Gibberellin induces non-reductional cell division and diploid pollen formation in Arabidopsis by interfering with DELLA-mediated male meiotic cytokinesis
Abstract
The plant hormone gibberellic acid (GA) controls many physiological processes, including cell differentiation, cell elongation, seed germination, and response to abiotic stress. In this study, we report that exogenous treatment of flowering Arabidopsis (Arabidopsis thaliana) plants with GA specifically affects the process of male meiotic cytokinesis leading to meiotic restitution and the production of diploid (2n) pollen grains. Similar defects in meiotic cell division and reproductive ploidy stability occur in Arabidopsis plants depleted of RGA and GAI, two members of the DELLA family that function as suppressor of GA signaling. Cytological analysis of the double rga-24 gai-t6 mutant revealed that defects in male meiotic cytokinesis are not caused by alterations in meiosis I (MI or meiosis II (MII) chromosome dynamics, but instead result from aberrations in the spatial organization of the phragmoplast-like radial microtubule arrays (RMAs) at the end of meiosis II. In line with a role for GA in the genetic regulation of the male reproductive system, we additionally show that DELLA downstream targets MYB33 and MYB65 are redundantly required for functional RMA biosynthesis and male meiotic cytokinesis. By analyzing the expression of pRGA::GFP-RGA in the wild-type Landsberg erecta background, we demonstrate that the GFP-RGA protein is specifically expressed in the anther cell layers surrounding the meiocytes and microspores, suggesting that appropriate GA signaling in the somatic anther tissue is critical for male meiotic cell wall formation and thus plays an important role in consolidating the male gametophytic ploidy consistency.
Polyploidization repeatedly occurred in the history of plant evolution, creating a basis for genetic diversification and speciation (Adams and Wendel, 2005). Different mechanisms may contribute to the formation of polyploid species (Ramsey and Schemske, 1998), yet the production of unreduced gametes is considered the main cause of polyploid induction (Bretagnolle and Thompson, 1995). Generally, both pre- and postmeiotic genome duplication events, as well as meiotic restitution, can lead to 2n gamete formation (Bretagnolle and Thompson, 1995; Ramsey and Schemske, 1998). In the tomato pmcd1 mutant, for example, ectopic defects in cell wall formation during mitotic cell division in meiotic founder cells lead to premeiotic genome duplication and the associated formation of tetraploid meiocytes and diploid pollen grains (De Storme and Geelen, 2013b). As the main cellular process driving sexual polyploidization, meiotic restitution can be classified into three types of mechanisms: omission of meiosis I or II, alteration in spindle organization, and unsuccessful or incomplete meiotic cytokinesis (Ramanna and Jacobsen, 2003).
Meiotic cell division and gametophytic ploidy stability are strictly controlled at the molecular level, and several factors operating in this process have already been identified (De Storme and Mason, 2014). In Arabidopsis (Arabidopsis thaliana), DYAD/SWITCH1 regulates meiotic chromatid cohesion and chromosome structure, and loss of function of this gene has been found to fully convert the reductional meiotic cell division into a mitotic one, eventually yielding clonal, diploid gametes in female sporogenesis (Ravi et al., 2008). The Arabidopsis OSD1/GIG1 and TAM/CYCA1;2 proteins promote the transition of meiosis I into meiosis II, and loss of function of each of them causes omission of the second meiotic cell division, resulting in 2n gamete formation in both male and female gametogenesis (d’Erfurth et al., 2009, 2010). Mutation in ps in potato and loss of function of JASON or AtPS1 in Arabidopsis cause an altered spindle configuration in male meiosis II, with parallel, tripolar, and fused spindles giving rise to restituted dyads and triads that contain 2n male gametes (Mok and Peloquin, 1975; d’Erfurth et al., 2008; De Storme and Geelen, 2011). Meiotic restitution may also result from defects in meiotic cytokinesis, either following MI or MII, with either a full or partial elimination of meiotic cell wall formation leading to 2n or polyploid gamete formation (De Storme and Geelen, 2013c). In Arabidopsis, a specific MAPK signaling cascade controls male meiotic cytokinesis (Takahashi et al., 2004; Takahashi et al., 2010). Loss of function of TES, MKK6, or MPK4 causes defects in male meiotic cell wall formation and eventually leads to the ectopic production of diploid or polyploid male gametes (Spielman et al., 1997; Kosetsu et al., 2010; Zeng et al., 2011). Besides being induced by genetic defects, meiotic restitution and alterations in the consistency of the gametophytic ploidy may also result from environmental stresses, such as heat and cold (Pécrix et al., 2011; De Storme et al., 2012; De Storme and Mason, 2014; Zhou et al., 2015). Although progress has been made in understanding the cellular mechanisms and genetic factors involved in 2n gamete formation, the molecular factors and signaling pathways involved remain largely unknown.
