end of transmission. In 2013, tags were duty-cycled to transmit 9 h on, 3 h off to increase data collection. In 2014, tags were duty-cycled to transmit 2 h on 5 h off during the day, and 2 h on 4 h off during the night. Without a long focal follow of a whale after tagging, it is impossible to know how long the tag disturbance lasted. During the focal follow period (approx. 10 min), responses of the whale to the tagging varied from none to short-term disturbance. In all cases in which the whale was actively engaged in a specific behaviour (e.g. within a competitive group), the whale continued with that behaviour pattern after a brief reaction to the immediate tagging event. No whale exhibited obvious signs of severe disturbance (e.g. leaving a group, flight or repeated percussive surface activity). As first whale positions are transmitted beyond 1 h after tagging; all data after tagging were used and considered undisturbed behaviour.
Table 1.
Summary of the tracking dataset of humpback whales equipped at Sainte Marie channel (SM) and Anakao (AK) and main characteristics of breeding movements based on switching state space model (SSSM) positions estimated every 12 h. Group types include singleton, S; pair, P; competitive group, CG; non-competitive group, NCG; mother--calf pair, MC; mother--calf escort, MCE; mother--calf with more than one escort, MCES. Whale's tracks are defined by coastal (C) or/and oceanic (O) movements. Means are expressed ± s.e. Asterisks indicate that mean values were computed on all location values, whereas mean values used in statistical tests were computed by individual.
| whale id | tag location | sex | group type | tag date | tag longevity (days) | number of location data points | number of estimated locations after application of SSSM | type of movements | travelled distance per day (km) | b-mode average | observed swimming speed (m s−1) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | SM | M | S | 24 July 2012 | 32 | 123 | — | — | |||
| 3 | — | — | — | — | |||||||
| 13 | — | 27 | C, O | 47 ± 6.88 | 1.26 | 1.02 ± 0.12 | |||||
| 2 | SM | M | CG | 30 July 2012 | 31 | 231 | 62 | C | 22 ± 2.4 | 1.88 | 0.46 ± 0.03 |
| 3 | SM | M | P | 30 July 2012 | 20 | 169 | 41 | C | 29 ± 2.97 | 1.63 | 0.62 ± 0.09 |
| 4 | SM | F | MC | 31 July 2012 | 25 | 58 | — | — | |||
| 8 | — | 17 | C | 34 ± 8.6 | 1.44 | 0.72 ± 0.17 | |||||
| 4 | — | — | — | — | |||||||
| 5 | SM | M | MCE | 01 August 2012 | 5 | 21 | — | — | — | ||
| 6 | SM | M | P | 31 July 2012 | 10 | 71 | 20 | C | 31 ± 4.4 | 1.79 | 0.65 ± 0.09 |
| 7 | SM | F | MC | 31 July 2012 | 13 | 102 | 27 | C, O | 38 ± 3.6 | 1.05 | 0.85 ± 0.09 |
| 8 | SM | M | CG | 01 August 2012 | 3 | 21 | — | — | — | ||
| 9 | SM | F | CG | 01 August 2012 | 58 | 368 | 116 | C, P | 44 ± 2.8 | 1.19 | 1 ± 0.06 |
| 10 | SM | F | MC | 01 August 2012 | 30 | 222 | 61 | C | 29 ± 3.2 | 1.32 | 0.6 ± 0.06 |
| 11 | SM | M | CG | 03 August 2012 | 15 | 104 | 30 | C | 28 ± 4.9 | 1.65 | 0.6 ± 0.12 |
| 12 | SM | F | CG | 03 August 2012 | 23 | 196 | 46 | C, O | 43 ± 4.8 | 1.05 | 0.9 ± 0.09 |
| 13 | AK | unknown | NCG | 16 July 2013 | 15 | 206 | 31 | C, O | 37 ± 3.6 | 1.25 | 0.86 ± 0.09 |
| 14 | AK | F | P | 17 July 2013 | 21 | 8 | — | — | — | ||
| 15 | AK | F | P | 17 July 2013 | 42 | 473 | 85 | C | 37. ± 3.1 | 1.17 | 0.8 ± 0.06 |
| 16 | AK | F | P | 17 July 2013 | 2 | 13 | — | — | — | ||
| 17 | AK | M | P | 17 July 2013 | 34 | 480 | 68 | C, O | 30 ± 2.6 | 1.24 | 0.71 ± 0.73 |
| 18 | AK | M | CG | 21 July 2013 | 23 | 307 | 45 | C | 30 ± 2.5 | 1.57 | 0.7 ± 0.06 |
| 19 | AK | M | CG | 21 July 2013 | 8 | 85 | 10 | C | 54 ± 9.3 | 1.39 | 0.96 ± 0.12 |
| 20 | AK | F | MBE | 23 July 2013 | 23 | 269 | 36 | C | 44 ± 5.7 | 1.03 | 0.83 ± 0.06 |
| 21 | AK | F | CG | 25 July 2013 | 17 | 129 | 34 | C | 28 ± 3.9 | 1.31 | 0.6 ± 0.06 |
| 22 | AK | F | NCG | 27 July 2013 | 56 | 786 | 105 | C, O | 32 ± 1.9 | 1.24 | 0.74 ± 0.06 |
| 23 | AK | F | MC | 28 July 2013 | 52 | 807 | 104 | C | 22 ± 1.8 | 1.17 | 0.48 ± 0.03 |
| 24 | SM | M | MCES | 21 August 2014 | 4 | 15 | — | — | — | ||
| 25 | SM | F | MC | 23 August 2014 | 37 | 367 | 74 | C | 23 ± 2.06 | 1.36 | 0.52 ± 0.06 |
| C | 1.34 ± 0.01* | 0.8 ± 0.02* | |||||||||
| O | 1.14 ± 0.01* | 1.12 ± 0.05* | |||||||||
| O (whale 22 not included) | 1.17 ± 0.02* | 1.21 ± 0.08* |
2.2. Data processing
All locations classified invalid by Argos (Argos location class ‘Z’) and all locations on land were removed from the dataset. We retained Argos locations, classes 3, 2, 1, 0, A and B (see http://www.argos-system.org/manual/3-location/34_location_classes.htm), and assumed as invalid any location implying a travel rate greater than 12 km h−1 (e.g. [19]) as indicated by R Package Trip (R Development Core Team 2006). The Bayesian Switching State Space Model (SSSM) was used to model estimated positions and associated errors based upon the raw Argos locations [65] to provide the best estimate of each whale's path. A 12 h time step was used to minimize the number of positions estimated when the tags were not transmitting. The model was fitted using WinBUGS v. 1.4. Two Markov Chain Monte Carlo (MCMC) chains were run in parallel, each for a total of 50 000 simulations. The first 20 000 samples were discarded as a ‘burn-in’ and the remaining samples were thinned, retaining every 30th sample to reduce autocorrelation. The 1000 retained iterations for each chain, giving a total of 2000 independent samples, were used to compute the posterior distribution of the parameters of interest, including behavioural mode. The behavioural mode parameter measures the likelihood of exhibiting localized movements based on the mean turning angle, and the autocorrelation of speed and direction into the first difference correlated random walk model within the SSSM [66]. It is a behaviour index estimated at each location by the SSSM. A b-mode approaching 1 means a highly directional and consistent long-distance movement that represents a ‘transiting’ behaviour. For example, animals tend to travel between/through breeding areas or migrate. A b-mode approaching 2 means more variable movements and marked changes in direction that represent a ‘localized’ behaviour. For example, animals are engaged in breeding activities and meandering movements within breeding habitat. A behavioural mode less than 1.25 was considered as an indication of a transiting behaviour [65]. Details on estimation procedure are presented in Jonsen et al. [66,67].
To avoid introduction of unrepresentative estimated locations or behavioural state values, all tracks shorter than 8 days were excluded from analysis (whales 5, 8, 14, 16, 24; table 1). For tracks showing long transmission gaps (e.g. 16 days and 12 days for whales 1 and 4, respectively; table 1), tracks were split into two segments that were analysed separately. The first segment of whale 1 and the second segment of whale 4 were excluded from subsequent analyses as they were less than 8 days of data.
2.3. Data organization
The whale position data were split into two categories: coastal and oceanic according to whale position relative to the bathymetric environment from which the whales evolved (figure 1). Whale movements were defined as coastal when located over the continental shelf (less than 200 m water depth, 78% of coastal positions) or around the shelf break (more than 200 m water depth, 22% of coastal positions). Whale movements were defined as oceanic when animals were definitively leaving the Madagascar continental shelf towards deep ocean waters (i.e. all whale locations that were over a depth more than 200 m and not associated with coastal movements).
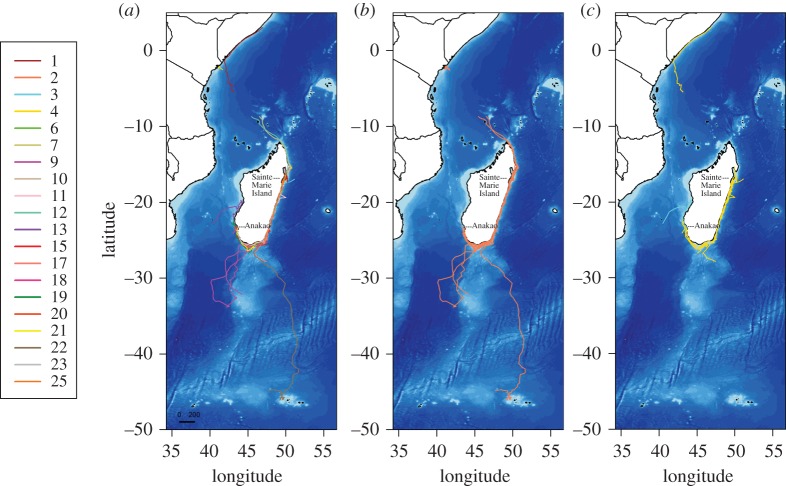
Figure 1.
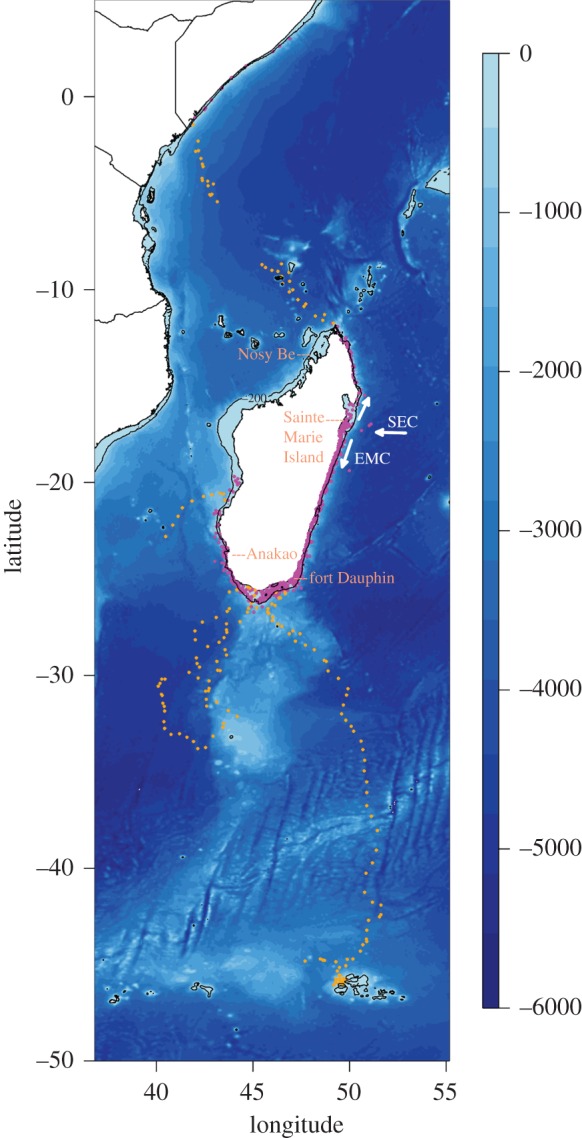
Whale locations associated with the type of movements (purple = coastal, orange = oceanic). Arrows illustrate the South Equatorial Current (SEC), and the southern branch of the SEC, known as the East Madagascar Current (EMC).
2.4. Environmental parameters
Three static variables (BAT, SL and DIST) and three dynamic variables (CHL, SST and SSC) were estimated at each whale time and position. The GEBCO bathymetry Grid-database (30 s per cell, http://www.gebco.net/) was used to compute bathymetry depth, bathymetry slope and DIST. Sea floor slope (hereafter ‘slope’) was derived using the R package raster (function terrain; [68]). The whale DIST was calculated as the distance between each location and the nearest coastline defined by a positive bathymetry.
CHL was estimated from the merged daily GlobColour product at a 4 km (0.1°) spatial resolution (http://www.globcolour.info/). We computed a spatial and temporal average around each estimated (or interpolated) whale position. The spatial means were calculated in 0.25° bins (approx. 27 km2), and the time mean within the 15-day period prior to the whale location. The temporal average was set 15 days prior to whale location as an attempt to consider the time of development of intermediate trophic levels in planktonic communities. These spatial and temporal means allowed us to fill gaps in satellite data availability due to cloud coverage, as well as to remove any potential local noise. We averaged over 0.25° bins rather than over 0.1° bins because this did not qualitatively change our results, and using the 0.1° bins removed some locations from the analysis.
SST was extracted from a daily SST dataset produced and distributed by JPL (http://ourocean.jpl.nasa.gov/SST/, G1SST product) at a 1 km spatial resolution. For consistency with the surface chlorophyll-a analysis, we used a mean value within a 0.25° bin around each whale location.
For the open-ocean dataset, daily geostrophic currents were computed from the daily merged and gridded satellite altimetry product produced by Aviso (http://www.aviso.altimetry.fr/) with a spatial resolution of 0.25° (latitude, longitude).
Because altimetry-derived ocean velocity measurements are noisier closer to a coast, surface currents for the coastal dataset were not available from Aviso products. Instead, we used a climatological estimate of a geostrophic current from a regional ocean model configuration. The model configuration is based on the NEMO ocean general circulation modelling system [69] and is a subset from the global 1/12° resolution (i.e. cell size approx. 9 km) configuration described by [70] covering the southwestern Indian Ocean subdomain (31–66.25° E; 3–29° S). This model successfully reproduces the major currents (mean and variability) as well as water masses’ properties. For the purpose of this study, only winter (June to October) surface currents are considered and used to calculate the temporal mean and the standard deviation over years (1995–2009), which constitute the climatology from which the current data were extracted underneath the whale tracks.
2.5. Movement datasets
From the 19 whales that displayed coastal movements, 11 were females (six females with calves and five females without calves; electronic supplementary material, figures S2a and S2b) and eight were males. Whale movements were analysed using linear mixed-effects models (LMMs; see below for details of analysis) considering the logit of behavioural mode values and whale active swimming speed values as response variables, and SST, bathymetry, DIST, slope and current speed as explanatory variables.
Seven whales displayed oceanic movements including six females and one male. These oceanic movements were analysed using LMMs considering the logit of behavioural mode values, whale active swimming speed and whale deviation from the current values as response variables, and SST, bathymetry, slope, current speed and the surface CHL concentration as covariates.
2.6. Behavioural metrics
Two metrics were used to characterize the whale movements: (i) the behavioural mode from SSSM outputs described earlier (see data processing), and (ii) the whale active swimming speed. The observed whale speed vector (T) was estimated by computing the distances, in the x and y directions, between the two consecutive locations and then dividing by the time elapsed. It was systematically computed as
| 2.1 |
where [X(t), X(t + Δt)] are the two successive positions over a time interval, t. The observed whale speed vector (T) can also be written as the sum of the whale's active swimming vector (A) and the current vector (C) (e.g. [71])
| 2.2 |
In other words, the mean active swimming speed (A) computed over the time interval [t, t + Δt] can be expressed as the difference between T(t) and the averaged current speed during the same time interval (namely [C(t) + C(t + Δt)]/2; e.g. Galli et al. [72]). To simplify the notation, A(t) will be denoted as A, T(t) as T and [C(t) + C(t + Δ1)]/2 as C; so equation (2.2) is rewritten as
| 2.3 |
The resulting tracks from the SSSM are made of positions estimated with a fixed sampling period Δt = 12 h. To extract environmental variables under the whale tracks, we first linearly interpolated locations every 6 h between the positions estimated every 12 h by the SSSM (using R package adehabitat, function redisltraj; [73]). Then the computed whale active swimming speeds were assigned to each interpolated location and environmental variables were extracted for these locations. The latter positions were used in the modelling analysis to link the active swimming speed metric and environmental variables.
2.7. Statistical analysis
All statistical analyses were performed using R Program v. 3.1.2 (R Core Team 2014). We fitted a series of LMMs using the R software package nlme (function lme; [74]) following the steps described in Zuur et al. [75] to examine the relationship between three response variables (behavioural mode, active swimming speed, angle between whale's heading and surface current) and the explanatory environmental variables. Behavioural state is the proportional likelihood of exhibiting localized movements, ranging between 1 (transiting) and 2 (localized). As a consequence, behavioural mode values were logit-transformed before the analysis [76]. An autocorrelation term (corAR1) was added to account for the lack of temporal independence within telemetry data for each whale [75]. The individual whale was included as a random term. Both predicted location associated with missing environmental values and outliers (values that were ecologically unreasonable to include) were removed from analysis. Non-collinearity was verified between continuous variables using Pearson's correlation (coef ≤ 0.5) and the variance inflection factor (VIF) [75], and one of each highly correlated pair was removed. Explanatory variables were standardized (centred and scaled) to facilitate model convergence and enable comparison of their contribution (using their corresponding slope coefficients). Model selection was performed using likelihood ratio tests starting from a full model with terms retained only if they improved the fit (p < 0.05, [77]). The results were then evaluated such that the most parsimonious model was also the model with the lowest Akaike's Information Criterion (AIC). The resulting optimal model was then fitted using restricted maximum-likelihood (REML). The normality and homogeneity of residuals were checked graphically [77,78].
2.8. Oceanic movements: surface current and trajectory
We calculated angles between A and C vectors (A–C angles, angles between current and whale), named compensation angles, as a means to estimate how whales orient their swimming direction with respect to the current. Angles close to 0° indicate that whale movements were following current direction, whereas angles close to 180° indicate movements oriented against the surface current. We assessed the respective contribution of currents (C) and whale's swimming (A) on observed whale movements (T) by projecting, respectively, the C-vector onto the T-vector (PCT) and A-vector onto the T-vector (PAT) (see Galli et al. [72]) and electronic supplementary material, S1).
3. Results
3.1. General tracking information
Whales were tracked for an average duration of 24.2 days (range 2–58 days) yielding 5631 locations raw Argos location. Five tracks shorter than 8 days were discarded from our analysis, providing us with 20 trips, or 1039 estimated locations after application of the SSSM (11 females, eight males and one of unknown sex; table 1, figure 2 and electronic supplementary material, figure S4a,b).
Figure 2.
Trajectories of tracked humpback whales in the Madagascar breeding ground (n = 20) (a). Trips shorter than 8 days are not represented. Females are represented in orange (b), males in yellow (c) and an individual of unknown sex in blue (c).
Whales travelled along the east, southwest and southern coasts of Madagascar with a high inter-individual variability in swimming direction (figure 2 and electronic supplementary material, figure S2a,b). The distances travelled during the tag transmission period (calculated from estimated SSSM positions) ranged from 488 to 4575 km, with an average (±s.e.) travelling speed of 31 ± 1 km per day (table 1). While most tagged individuals stayed relatively close to the Madagascar coast, one displayed a southward migration and reached as far as the Crozet Islands (approx. 2000 km south of Madagascar coast; whale 22 and another one (whale 9) visited the Walters Shoals seamount before coming back to the south of Madagascar (figure 2); Cerchio et al. [62] discuss in greater detail the movements of tagged whales within Madagascar waters and destinations of whales departing from Madagascar.
All humpback whales in our study spent time in the coastal waters of Madagascar, with the vast majority of received locations (78%) classified as coastal (figure 1). Among those, 12 whales (2, 3, 6, 10, 11, 15, 18, 19, 20, 21, 23 and 25) remained exclusively over the continental shelf of Madagascar for the duration of tag transmission (electronic supplementary material, figure S2a,b). Seven whales (1, 7, 9, 12, 13, 17 and 22) stayed within coastal waters of Madagascar before heading toward the deep waters (electronic supplementary material, figure S2a,b). The mean tag transmission was slightly longer (Wilcoxon test, p < 0.001) for tracks containing an oceanic part (33 ± 7 days) than for tracks that were only coastal (26 ± 3 days). Most animals that undertook oceanic movements were females (five), with one male, and one was of unknown sex. Whales spent more time per unit area in coastal waters (4 ± 0.12 h per 0.1° × 0.1° bins) than in oceanic waters (2 ± 0.07 h per 0.1° × 0.1° bins).