Gibberellins (GAs) are endogenous plant hormones that play an important role in many aspects of plant growth and development, including seed germination, leaf expansion, trichome development, pollen maturation, and floral transition (Debeaujon and Koornneef, 2000; Davière and Achard, 2013; Claeys et al., 2014). The Arabidopsis GA biosynthetic mutant ga1-3 displays defects in stem elongation, flowering time, and leaf abaxial trichome initiation (Silverstone et al., 1998). Besides mediating somatic tissue growth and development, GA is also involved in the control of reproductive system growth and floral morphogenesis (Fleet and Sun, 2005). GA positively regulates the development of the Arabidopsis stamen and petal by promoting cell elongation and also regulates microsporogenesis and pollen development (Cheng et al., 2004; Plackett et al., 2014). In multiple species, GA signaling regulates both the development and programmed cell death of tapetal cells, which is required for the growth and maturation of the developing microspores. Moreover, GA is also known to operate as a stress signaling molecule upon exposure to abiotic stresses (cold, salt, and osmotic stresses) triggered by either alterations in GA biosynthesis or its downstream signaling pathway. For example, cold stress inhibits root growth and disrupts pollen development by reducing the plant’s endogenous GA levels (Achard et al., 2008; Sakata et al., 2014). However, although various significances of GA in both vegetative and reproductive growth and development have been identified, it is unknown whether GA plays a role in the control of meiotic cell division.
The DELLA family, which consists of five members (RGA, GAI, RGL1, RGL2, and RGL3), acts as the main repressor of the GA signaling pathway by negatively regulating the expression of GA responsive genes (Davière and Achard, 2013). The positive effect of GA on plant growth is achieved by stimulating the degradation of DELLAs and hence by counteracting their growth-suppressive effect (Silverstone et al., 2001; Xu et al., 2014). The majority of GA-dependent growth and development processes are mediated by DELLAs and their promiscuous interaction with transcription factors (Fleet and Sun, 2005; Daviere and Achard, 2015). The GA receptor GID1-DELLA complex constitutes an important regulatory pathway in GA signaling. GA stimulates the formation of the GA-GID1-DELLA complex that is a target for ubiquitination and subsequent 26S proteasome (Sun, 2010, 2011).
In barley and rice, GA positively regulates the expression of a specific group of MYB transcription factors, sometimes referred to as GAMYB, by relieving the repressive action of DELLAs (Gubler et al., 2002; Huang et al., 2015). Arabidopsis MYB33 and MYB65 are two of the GAMYB-like DELLA downstream target genes that are essential for anther development (Millar and Gubler, 2005; Cheng et al., 2009) and are involved in various other development processes (Jin and Martin, 1999; Gocal et al., 2001; Stracke et al., 2001). Several studies have shown that MYB33 and MYB65 are negatively controlled by miRNA159 (Millar and Gubler, 2005; Alonso-Peral et al., 2010; Alonso-Peral et al., 2010), whose expression is suppressed by DELLAs (Achard et al., 2004), suggesting a positive role of DELLA in regulating MYB33 and MYB65.