3.2. Coastal movements
3.2.1. General characteristics of movements and habitat in coastal areas according to sex and reproductive status
Overall, females used shallower waters (191 ± 20 m) than males (418 ± 48 m) on the shelf and in adjacent areas (electronic supplementary material, table S1 and figures S2a and S2b). Females spent a significantly higher portion of their time over the shelf (less than or equal to 200 m water depth) than males (69 ± 5%, and 45 ± 10%, respectively, permutation t-test, p = 0.034). However, there was no significant difference in the mean DIST between females (15.1 ± 2.8 km) and males (20.58 ± 3.9 km) (permutation t-test, p = 0.28) (electronic supplementary material, table S1 and figure S3). Similarly, there was no significant difference between females with calves and females without calves in the mean DIST (12.1 ± 3 km and 19 ± 4 km, respectively, permutation t-test, p = 0.2) or mean water depth (187 ± 58 m and 233 ± 66 m, respectively, permutation t-test, p = 0.5). Mean SST in the area used by whales ranged between 21 and 26°C (24 ± 0.04°C). The mean current speed in the same area was 0.29 ± 0.01 m s−1.
Transiting behaviour (consistent/directional movement, behavioural mode approaching 1; see data processing) was found in higher proportion in females (58%) than males (38%) (electronic supplementary material, figures S4–S6). Likewise, in coastal habitat, the mean behavioural mode of females was significantly lower than the mean behavioural mode of males (1.25 ± 0.12 and 1.52 ± 0.39, respectively, permutation t-test, p = 0.016), suggesting that males performed more localized movements (variable/non-directional movement, behavioural mode approaching 2; see data processing) in coastal areas than females (electronic supplementary material, figure S4a,b). There was no significant difference between the behavioural mode of females with a calf (1.30; 6 individuals), and females without a calf (1.1; 5 individuals) (permutation t-test, p = 0.26). The females' observed speed was not significantly lower than males' (0.85 ± 0.11 m s−1, 0.88 ± 0.11 m s−1, respectively; permutation t-test, p = 0.8). Additionally, the observed speed of females with a calf (0.82 ± 0.08 m s−1) was not significantly different from females without a calf (0.89 ± 0.13 m s−1) (permutation t-test, p = 0.69).
3.2.2. Influence of environmental conditions on both female and male behavioural mode and swimming speed
The influence of environmental variables on the behavioural mode of females was most parsimoniously described by a model including water depth and distance to shore (table 2; electronic supplementary material, table S2). Namely, the behavioural mode (i.e. probability of performing localized movements) was positively correlated to depth and to DIST. By contrast, the most parsimonious model describing the behavioural mode of males included all environmental variables except DIST (collinear with the depth) but none was associated significantly with movement patterns (table 2).
Table 2.
Summary of regression coefficients from the most parsimonious models (LMMs) relating b-mode (logit) or whale swimming speed to environmental parameters during coastal movements (for the females and males) and oceanic movements. Coefficients are presented ± s.e. with their p-value associated. Significant parameters are highlighted in italicized characters. Parameters not included in the full model are indicated by a black box, and those included in the full models but not retained in the model selection by an em-dash.
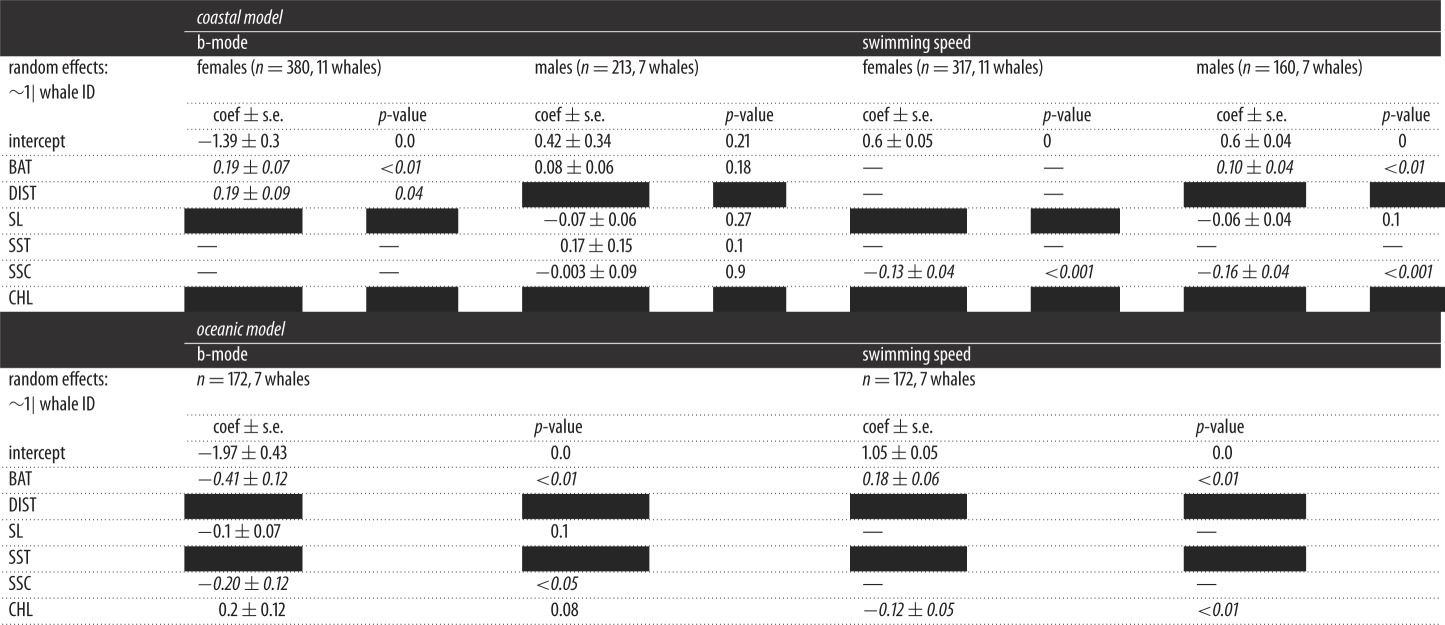 |
The active swimming speed of females was most parsimoniously described by a model including only the speed of the surface current (table 2; electronic supplementary material, table S2): female active swimming speed increased with decreasing current speed. The most parsimonious model describing the behavioural mode of males included all environmental variables except DIST (collinear with the depth; table 2). The model indicated swimming speed was positively correlated with water depth and negatively correlated with current intensity.
3.3. Oceanic movements
3.3.1. General characteristics of movements in oceanic areas
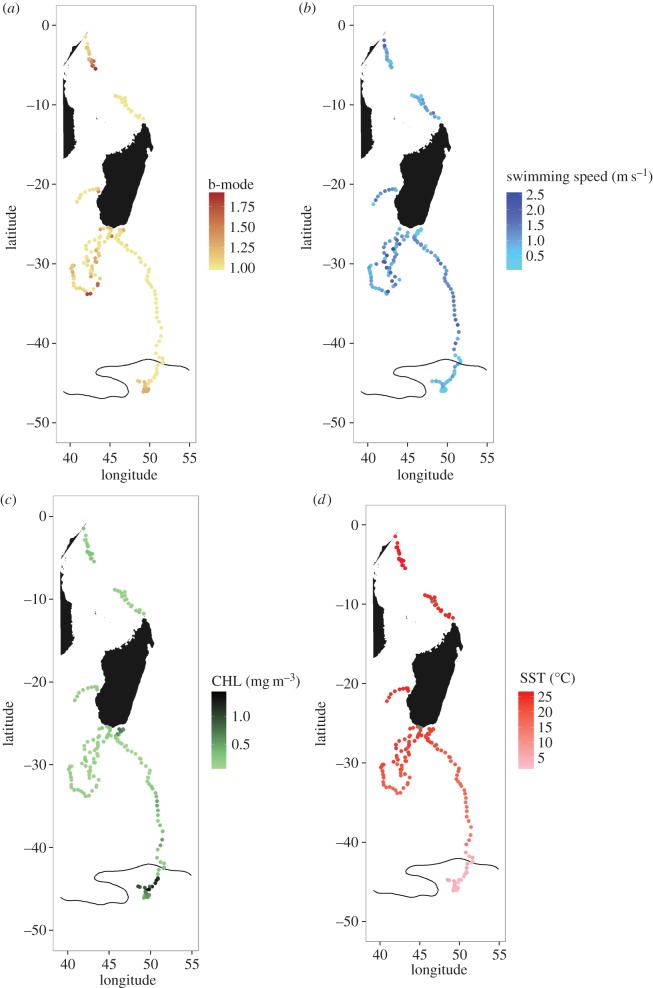
During oceanic movements, humpback whales travelled over deep waters (mean depth 2944 ± 105 m), and encountered an average current speed of 0.3 ± 0.02 m s−1 (electronic supplementary material; table S3). The CHL along their tracks ranged between 0.08 and 1.4 mg m−3 (mean of 0.3 ± 0.02 mg m−3). The mean SST values ranged from 2 to 26°C (mean of 18 ± 0.6°C). The highest mean CHL concentration (0.5 ± 0.04 mg m−3, max: 1.4 mg m−3) and the lowest mean SST (10.8 ± 0.9°C, min: 2°C) were encountered by whale 22, where it arrived at the Polar Frontal Zone (approx. 45° S) in late September (electronic supplementary material, figure S2b; figure 3).
Figure 3.
Locations for whale oceanic movements. Values of the b-mode (a), the active swimming speed (b), the CHL (c) and the SST (d) are expressed for each location. Polar Frontal Zone from Roquet et al. [79] is represented by the black line.
The behavioural mode associated with oceanic movements of whales ranged from 1 to 1.9 (1.14 ± 0.01). Transiting behaviour (consistent/directional movement, defined here as a behavioural mode less than 1.25) was found during 79% of the oceanic movements (figure 3). Observed speed was significantly higher in deep waters (migration track not included) than in coastal waters (1.15 ± 0.08 m s−1 and 0.86 ± 0.08 m s−1, respectively, permutation t-test, p = 0.03).
3.3.2. Influence of environmental conditions on behavioural mode and swimming speed
The behavioural mode of oceanic movements was most parsimoniously described by a model including only two environmental variables: current speed and water depth; no significant influence of slope or CHL concentration was detected (table 2; electronic supplementary material, table S2). The behavioural mode (i.e. transiting behaviour, approaching 1, and localized behaviour, approaching 2) was negatively related to both water depth and current speed.
Active swimming speed was most parsimoniously described by a model including only water depth and CHL concentration (table 2; electronic supplementary material, table S2). Whales swam faster in deeper waters and in areas with lower CHL concentration (figure 3). No significant effect of slope and current speed was detected.
It is noteworthy that for all oceanic movements, the mean active swimming speed was always found to be twofold to fivefold greater than the mean surface current speed they encountered.
3.3.3. Ocean currents’ influence on animal direction in oceanic habitat
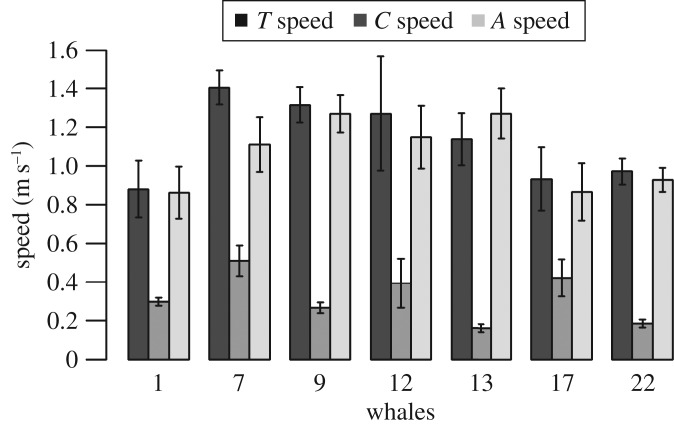
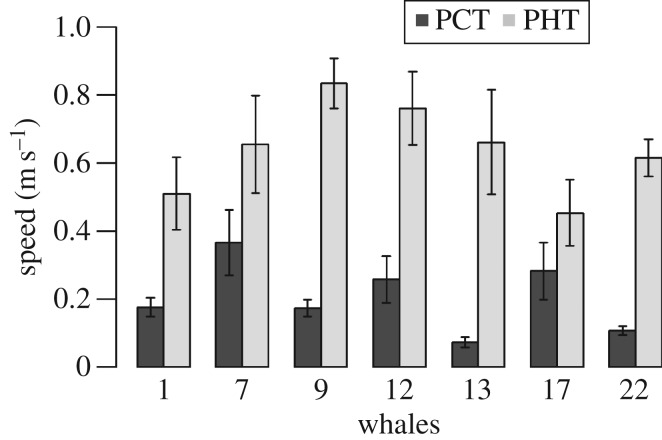
The mean magnitude of C, the surface current, along oceanic whale tracks ranged from 0.16 to over 0.51 m s−1, while the mean magnitude of A, the active swimming vector, ranged from 0.87 to over 1.27 m s−1 and was larger than C for all whales (figure 4). Only whale 13, which moved offshore towards the Mozambique Channel showed an A higher (1.27 m s−1) than T (0.97 m s−1). In addition, in all pelagic whale movements, the PATs (the projection of the active swimming vector onto the observed direction vector) were higher than the PCT (the projection of the current vector onto the observed direction vector (figure 5). This demonstrates that the whales actively swam regardless of current speed.
Figure 4.
Mean speed ± s.e. of the observed whale speed (T), the current (C) and whale's active swimming speed (A) vectors for each individual whale during oceanic movement. The value of C speed shows the mean intensities of the currents along each whale trajectory, while A provides a measure of each whale's active swimming effort.
Figure 5.
Mean ± s.e. projections of the C vector on the T vector (PCT) and the A vector on the T vector (PAT) for each whale.
As shown in figure 6, even though compensation angle and current speed were negatively correlated (Pearson's r = −0.27, p < 0.001; d.f. = 173), the whale active swimming speed did not depend on current speed, and plateaued at around 1 m s−1 for current speeds increasing from 0.2 to 1 m s−1. As further shown in figure 7, when whales moved away from Madagascar to the north or the south, the compensation angle was low and then varied according to each individual whale's path (electronic supplementary material, figures S7a and 7b). Whales were oriented in the same direction as prevailing currents when the currents were strong in the oceanic sectors (figure 7). We note, however, that no environmental parameter was retained in the deviation model with compensation angle and current speed.
Figure 6.
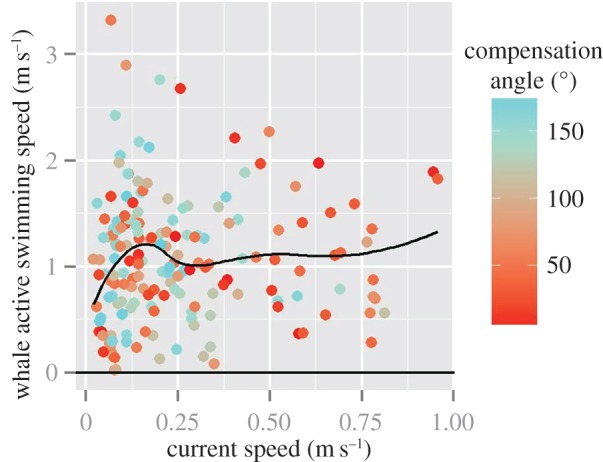
Whale active swimming speed (A) by current speed (C). Colour scale shows the compensation angles.
Figure 7.
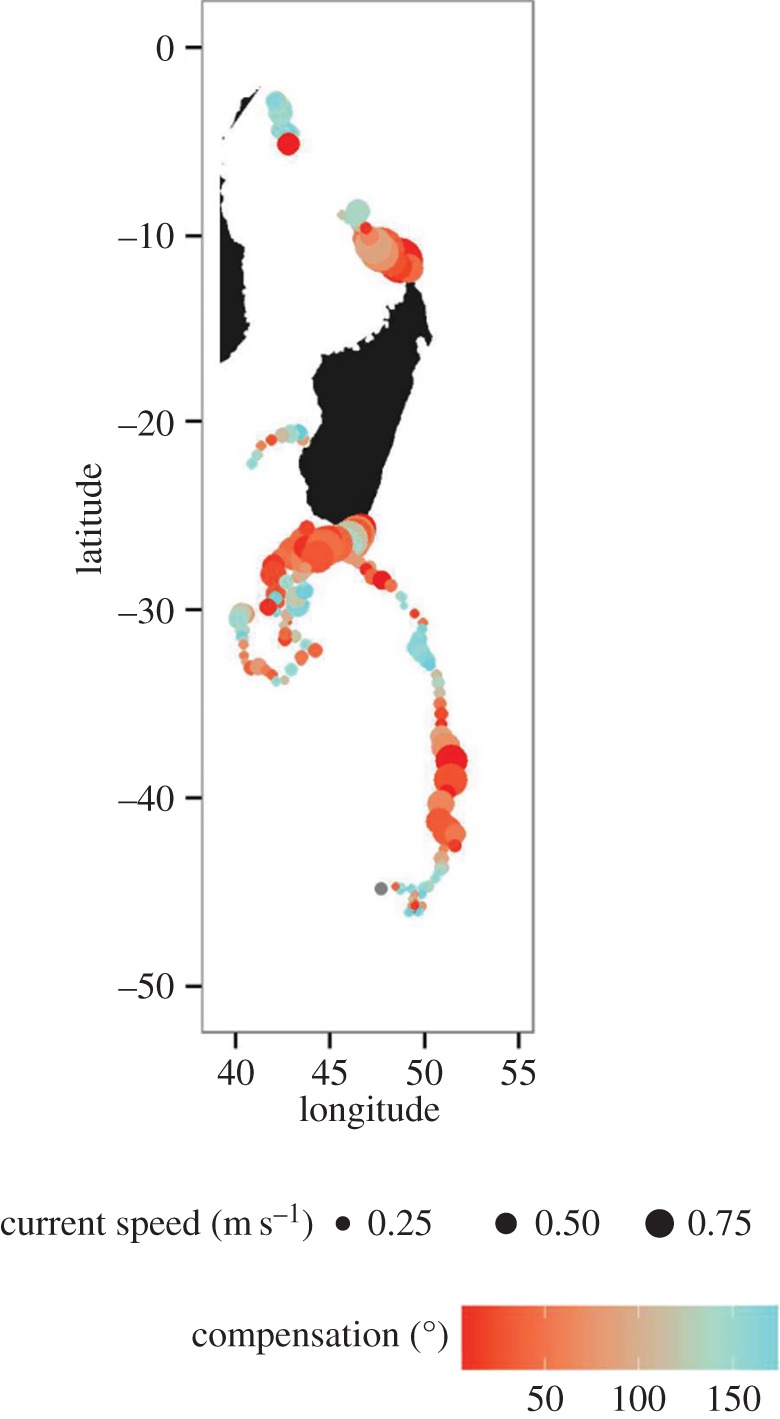
Current speed for each whale oceanic location. Colour scale shows the compensation angles.
4. Discussion
In this study, we used satellite tracking of 25 humpback whales to investigate the links between breeding movements and habitat use of humpback whales according to sex and breeding status in Madagascar breeding grounds. By modelling movements of tagged humpback whales against a suite of environmental parameters, we were able to make inferences on habitat preferences between males and females during the breeding season in Madagascar. Our results showed no significant habitat differences (i.e. BAT, DIST) between females without calves and females with calves, possibly due to a low resolution from available data. However, our models suggested that female breeding habitat extends beyond the shallow coastal waters and males tend to not be very much influenced by oceanographic factors in their breeding habitat, at least for the parameters that we examined at this spatial scale. In oceanic habitats, the active swimming speed accounts for a large proportion of the whale's observed speed, while the observed direction of tagged whales tends to align to the current direction when the current intensity was high.
4.1. General patterns of coastal and oceanic movements
Humpback whales preferentially used the continental shelf, which is consistent with what has been observed in many other breeding areas. In addition, they spent, on average, more time per unit area when engaged in coastal movements than during oceanic movements. This concurs with the lower observed whale speed and more localized movements (i.e. variable/non-directional movement, lower behaviour mode) found in coastal waters. Altogether, these results suggest that the shelf is a key habitat on which whales exhibited a large amount of localized movements, most likely related to breeding activities (e.g. searching, pairing, mating and resting; [80]). Both females and males increased their active swimming speed with decreased current speed, suggesting that in coastal habitats when whales were engaged in movements that could correspond to mate-searching movements, current speed did not influence their movement patterns [81]. By contrast, in oceanic habitats currents influenced whale behavioural mode and could impact the whale headings. Our models did not show any influence of SST on humpback whale movement patterns, perhaps as a result of the low variations in SST within the Madagascar coastal region, and/or because SST does not impact the way whales use their habitat once they reach the breeding grounds.
4.2. Coastal movement of females and males in relation to habitat characteristics
While all whales spent more time visiting coastal than oceanic areas, females performed more transiting than localized movements during their time in the coastal environment, as indicated by their mean behavioural mode [62]. Although no significant differences were found in behaviours and habitat use between females with calves and females without calves, females with calves tended to perform more localized movements and occurred in nearer-shore areas than females without calves. The most important environmental parameters affecting the movement patterns of females were the water depth and the distance from shore. Within coastal habitat, localized movements of females were performed at greater distances from the shoreline and in deeper waters, suggesting that they used the offshore habitat more intensively than expected. Because of the difficulty of identifying females in the field in the absence of a calf, little information exists on non-parous females' movements and the potential influence of habitat features. Non-parous females (migrating to the breeding grounds solely to mate) can be found in competitive groups or pairs that are mostly observed in deeper waters, farther from shore [28,29,82]. This is consistent with our results, suggesting that non-parous females preferentially use areas favourable for reproductive behaviours, i.e. the deeper offshore coastal waters where competitive groups and possibly singing males are likely to be found. Previous studies show that mother–calf pairs have a preference for shallow coastal waters and areas close to shore [16,28,29,83,84]. Although many previous research studies including boat-based and shore-based studies of humpback whales spent little time in offshore areas, other studies sampled both inshore and offshore areas in a relatively comparable way and found mother–calf pairs preferentially in coastal waters (e.g. [67,72,74,75,85,86]). It may be that females with calves change movement patterns and habitat use across the season as the calf matures by moving more extensively into offshore areas and deeper waters on the shelf. It could be expected that females introduce breeding locations to their newborns although this has never been demonstrated [29,87]. Our tracking data revealed that females, including mothers with calves, use offshore areas on the shelf, suggesting that females probably exploit a more extended range of reproductive habitat than previously thought.