In this study, we demonstrate that exogenous GA treatment of flowering Arabidopsis plants induces meiotic restitution in male sporogenesis and subsequently generates viable 2n pollen. Using a combination of genetic and cytological analyses, we found that the combined loss of the DELLA transcription factors RGA and GAI causes defective cytokinesis and aberrant callosic cell wall formation at the male meiotic tetrad stage, yielding a subset of diploid, bean-shaped microspores. In agreement with the cytokinesis defects, the formation of the radial microtubule arrays (RMAs) at the end of male meiosis is altered or absent between adjacent nuclei. In addition, combined loss of function of the downstream DELLA targets MYB33 and MYB65 causes similar defects in male meiotic cytokinesis and gametophytic ploidy stability as observed in the DELLA double mutant, suggesting that MYB33 and MYB65 are downstream targets of DELLA in controlling male meiotic cytokinesis. Based on our findings, we propose that gametophytic ploidy consistency depends on a functional GA-DELLA-MYBs regulatory module.
RESULTS
GA3 Treatment Induces Diploid Pollen Formation in Arabidopsis
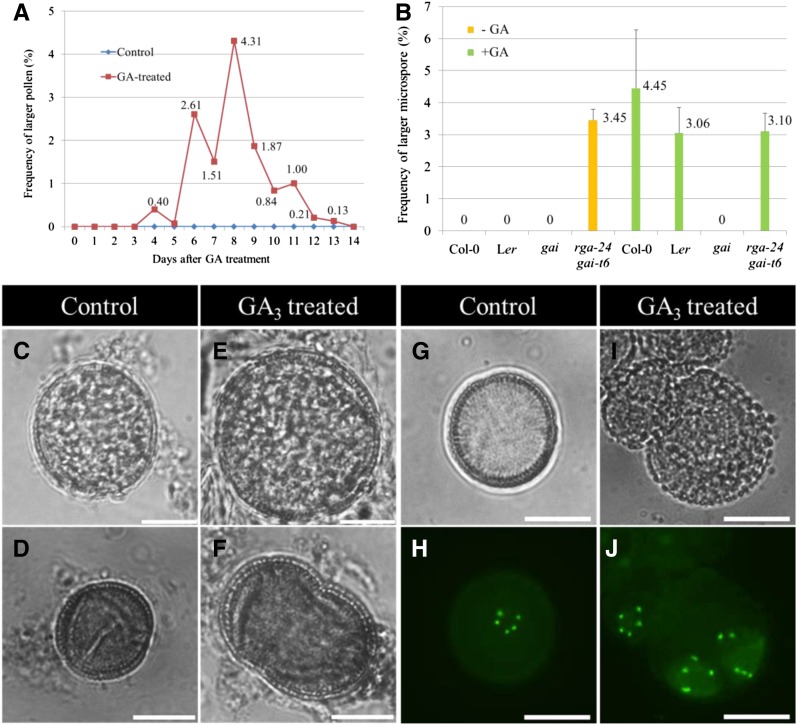
To determine the effect of the plant hormone GA on male sporogenesis in Arabidopsis, we sprayed wild-type Col-0 plants with 100 µM GA3 and water. To assess for putative alterations in male spore formation, the ploidy of Arabidopsis pollen was analyzed during the 14 d following GA3 treatment (Fig. 1A). The ploidy level of resulting pollen grains was monitored by assessing pollen diameter, which is a proxy for the gametophytic ploidy level (De Storme et al., 2013). During the first 5 days following GA treatment, pollen grains appeared normally sized, similar to pollen isolated from nontreated control plants. However, from the sixth day (2.61%) to the thirteenth day (0.13%) after GA3 treatment, a variable number of oversized mature pollen grains was found, with the highest frequency of larger pollen (4.31%) at day 8 post treatment (dpt; Fig. 1, A, C, and E). To monitor whether GA-induced larger pollen resulted from enlarged microspores, spore size at the early microspore stage was analyzed during the first 7 days following GA3 treatment. Three to four days post treatment, a subpopulation of microspores at the unicellular stage (4.45%) appeared larger than similar stage spores isolated from a nontreated control (Fig. 1, B, D, and F). These findings demonstrate that GA induces the formation of larger pollen grains, indicating an increased gametophytic ploidy level and that the GA-induced larger pollen grains originate from a cellular defect occurring before pollen mitosis I.