Our models did not reveal any significant effect of environmental parameters on the type of movements (localized or transiting) performed by males. However, male active swimming speed was positively influenced by depth, indicating that animals slowed down in shallow waters. The absence of influence of key environmental parameters on the behavioural mode suggests that males were more influenced by social factors such as female occurrence, possibly in order to increase the number of opportunities to interact with receptive females. The mating system of humpbacks whales includes alternative mating strategies adopted by males (e.g. engaging direct competition with other males, singing or escorting) presumably to successfully obtain mating with reproductive females [8,88]. Male songs are known to play a role in the humpback whale mating system [8,89], suggesting that acoustic conditions could influence the occurrence and habitat utilization of singing males [82,90]. For example, in Puerto Rico, singers tend to be found on ledges, close to the shelf, which may be favourable geographical features to spread out the song or detect songs of other males [5]. However, several studies have suggested that male movement patterns may be mostly driven by mating prospects, and by the temporal distribution of receptive females, rather than by favourable singing habitats [13,82,91–93].
4.3. Oceanic movements in relation to habitat characteristics
Overall, in oceanic habitat, whales displayed highly directional movements in deep waters between breeding sites but also performed more erratic movements in oceanic shallow habitats such as Walters Shoals seamount or the Crozet Plateau during migration.
4.3.1. Directional movements between breeding sites
The tracks of tagged whales revealed that whales used oceanic habitats during the breeding season and performed highly directional and consistent movements (i.e. transiting behaviours) both in deep open waters and in areas with strong currents. Non-migrating animals travelled faster in oceanic than in coastal habitats with an average observed speed of 1.21 ± 0.08 m s−1. This value is slightly lower than the speed found in whales migrating southwards from Moheli and Mayotte in the Comoros Archipelago [94], higher than whales from the eastern South Atlantic [95], but is comparable to swimming speeds of humpback whales migrating between breeding and feeding grounds in the South Atlantic (Brazil; [57]), the North Atlantic (Caribbean Sea; [26]) and in the North Pacific Ocean (Hawaii; [96], Mexico; [97]). This study was the first to assess humpback whale swimming speed between different breeding sites within a breeding region. Our findings suggest that during offshore movements between breeding sites, humpback whales generally swam at a rate of travel similar to the mean travelling speeds of migrating whales.
4.3.2. Movement on Walters Shoals seamount
One individual (whale 9) displayed localized movement patterns and moved slowly over the offshore Walters Shoals seamount. Our models indicate that humpback whales exhibited localized movements and a slower active swimming speed in response to shallow sea floor depth, chlorophyll concentration and low current speed. Walters Shoals seamount may constitute either previously undescribed breeding habitat or potential opportunistic feeding habitat. Seamounts are shallow geographical features frequently found at low latitudes, suggesting that they represent important habitats for humpback whales during both the breeding and migratory periods [20]. Humpback whales have been previously observed in the Walters Shoals seamount in September, the same period as whale 9 (M. Le Corre, 28 October 2014, personal communication). Furthermore, occurrence of humpback whales over shallow seamounts during the breeding period has been documented elsewhere in the southwestern Indian Ocean (La Perouse seamount off Reunion Island; [98]) and in the Pacific Ocean (Antigonia seamounts; [19]). While relatively low instantaneous chlorophyll concentrations (ranging from 0.1 to 0.2 mg m−3) were associated with the whale positions on the Walters Shoals seamount, the monthly mean chlorophyll concentrations were higher (ranging from 0.5 to 1 mg m−3; averaged for September 2013) than adjacent waters located further north (electronic supplementary material, figure S8). The Walters Shoals are located downstream of productive regions: south of Madagascar and the subtropical South Indian Ocean countercurrent [53,99,100]. A number of humpback whales have also been sighted on the Walters Shoals seamount in summer (November and December), a time of increased productivity in the area [31,50,101]. The shallow waters of seamount habitats are favourable for humpback whale breeding activities, yet the abrupt topographies and the occurrence of localized physical processes (e.g. tides, eddies and upwelling) surrounding seamounts are known to favour prey aggregation attracting marine mammals [102–104] such as humpback whales [56,105]. Consistently, the Walters Shoals area is also known to be a seabird foraging hot spot [106,107]. Further investigation and larger sample sizes are needed to fully understand the presence of humpback whales in that region at that time of the year.
4.3.3. Migration
Our results indicate that multiple whales engaged in oceanic movements decreased their swimming speed in response to high chlorophyll concentrations. One individual (whale 22) migrated south and spent 6 days in productive waters (i.e. higher chlorophyll concentration) in the west part of the Crozet Plateau before the tag stopped transmitting. This suggests that this individual very likely stopped to feed in this area where a phytoplankton bloom is present all year long [108] and supports zooplanktonic populations such as copepods [109]. Fossette et al. [94] reported a humpback whale migration from Mayotte towards northwest of the Crozet Plateau, and also suggested that humpback whales could forage in that area either before migration to Antarctic feeding grounds, or could remain within the plateau area during summer [94]. Humpback whales are also regularly observed from fishing vessels operating on the Crozet Island Plateau in spring and early summer [110]. Our individual movement data support that humpback whale stop over on the Crozet Plateau probably to feed, at least en route to high latitude foraging areas.
4.3.4. Movement direction
We investigated the influence of currents on the whale active swimming speed and direction of travel during the offshore sections of tagged whale non-migratory movements and one migratory movement. We note that, to our knowledge, this is the first time current influence on whale movements has been investigated using current values extracted under the whale positions. Humpback whales exhibited highly directional travelling movements over deep waters. Our results showed that active swimming speed stayed around 1 m s−1 across the range of current speed encountered by the whales (up to 1 m s−1) close to the optimal velocity of 1.1 m s−1 found by Braithwaite et al. [111]. By contrast, the direction of tagged whales tended to be closer to the current direction when the current intensity was high, such as at the northern and southern tip of Madagascar. We found that whales tended to follow the prevailing currents when they moved away from Madagascar, thereby potentially using a strategy for minimizing their energy expenditure. Then, depending on their headings, they may adjust their movement direction to the local current conditions. Few studies have investigated the influence of ocean currents on swimming speed and direction of large migratory species, and previous work has mostly concerned leatherback turtle (Dermochelys coriacea, [70,71,111–114]). A tracking study of humpback whales migrating from the Brazilian breeding grounds to the South Atlantic feeding grounds has suggested that whales would tend to keep a constant heading regardless of current direction, but this analysis was based on mean currents at the regional scale [115]. Chapman et al. [81] discussed the same results from a theoretical point of view and suggested that whales could use a compensation strategy that involves an animal altering its heading into the flow to achieve a track coincident with its desired direction, regardless of current direction. However, these authors also noted that animals could not persistently compensate for currents over very long journeys, and might adjust their strategy to the local ocean circulation context, thereby alternating phases of active downstream strategy (heading alignment along current direction) and compensation strategy. In our study based on real-time, local current data extracted from underneath the whale oceanic tracks, we found that whales did not compensate for current direction in the strongest current area. This suggests that whales aligned their headings with the local current directions to exploit strong favourably directed ocean surface currents, and maximized their displacement speed and travelled distance in a given time. Thus, in non-migratory offshore movements (e.g. travel between breeding sites, short-term offshore travel), humpback whales could stop compensating at some point along the pathway by taking up their headings along with the current such as in the presence of strong currents.
5. Conclusion
Our satellite tracking-based study described movement patterns and habitat utilization of humpback whales on the Madagascar breeding grounds. During the winter breeding season, humpback whale habitat was not restricted to coastal waters, but also included offshore habitat. Whales from Madagascar used offshore habitats and moved to other coastal areas in the Indian Ocean (e.g. east coast of Africa). Humpback whale movement patterns were significantly related to bathymetric features, in both coastal and oceanic habitats. In the former, females spent most of their time on the shelf where they performed localized movements while at a distance from inshore areas. Our study showed that females (with or without a calf) use a wider range of breeding habitats than near-shore waters only. Unfortunately, our sample size was too small to investigate in detail the influence of environmental parameters on movements according to female subclasses. We also found that whales performed offshore movements during the breeding season and that their observed swim speeds were represented in large part by their active swimming speeds. While only seven whales performed offshore movements, we found that humpback whales do not constantly employ a compensation strategy but can alter their direction based on local currents. This study highlights the need to increase the sample size of tagged whales and extend the duration of the tag anchoring system to better identify different strategies of females (with or without a calf) within breeding seasons and to better assess the different winter movements. Our results improve our understanding on humpback whale movement patterns and habitat use in critical breeding coastal regions around Madagascar and adjacent oceanic habitat, which need to be taken into account to implement effective management and conservation strategies
Supplementary Material
Acknowledgements
Many individuals made this work possible. This study represents a collaborative effort between the Institute of Neurosciences Paris Saclay (NeuroPSI, France), the association Cetamada (Madagascar), the Wildlife Conservation Society (WCS, USA) and the NOAA National Marine Mammal Lab (NMML, USA). Maria Faria and Henry Bellon provided critical field logistic support and participated in the fieldwork; further logistic and administrative support was provided by Sophia Rakotoharimalala, Anjara Salomavola, Norbert Andrianarivelo, Boris Andrianantenaina, Sylviane Raharivelo, Luccianie Raonison, Cesaire Ramilison, Rina Ralison, Devon Litherland, Victoria Cordi, Ambroise Brenier and Christopher Holmes. For logistical support in the Anakao region, we thank the staff of IHSM in Toliara, in particular Daniel Ramampiherika and Thierry Lavitra, Thierry Bournonville and the staff of Lalandaka Lodge, Michel Agou and Madam Diamondra from Le Prince Hotel, and the Ministry of Fisheries PACP project. Amy Kennedy provided valuable guidance in analysis. Loriane Mendez, Eric Alfonsi, Eléonore Méheust and François-Gilles Carpentier provided assistance with molecular analysis. We also thank Kate Stafford for editorial help. All work was conducted under permits from the Madagascar Ministry of the Environment and Ministry of Fisheries.
Ethics
All fieldwork was conducted in accordance with relevant guidelines, as defined by the Society for Marine Mammalogy's Guidelines for the Treatment of Marine Mammals in Field Research (https://www.marinemammalscience.org/about-us/ethics/marine-mammal-treatment-guidelines/), and was approved under permit from the Madagascar Ministry of the Environment issued to Cetamada for work around Ile Saint Marie and to WCS for work around Anakao.
Data accessibility
Our data are available at the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.73h36/1 [116].
Authors' contributions
L.T. developed research hypotheses, carried out fieldwork, prepared the data, contributed to data processing and analysis and drafted the initial manuscript. S.C. conceived the study, developed research hypotheses, carried out fieldwork, and contributed to data analysis and manuscript preparation. A.N.Z. contributed to data processing and analysis and assisted with manuscript redaction. Y.G. carried out fieldwork. F.-X.M. carried out fieldwork and managed field logistics. J.-L.J. carried out the molecular laboratory work and sequence alignment. M.R.H. contributed to statistical analysis. S.P contributed to open-ocean data processing, and the model experiments that were performed using HPC resources from GENCI-IDRIS (Grant 2010-011140). J.-B.S. contributed to open-ocean data processing. H.C.R. conceived the study with collaborators, raised funds for the study and helped draft the manuscript. O.A. conceived of, raised funds for and coordinated the study. J.-B.C. developed the research hypothesis, participated in data analysis and helped draft the manuscript. All authors gave their final approval for publication.
Competing interests
The authors declare no competing interests.
Funding
Funding was provided by Total Foundation to NeuroPSI, and by individuals and foundations to the WCS Ocean Giants Program.
References
- 1.Acevedo-Gutiérrez A. 2009. Habitat use. In Encyclopedia of marine mammals (eds Perrin WF, Wursig B), pp. 545–548. San Diego, CA: Academic Press. [Google Scholar]
- 2.Borcard D, Legendre P, Drapeau P. 1992. Partialling out the spatial component of ecological variation. Ecology 73, 1045 (doi:10.2307/1940179) [Google Scholar]
- 3.Stevick P, McConnell BJ, Hammond PS. 2002. Patterns of movement. In Marine mammal biology: an evolutionary approach (ed. Hoelzel AR.), pp. 185–216. Oxford, UK: Blackwell Science. [Google Scholar]
- 4.Oña J, Garland EC, Denkinger J. 2016. Southeastern Pacific humpback whales (Megaptera novaeangliae) and their breeding grounds: distribution and habitat preference of singers and social groups off the coast of Ecuador. Mar. Mamm. Sci. (doi:10.1111/mms.12365) [Google Scholar]
- 5.MacKay MM, Würsig B, Bacon CE, Selwyn JD. 2016. North Atlantic humpback whale (Megaptera novaeangliae) hotspots defined by bathymetric features off western Puerto Rico. Can. J. Zool. 94, 517–527. (doi:10.1139/cjz-2015-0198) [Google Scholar]
- 6.Ford JK, Reeves RR. 2008. Fight or flight: antipredator strategies of baleen whales. Mamm. Rev. 38, 50–86. (doi:10.1111/j.1365-2907.2008.00118.x) [Google Scholar]
- 7.Dawbin WH. 1966. The seasonal migratory cycle of humpback whales. In Whales, dolphins and porpoises (ed. Norris KS.), pp. 145–170. Berkeley, CA: University of California Press. [Google Scholar]
- 8.Clapham PJ. 1996. The social and reproductive biology of humpback whales: an ecological perspective. Mamm. Rev. 26, 27–49. (doi:10.1111/j.1365-2907.1996.tb00145.x) [Google Scholar]
- 9.Corkeron PJ, Connor RC. 1999. Why do baleen whales migrate? Mar. Mamm. Sci. 15, 1228–1245. (doi:10.1111/j.1748-7692.1999.tb00887.x) [Google Scholar]
- 10.Clapham P. 2001. Why do baleen whales migrate? Mar. Mamm. Sci. 17, 432–436. (doi:10.1111/j.1748-7692.2001.tb01289.x) [Google Scholar]
- 11.Connor RC, Corkeron PJ. 2001. Predation past and present. Mar. Mamm. Sci. 17, 436–439. (doi:10.1111/j.1748-7692.2001.tb01290.x) [Google Scholar]
- 12.Rasmussen K, Palacios DM, Calambokidis J, Saborío MT, Dalla Rosa L, Secchi ER, Steiger GH, Allen JM, Stone M. 2007. Southern Hemisphere humpback whales wintering off Central America: insights from water temperature into the longest mammalian migration. Biol. Lett. 3, 302–305. (doi:10.1098/rsbl.2007.0067) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tyack P, Whitehead H. 1983. Male competition in large groups of wintering humpback whales. Behaviour 83, 132–154. (doi:10.1163/156853982X00067) [Google Scholar]
- 14.Whitehead H. 1983. Structure and stability of humpback whale groups off Newfoundland. Can. J. Zool. 61, 1391–1397. (doi:10.1139/z83-186) [Google Scholar]
- 15.Ersts PJ, Rosenbaum HC. 2003. Habitat preference reflects social organization of humpback whales (Megaptera novaeangliae) on a wintering ground. J. Zool. 260, 337–345. (doi:10.1017/S0952836903003807) [Google Scholar]
- 16.Craig AS, Herman LM. 2000. Habitat preferences of female humpback whales Megaptera novaeangliae in the Hawaiian Islands are associated with reproductive status. Mar. Ecol. Prog. Ser. 193, 209–216. (doi:10.3354/meps193209) [Google Scholar]
- 17.Mattila DK, Clapham PJ, Katona SK, Stone GS. 1989. Population composition of humpback whales, Megaptera novaeangliae, on Silver Bank, 1984. Can. J. Zool. 67, 281–285. (doi:10.1139/z89-041) [Google Scholar]
- 18.Félix F, Haase B. 1997. Spatial distribution of different age groups of humpback whales along the Ecuadorian coast. Eur. Res. Cetaceans 11, 129–132. [Google Scholar]
- 19.Garrigue C, Zerbini AN, Geyer Y, Heide-Jørgensen MP, Hanaoka W, Clapham P. 2010. Movements of satellite-monitored humpback whales from New Caledonia. J. Mamm. 91, 109–115. (doi:10.1644/09-MAMM-A-033R.1) [Google Scholar]
- 20.Garrigue C, Clapham PJ, Geyer Y, Kennedy AS, Zerbini AN. 2015. Satellite tracking reveals novel migratory patterns and the importance of seamounts for endangered South Pacific humpback whales. R. Soc. open sci. 2, 150489 (doi:10.1098/rsos.150489) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herman L, Antinoja R. 1977. Humpback whales in the Hawaiian breeding waters: population and pod characteristics. Sci. Rep. Whales Res. Inst. 29, 59–85. [Google Scholar]
- 22.Herman LM. 1979. Humpback whales in Hawaiian waters: a study in historical ecology. Pac. Sci. 33, 1–15. [Google Scholar]
- 23.Mobley JR, Bauer GB, Herman LM. 1999. Changes over a ten-year interval in the distribution and relative abundance of humpback whales (Megaptera novaeangliae) wintering in Hawaiian waters. Aquat. Mamm. 25, 63–72. [Google Scholar]
- 24.Félix F, Haase B. 2001. The humpback whale off the coast of Ecuador, population parameters and behavior. Rev. Biol. Mar. Oceanogr. 36, 61–74. (doi:10.4067/S0718-19572001000100006) [Google Scholar]
- 25.Cartwright R, Gillespie B, LaBonte K, Mangold T, Venema A, Eden K, Sullivan M, Fenton B. 2012. Between a rock and a hard place: habitat selection in female-calf humpback whale (Megaptera novaeangliae) pairs on the Hawaiian breeding grounds (ed. B Fenton). PLoS ONE 7, e38004 (doi:10.1371/journal.pone.0038004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kennedy AS, Zerbini AN, Vásquez OV, Gandilhon N, Clapham PJ, Adam O. 2013. Local and migratory movements of humpback whales (Megaptera novaeangliae) satellite-tracked in the North Atlantic Ocean. Can. J. Zool. 26, 8–17. (doi:10.1139/cjz-2013-0161) [Google Scholar]
- 27.Zerbini AN, et al. 2006. Satellite-monitored movements of humpback whales Megaptera novaeangliae in the Southwest Atlantic Ocean. Mar. Ecol. Prog. Ser. 313, 295–304. (doi:10.3354/meps313295) [Google Scholar]
- 28.Martins CCA, Morete ME, Coitinho MHE, Freitas A, Secchi ER, Kinas PG. 2001. Aspects of habitat use patterns of humpback whales in the Abrolhos Bank, Brazil, breeding ground. Mem. Queensl. Mus. 47, 563–570. [Google Scholar]
- 29.Félix F, Botero-Acosta N. 2011. Distribution and behaviour of humpback whale mother–calf pairs during the breeding season off Ecuador. Mar. Ecol. Prog. Ser. 426, 277–287. (doi:10.3354/meps08984) [Google Scholar]
- 30.Smith J, Grantham HS, Gales N, Double MC, Noad MJ, Paton D. 2012. Identification of humpback whale breeding and calving habitat in the Great Barrier Reef. Mar. Ecol. Prog. Ser. 447, 259–272. [Google Scholar]
- 31.Best PB, Findlay KP, Sekiguchi K, Peddemors VM, Rakotonirina B, Rossouw A, Gove D. 1998. Winter distribution and possible migration routes of humpback whales Megaptera novaeangliae in the southwest Indian Ocean. Mar. Ecol. Prog. Ser. 162, 287–299. (doi:10.3354/meps162287) [Google Scholar]
- 32.IWC. International Whaling Commission. Annex G. 1998. Report of the sub-committee on comprehensive assessment of Southern Hemisphere humpback whales. J. Cetacean Res. Manage. 48, 170–182. [Google Scholar]
- 33.Bermond M. 1950. Campagne baleiniere dans les eaux de Madagascar (saison 1949). Cybium 5, 31–47. [Google Scholar]
- 34.Angot M. 1951. Rapport scientifique sur les expeditions baleinieres autour de Madagascar (saisons 1949 et 1950). Mem. Lnst. Scient. Madagascar Ser. A 2, 439–486. [Google Scholar]
- 35.Best P, Sekiguchi K, Findlay K. 1995. A suspended migration of humpback whales Megaptera novaeangliae on the west coast of South Africa. Mar. Ecol. Prog. Ser. 118, 1–12. (doi:10.3354/meps118001). [Google Scholar]
- 36.Hart T, et al. 1997. Conservation and civil strife: two perspectives from Central Africa. Conserv. Biol. 11, 308–314. (doi:10.1046/j.1523-1739.1997.011002308.x) [Google Scholar]
- 37.Rosenbaum H. 2003. Humpback whales of Madagascar. In Natural history of Madagascar (eds Goodman S, Bengston J), pp. 217–221. Chicago, IL: University of Chicago Press. [Google Scholar]
- 38.Best PB, Brandão A. 2009. Humpback whaling at Madagascar, 1910–1950. Report SCF09SH2. presented to the IWC Scientific Committee. See http://iwcoffice.co.uk/_documents/sci_com/workshops/SC-F09-SH2.pdf.