Figure 1.
GA3 treatment induces the formation of diploid pollen in Arabidopsis. A, Histogram showing the frequency of larger pollen produced in Arabidopsis Col-0 plants 1 to 14 d after exogenous GA3 treatment (100 µM); see exact numbers in the histogram. B, Histogram showing the frequency of enlarged unicellular-stage microspores yielded 3 to 4 d after GA3 treatment (100 µM). Bright field image of haploid mature pollen grain (C) and unicellular-stage microspore (D) produced in untreated Arabidopsis plants. Enlarged mature pollen grain (E) and unicellular-stage microspore (F) from Arabidopsis plants treated by 100 µM GA3. G and H, Haploid microspore displaying five centromeric GFP dots in control, untreated plants harboring the pWOX2::CENH3-GFP reporter construct. I and J, Diploid microspores in pWOX2::CENH3-GFP transgenic plants treated by 100 µM GA3, exhibiting two syncytial groups of five CENH3-GFP dots. Scale bars = 10 µm.
To determine the gametophytic ploidy level of the GA3-induced enlarged microspores, the chromosome number in male gametes at the unicellular microspore stage was quantified using the pWOX2::CENH3-GFP reporter line (De Storme et al., 2016). Under control conditions, unicellular microspores isolated from diploid Arabidopsis plants (2× = 10) harboring the pWOX2::CENH3-GFP construct consistently show five distinct GFP foci, reflecting the haploid chromosome number in their gametophytic nucleus (Fig. 1, G and H). In contrast, the enlarged microspores in GA3-treated plants revealed an increased number of GFP dots, specifically showing 10 distinct foci organized in two spatially separated but syncytial clusters of five GFP dots (Fig. 1, I and J). The occurrence of 10 CENH3-GFP dots provides evidence for a doubled chromosome number, indicating that the enlarged pollen grains are diploid. The specific localization pattern of the centromeric CENH3-GFP foci in GA3-induced enlarged spores suggests the presence of two syncytial haploid nuclei, indicating that diploid microspores either result from a nuclear duplication event (endomitosis) during early microgametogenesis or, alternatively, from of meiotic nonreduction through defects in meiotic cell wall formation.