- 39.Cerchio S, et al. 2009. Updated estimates of abundance for humpback whale breeding stock C3 off Madagascar, 2000–2006. Report SC61SH7 presented to the IWC Scientific Committee.
- 40.Rosenbaum HC, et al. 2009. Population structure of humpback whales from their breeding grounds in the South Atlantic and Indian Oceans. PLoS ONE 4, e7318 (doi:10.1371/journal.pone.0007318) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jackson J, et al. 2015. Southern hemisphere humpback whale comprehensive assessment. A synthesis and summary: 2005–2015. (San Diego, USA). Report SC-66a-SH03 presented to the IWC Scientific Committee.
- 42.Starbuck RS. 1878. History of the American whale fishery from its earliest inception to the year 1876. United States Commission of Fish and Fisheries, Report of the Commissioner for 1875–1876, Appendix A. Government Printing Office, Washington, DC.
- 43.Braleys ST. 1849. Journal kept on board the ship Arab of Fairhaven, 1849–1853. Sharon, MA: The Kendall Whaling Museum. [Google Scholar]
- 44.Rosenbaum H, Walsh P, Razafindrakoto Y, Vely M, Desalle R. 1997. First description of a humpback whale wintering ground in Baie d'Antongil, Madagascar. Conserv. Biol. 11, 308–314. [Google Scholar]
- 45.Razafindrakoto Y, Rosenbaum H, Helweg D. 2001. First description of humpback whale song from Antongil Bay, Madagascar. Mar. Mamm. Sci. 17, 180–186. (doi:10.1111/j.1748-7692.2001.tb00990.x) [Google Scholar]
- 46.Best PB, Sekiguchi K, Rakotonirina B, Rossouw A. 1996. The distribution and abundance of humpback whales off southern Madagascar, August-September 1994. Rep. Int. Whale Comm. 46, 323–331. [Google Scholar]
- 47.Pripp T, Gammelsrød T, Krakstad JO. 2014. Physical influence on biological production along the western shelf of Madagascar. Deep Sea Res. Part II Top. Stud. Oceanogr. 100, 174–183. (doi:10.1016/j.dsr2.2013.10.025) [Google Scholar]
- 48.Katz MB, Premoli C. 1979. India and Madagascar in Gondwanaland based on matching Precambrian lineaments. Nature 279, 312–315. (doi:10.1038/279312a0) [Google Scholar]
- 49.Goslin J, Segoufin J, Schlich R, Fisher RL. 1980. Submarine topography and shallow structure of the Madagascar Ridge, western Indian Ocean. Geol. Soc. Am. Bull. 91, 741–753. (doi:10.1130/0016-7606(1980)9) [Google Scholar]
- 50.Collette BB, Parin NV. 1941. Shallow-water fishes of Walters Shoals, Madagascar Ridge. Bull. Mar. Sci. 48, 1–22. [Google Scholar]
- 51.Lutjeharms JR, Machu E. 2000. An upwelling cell inshore of the East Madagascar Current. Deep Sea Res. I 47, 2405–2411. (doi:10.1016/S0967-0637(00)00026-1) [Google Scholar]
- 52.De Ruijter WPM, Aken HM, Beier EJ, Lutjeharms JRE, Matano RP, Schouten MW. 2004. Eddies and dipoles around South Madagascar: formation, pathways and large-scale impact. Deep Sea Res. Part I Oceanogr. Res. Pap. 51, 383–400. (doi:10.1016/j.dsr.2003.10.011) [Google Scholar]
- 53.Lutjeharms JR. 2006. The Agulhas Current, p. 329 New York, NY: Springer. [Google Scholar]
- 54.Braby L. 2014. Dynamics, interactions and ecosystem implications of mesoscale eddies formed in the southern region of Madagascar. Doctoral dissertation, University of Cape Town.
- 55.Clapham PJ. 2000. The humpback whale: seasonal feeding and breeding in a baleen whale. In Cetacean societies: field studies of dolphins and whales (eds Mann J, Connor RC, Tyack PL, Whitehead H), pp. 173–196. Chicago, IL: University of Chicago Press. [Google Scholar]
- 56.Mate B, Mesecar R, Lagerquist B. 2007. The evolution of satellite-monitored radio tags for large whales: one laboratory's experience. Deep Sea Res. Part II Top. Stud. Oceanogr. 54, 224–247. (doi:10.1016/j.dsr2.2006.11.021) [Google Scholar]
- 57.Zerbini AN, Andriolo A, Heide-Jørgensen MP, Moreira S, Pizzorno JL, Maia YG, Vanblaricom GR, Demaster DP. 2011. Migration and summer destinations of humpback whales (Megaptera novaeangliae) in the western South Atlantic Ocean. J. Cetacean Res. Manage. Spec. Issue 3, 113–118. [Google Scholar]
- 58.Gales N, Double MC, Robinson S, Jenner C, Jenner M, King E, Gedamke J, Paton D, Raymond B. 2009. Satellite tracking of southbound East Australian humpback whales (Megaptera novaeangliae): challenging the feast or famine model for migrating whales. Int. Whale Comm. SC61/SH17. [Google Scholar]
- 59.De Sá Alves LCP, Andriolo A, Zerbini AN, Pizzorno JLA, Clapham PJ. 2009. Record of feeding by humpback whales (Megaptera novaeangliae) in tropical waters off Brazil. Mar. Mamm. Sci. 25, 416–419. (doi:10.1111/j.1748-7692.2008.00249.x) [Google Scholar]
- 60.Swingle WM, Barco SG, Pitchford TD, Mclellan WA, Pabst D. 1993. Appearance of juvenile humpback whales feeding in the nearshore waters of Virginia. Mar. Mamm. Sci. 9, 309–315. (doi:10.1111/j.1748-7692.1993.tb00458.x) [Google Scholar]
- 61.Stockin KA, Burgess EA. 2005. Opportunistic feeding of an adult humpback whale migrating along the coast of Southeastern Queensland, Australia. Aquat. Mamm. 31, 120–123. (doi:10.1578/AM.31.1.2005.120) [Google Scholar]
- 62.Cerchio S, Trudelle L, Zerbini AN, Geyer Y, Mayer F, Charrassin JB, Jung JL, Adam O, Rosenbaum H. In press. Satellite tagging of humpback whales off Madagascar reveals long range movements of individuals in the Southwest Indian Ocean during the breeding season. Mar. Ecol. Prog. Ser. [Google Scholar]
- 63.Jayasankar P, Anoop B, Rajagopalan M. 2008. PCR-based sex determination of cetaceans and dugong from the Indian seas. Curr. Sci. 94, 1513–1516. [Google Scholar]
- 64.Argos. 1990. User's manual. Landover, MD: Service Argos. [Google Scholar]
- 65.Jonsen ID, Myers RA, James MC. 2007. Identifying leatherback turtle foraging behaviour from satellite telemetry using a switching state-space model. Mar. Ecol. Prog. Ser. 337, 255–264. (doi:10.3354/meps337255) [Google Scholar]
- 66.Jonsen ID, Flemming JM, Myers RA. 2005. Robust state-space modeling of animal movement data. Ecology 86, 2874–2880. (doi:10.1890/04-1852) [Google Scholar]
- 67.Jonsen ID, Myers RA, James MC. 2006. Robust hierarchical state-space models reveal diel variation in travel rates of migrating leatherback turtles: robust hierarchical state-space models. J. Anim. Ecol. 75, 1046–1057. (doi:10.1111/j.1365-2656.2006.01129.x) [DOI] [PubMed] [Google Scholar]
- 68.Hijmans R, Van Etten J. 2014. Raster: geographic data analysis and modeling. R package version 2, 15. [Google Scholar]
- 69.Madec G. 2008. ‘NEMO ocean engine’. Note du Pole de modélisation, Institut Pierre-Simon Laplace (IPSL), France. Tech. Rep. No. 27. ISSN No. 1288-1619.
- 70.Deshayes J, et al. 2013. Oceanic hindcast simulations at high resolution suggest that the Atlantic MOC is bistable. Geophys. Res. Lett. 40, 3069–3073. (doi:10.1002/grl.50534) [Google Scholar]
- 71.Gaspar P, Georges J-Y, Fossette S, Lenoble A, Ferraroli S, Le Maho Y. 2006. Marine animal behaviour: neglecting ocean currents can lead us up the wrong track. Proc. R. Soc. B. 273, 2697–2702. (doi:10.1098/rspb.2006.3623) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Galli S, Gaspar P, Fossette S, Calmettes B, Hays GC, Lutjeharms JRE, Luschi P. 2012. Orientation of migrating leatherback turtles in relation to ocean currents. Anim. Behav. 84, 1491–1500. (doi:10.1016/j.anbehav.2012.09.022) [Google Scholar]
- 73.Calenge C. Home range estimation in R: the adehabitatHR package. R package version 0.4. 4. The Comprehensive R Archive Network.
- 74.Pinheiro J, Bates D, DebRoy S, Sarkar D.2007. nlme: linear and nonlinear mixed effects models. R package version 3.1–117. See http://CRAN.R-project.org/package=nlme .
- 75.Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM. 2009. Mixed effects models and extensions in ecology with R, 574 New York, NY: Springer. [Google Scholar]
- 76.O'Toole MD, Lea M-A, Guinet C, Schick R, Hindell MA. 2015. Foraging strategy switch of a top marine predator according to seasonal resource differences. Front. Mar. Sci. 2, 21 (doi:10.3389/fmars.2015.00021) [Google Scholar]
- 77.Zuur AF, Ieno EN, Elphick CS. 2010. A protocol for data exploration to avoid common statistical problems: data exploration. Methods Ecol. Evol. 1, 3–14. (doi:10.1111/j.2041-210X.2009.00001.x) [Google Scholar]
- 78.Herve M. 2014. Aide-mémoire de statistique appliquée à la biologie. Constr Son Étude Anal Résultats à l'aide du logiciel R. Version, 5.