Constitutive GA Signaling in the Arabidopsis DELLA rga-24 gai-t6 Mutant Leads to Diploid Pollen Formation
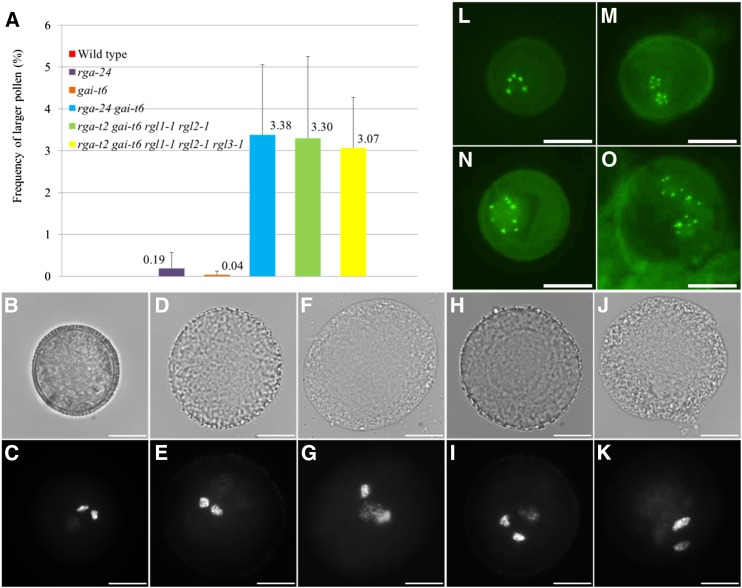
GA3-mediated activation of the GA signaling pathway generally occurs through degradation of DELLA proteins that mainly act as negative transcriptional regulators of the GA response. To determine whether GA3-induced formation of diploid pollen is mediated by DELLAs, we further analyzed a series of single, double, quadruple, and pentuple null DELLA mutants for alterations in pollen morphology. In contrast to the single rga-24 and gai-t6 Arabidopsis mutants, which only produce a very low number of larger pollen grains (Fig. 2A), the double null rga-24 gai-t6 mutant generates a significant number of larger pollen (3.38%), similar to the frequency of larger pollen grains induced by exogenous GA3 treatment (Fig. 2, A, B–G). Similar frequencies of larger pollen grains were observed in the quadruple rga-t2 gai-t6 rgl1-1 rgl2-1 mutant (3.30%) and the pentuple rga-t2 gai-t6 rgl1-1 rgl2-1 rgl3-1 mutant (3.07%; Fig. 2, A, H–K; Supplemental Fig. S1F), indicating that except for RGA and GAI, the mutations in other DELLA genes (e.g. RGL1, RGL2, and RGL3) do not contribute to the larger pollen phenotype.
Figure 2.
The DELLA double rga-24 gai-t6, quadruple rga-t2 gai-t6 rgl1-1 rgl2-1, and pentuple rga-t2 gai-t6 rgl1-1 rgl2-1 rgl3-1 mutants produce a subset of diploid pollen. A, Histogram showing the percentage of larger pollen grains produced in rga-24 and gai-t6 single and double mutants, rga-t2 gai-t6 rgl1-1 rgl2-1 quadruple mutant, and in the rga-t2 gai-t6 rgl1-1 rgl2-1 rgl3-1 pentuple mutant. B to K, DAPI staining of mature pollen grains isolated from wild-type Ler plants (B and C), the rga-24 gai-t6 double mutant (D–G), and the rga-t2 gai-t6 rgl1-1 rgl2-1 quadruple mutant (H–K). Unicellular microspores of control plants (L) and the rga-24 gai-t6 double mutant (M–O) harboring the pWOX2::CENH3-GFP reporter construct. Enlarged spores in the rga-24 gai-t6 double mutant either show 10 (M and N) or 15 (O) centromeric GFP foci. Enlarged GFP dots in picture N result from an overlay of two single GFP foci. Scale bars = 10 µm.
To analyze the size and organization of the gametophytic nuclei in the larger pollen grains of the DELLA double and quadruple mutants, nuclei were stained and visualized using 4’,6-diamidino-2-phenylindole (DAPI). The nuclear configuration of the enlarged pollen in both types of DELLA mutants appears highly similar to that of wild-type haploid pollen, displaying one less-condensed vegetative nucleus and two highly condensed sperm nuclei. However, both the vegetative nucleus and the sperm nuclei in rga-24 gai-t6 and rga-t2 gai-t6 rgl1-1 rgl2-1 larger pollen appear significantly larger compared to their corresponding counterparts in wild-type haploid pollen, indicating that the enlarged pollen have a higher nuclear DNA content. These findings, together with relative pollen size increase, suggest that the enlarged pollen grains in both types of DELLA mutants are diploid (Fig. 2, D, E, H and I) or triploid (Fig. 2, F, G, J and K). In addition to this ectopic induction of gametophytic instability, it was also noted that all pollen in both DELLA double and quadruple mutants display a defective pollen wall morphology (Fig. 2, D, F, H and J), typically lacking the thick, protein-rich outer cell wall. A similar defect has been observed in the enlarged pollen isolated from wild-type Landsberg erecta (Ler) plants sprayed with 100 µM GA3 (Supplemental Fig. S2), indicating that alterations in GA signaling not only interfere with male gametophytic ploidy stability but also affect processes in pollen wall formation.