- 79.Roquet F, et al. 2013. Estimates of the Southern Ocean general circulation improved by animal-borne instruments. Geophys. Res. Lett. 40, 6176–6180. (doi:10.1002/2013GL058304) [Google Scholar]
- 80.Jenner KCS, Jenner MN, McCabe KA. 2001. Geographical and temporal movements of humpback whales in Western Australian waters. APPEA J. 38, 692–707. [Google Scholar]
- 81.Chapman JW, Klaassen RHG, Drake VA, Fossette S, Hays GC, Metcalfe JD, Reynolds AM, Reynolds DR, Alerstam T. 2011. Animal orientation strategies for movement in flows. Curr. Biol. 21, R861–R870. (doi:10.1016/j.cub.2011.08.014) [DOI] [PubMed] [Google Scholar]
- 82.Frankel AS, Clark CW, Herman L, Gabriele CM. 1995. Spatial distribution, habitat utilization, and social interactions of humpback whales, Megaptera novaeangliae, off Hawai'i, determined using acoustic and visual techniques. Can. J. Zool. 73, 1134–1146. (doi:10.1139/z95-135) [Google Scholar]
- 83.Smultea MA. 1994. Segregation by humpback whale (Megaptera novaeangliae) cows with a calf in coastal habitat near the island of Hawaii. Can. J. Zool. 72, 805–811. (doi:10.1139/z94-109) [Google Scholar]
- 84.Zerbini AN, Andriolo A, Da Rocha JM, Simões-Lopes PC, Siciliano S, Pizzorno JL, Waite JM, DeMaster DP, Vanblaricom GR. 2004. Winter distribution and abundance of humpback whales (Megaptera novaeangliae) off Northeastern Brazil. J. Cetacean Res. Manage. 6, 101–107. [Google Scholar]
- 85.Andriolo A, Martins CCA, Engel MH, Pizzorno JL, Más-Rosa S, Freitas AC, Morete ME, Kinas PG. 2006. The first aerial survey to estimate abundance of humpback whales (Megaptera movaeangliae) in the breeding ground off Brazil (Breeding Stock A). J. Cetacean Res. Manage. 8, 307–311 [Google Scholar]
- 86.Findlay KP, Meyer M, Elwen S, Kotze D. 2011. Distribution and abundance of humpback whales, Megaptera novaeanglie, off the coast of Mozambique, 2003. J. Cetacean Res. Manage. (special issue) 3, 163–174. [Google Scholar]
- 87.Darling J. 2001. Characterization of behavior of humpback whales in Hawaiian waters. Report to Hawaiian Islands Humpback Whale National Marine Sanctuary. Honolulu, HI.
- 88.Cerchio S, Jacobsen JK, Cholewiak DM, Falcone EA, Merriwether DA. 2005. Paternity in humpback whales, Megaptera novaeangliae: assessing polygyny and skew in male reproductive success. Anim. Behav. 70, 267–277. (doi:10.1016/j.anbehav.2004.10.028) [Google Scholar]
- 89.Payne R, McVay S. 1971. Songs of humpback whales. Humpbacks emit sounds in long, predictable patterns ranging over frequencies audible to humans. Science 173, 585–597. [DOI] [PubMed] [Google Scholar]
- 90.Bass AH, Clark CW. 2003. The physical acoustics of underwater sound communication. In Acoustic communication (eds Simmons AM, Fay RR, Popper AN), pp. 15–64. New York, NY: Springer. [Google Scholar]
- 91.Baker CS, Herman LM. 1984. Aggressive behavior between humpback whales (Megaptera novaeangliae) wintering in Hawaiian waters. Can. J. Zool. 62, 1922–1937. (doi:10.1139/z84-282) [Google Scholar]
- 92.Weinrich M. 1995. Humpback whale competitive groups observed on a high-latitude feeding ground. Mar. Mamm. Sci. 11, 251–254. (doi:10.1111/j.1748-7692.1995.tb00524.x) [Google Scholar]
- 93.Smith JN, Goldizen AW, Dunlop RA, Noad MJ. 2008. Songs of male humpback whales, Megaptera novaeangliae, are involved in intersexual interactions. Anim. Behav. 76, 467–477. (doi:10.3354/meps09462) [Google Scholar]
- 94.Fossette S, Heide-Jørgensen M-P, Jensen MV, Kiszka J, Bérubé M, Bertrand N, Vely M. 2014. Humpback whale (Megaptera novaeangliae) post breeding dispersal and southward migration in the western Indian Ocean. J. Exp. Mar. Biol. Ecol. 450, 6–14. (doi:10.1016/j.jembe.2013.10.014) [Google Scholar]
- 95.Rosenbaum HC, Maxwell SM, Kershaw F, Mate B. 2014. Long-range movement of humpback whales and their overlap with anthropogenic activity in the South Atlantic Ocean: whale habitat overlap with human activity. Conserv. Biol. 28, 604–615. (doi:10.1111/cobi.12225) [DOI] [PubMed] [Google Scholar]
- 96.Mate B, Gisiner G, Mobley J. 1998. Local and migratory movements of Hawaiian humpback whales tracked by satellite telemetry. Revue Canadienne de Zoologie 76, 863–868. (doi:10.1139/z98-008) [Google Scholar]
- 97.Lagerquist BA, Mate BR, Ortega-Ortiz JG, Winsor M, Urbán-Ramirez J. 2008. Migratory movements and surfacing rates of humpback whales (Megaptera novaeangliae) satellite tagged at Socorro Island, Mexico. Mar. Mamm. Sci. 24, 815–830. (doi:10.1111/j.1748-7692.2008.00217.x) [Google Scholar]
- 98.Dulau-Drouot S, Cerchio S, Pinet P, Geyer Y, Mongin P, Fayan J, Cottarel G, Zerbini A. In preparation. Humpback whale satellite tagging in Réunion: where do they go next?
- 99.Penven P, Lutjeharms J, Florenchie P. 2006. Madagascar: a pacemaker for the Agulhas Current system? Geophys. Res. Lett. 33, GL026854 (doi:10.1029/2006GL026854) [Google Scholar]
- 100.Siedler G, Rouault M, Lutjeharms JR. 2006. Structure and origin of the subtropical South Indian Ocean Countercurrent. Geophys. Res. Lett. 33, pGL027399 (doi:10.1029/2006GL027399) [Google Scholar]
- 101.Shotton R. 2006. Management of demersal fisheries resources of the southern Indian Ocean, p. 90. FAO Fisheries Circular. Rome, Italy: Food and Agriculture Organization of the United Nations; See http://afrilib.odinafrica.org/handle/0/16947. [Google Scholar]
- 102.Kaschner K. 2008. Air-breathing visitors to seamounts: marine mammals. In Seamounts: ecology, fisheries and conservation (eds Pitcher TJ, Morato T, Hart PJB, Clark MR, Haggan N, Santos RS), pp. 245–251. Oxford, UK: Blackwell Publishing Ltd. [Google Scholar]
- 103.Porteiro FM, Sutton T. 2008. Midwater fish assemblages and seamounts. In Seamounts: ecology, fisheries and conservation (eds Pitcher TJ, Morato T, Hart PJB, Clark MR, Haggan N, Santos RS), pp. 245–251. Oxford, UK: Blackwell Publishing Ltd. [Google Scholar]
- 104.Morato T, Hoyle SD, Allain V, Nicol SJ. 2010. Seamounts are hotspots of pelagic biodiversity in the open ocean. Proc. Natl Acad. Sci. USA 107, 9707–9711. (doi:10.1073/pnas.0910290107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tynan CT, Ainley DG, Barth JA, Cowles TJ, Pierce SD, Spear LB. 2005. Cetacean distributions relative to ocean processes in the northern California Current System. Deep Sea Res. Part II Top. Stud. Oceanogr. 52, 145–167. (doi:10.1029/97GL02860) [Google Scholar]
- 106.Le Corre M, et al. 2012. Tracking seabirds to identify potential Marine Protected Areas in the tropical western Indian Ocean. Biol. Conserv. 156, 83–93. (doi:10.1016/j.biocon.2011.11.015) [Google Scholar]
- 107.Pinet P, Jaquemet S, Phillips RA, Le Corre M. 2012. Sex-specific foraging strategies throughout the breeding season in a tropical, sexually monomorphic small petrel. Anim. Behav. 83, 979–989. (doi:10.1016/j.anbehav.2012.01.019) [Google Scholar]
- 108.Venables HJ, Pollard RT, Popova EE. 2007. Physical conditions controlling the development of a regular phytoplankton bloom north of the Crozet Plateau, Southern Ocean. Deep Sea Res. Part II Top. Stud. Oceanogr. 54, 1949–1965. (doi:10.1016/j.dsr2.2007.06.014) [Google Scholar]
- 109.Fielding S, Ward P, Pollard RT, Seeyave S, Read JF, Hughes JA, Smith T, Castellani C. 2007. Community structure and grazing impact of mesozooplankton during late spring/early summer 2004/2005 in the vicinity of the Crozet Islands (Southern Ocean). Deep Sea Res. Part II Top. Stud. Oceanogr. 54, 2106–2125. (doi:10.1016/j.dsr2.2007.06.016) [Google Scholar]
- 110.Martin A, Pruvost P. 2007. Pecheker, relational database for analysis and management of fisheries and related biological data from the French Southern Ocean fisheries monitoring scientific programs. Paris, France: Muséum National d'Histoire Naturelle. [Google Scholar]
- 111.Braithwaite JE, Meeuwig JJ, Hipsey MR. 2015. Optimal migration energetics of humpback whales and the implications of disturbance. Conserv. Physiol. 3, cov001 (doi:10.1093/conphys/cov001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Luschi P, Sale A, Mencacci R, Hughes GR, Lutjeharms JRE, Papi F. 2003. Current transport of leatherback sea turtles (Dermochelys coriacea) in the ocean. Proc. R. Soc. Lond. B 270, S129–S132. (doi:10.1098/rsbl.2003.0036) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lambardi P, Lutjeharms JR, Mencacci R, Hays GC, Luschi P. 2008. Influence of ocean currents on long-distance movement of leatherback sea turtles in the Southwest Indian Ocean. Mar. Ecol. Prog. Ser. 353, 289–301. (doi:10.3354/meps07118) [Google Scholar]
- 114.Fossette S, Hobson VJ, Girard C, Calmettes B, Gaspar P, Georges J-Y, Hays GC. 2010. Spatio-temporal foraging patterns of a giant zooplanktivore, the leatherback turtle. J. Mar. Syst. 81, 225–234. (doi:10.1016/j.jmarsys.2009.12.002) [Google Scholar]
- 115.Horton TW, Holdaway RN, Zerbini AN, Hauser N, Garrigue C, Andriolo A, Clapham P. 2011. Straight as an arrow: humpback whales swim constant course tracks during long-distance migration. Biol. Lett. 7, 674–679. (doi:10.1098/rsbl.2011.0279) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Trudelle L, et al. 2016. Data from: Influence of environmental parameters on movements and habitat utilization of humpback whales (Megaptera novaeangliae) in the Madagascar breeding ground. Dryad Digital Repository. (doi:10.5061/dryad.73h36). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Our data are available at the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.73h36/1 [116].