The gametophytic ploidy level of the larger pollen in the rga-24 gai-t6 double mutant was accurately determined by quantifying the chromosome number in early-stage microspores using the pWOX2::CENH3-GFP. Contrary to the haploid unicellular microspores from control plants, which consistently show five distinct centromeric GFP dots (Fig. 2L), the oversized microspores in the rga-24 gai-t6 double mutant harbor either two or three nuclei that each exhibits five distinct GFP foci (Fig. 2, M and O) or alternatively contain one single enlarged nucleus with 10 GFP dots reflecting a fusion of two haploid nuclei (Fig. 2N). These observations demonstrate that the rga-24 gai-t6 double mutant generates a subset of microspores containing two or more sets of haploid nuclei that subsequently fuse to yield diploid or triploid pollen grains.
To determine the viability of the diploid pollen grains induced by RGA and GAI mutation, we evaluated cellular activity of mature pollen grains by fluorescein diacetate (FDA) staining (Heslop-Harrison and Heslop-Harrison, 1970; Trognitz, 1991; Abdul-Baki, 1992; Supplemental Fig. S3A–F). FDA staining of both mature haploid Ler pollen grains (Supplemental Fig. S3, C and D) and enlarged rga-24 gai-t6 mutant pollen (Supplemental Fig. S3, E and F) showed clear cytoplasmic fluorescence, indicating that haploid, diploid, and polyploid pollen grains were biologically active and thus viable. In addition, the germination ability of the larger pollen grains in the rga-24 gai-t6 double mutant plants was examined in vitro (Supplemental Fig. S3G–J). Following soiling on pollen growth medium and incubation in the dark for 24 h, enlarged rga-24 gai-t6 pollen grains germinated and produced pollen tubes (Supplemental Fig. S3H and J; red dot labels larger pollen grains in J) similar to the control Ler haploid pollen (Supplemental Fig. S3G and I). Altogether, these data indicate that diploid pollen grains produced by rga-24 gai-t6 Arabidopsis plants are viable and may evoke sexual polyploidization events.
GA3 Induces the Formation of Diploid Pollen by Promoting DELLA Degradation
To determine whether GA induces diploid pollen formation through degradation of DELLA, we next examined the GA response of male sporogenesis in the GA-insensitive gai mutant and the DELLA rga-24 gai-t6 double mutant. In contrast to the gai-t6 loss-of-function mutation, the allelic gai mutation is localized in the N-terminal DELLA domain of GAI and thus acts as a gain-of-function mutation, impairing the GA response in multiple GA-mediated processes (seed germination, stem elongation, and onset of flowering), even under exogenous GA treatment conditions (Wilson and Somerville, 1995; Willige et al., 2007). After spraying both wild-type Ler plants and gai mutant plants with 100 µM GA3, the frequency of enlarged uninuclear stage microspore was quantified 4 d post treatment. Under normal growth conditions, gai plants produce normal-sized haploid microspores (Fig. 1B), indicating that an impaired GA response ability in Arabidopsis does not affect male sporogenesis. However, in contrast to GA-treated wild-type Ler plants, which produce a subset of enlarged unicellular-stage microspores (3.06%) (Fig. 1B), no oversized microspores were observed in gai plants after GA treatment (Fig. 1B). As male sporogenesis in gai plants is not responsive to exogenous GA application, these findings demonstrate that GA-induced production of 2n pollen in Arabidopsis requires a functional, targetable GAI DELLA domain and thus indicate that GA-based induction of 2n pollen in Arabidopsis is mediated through GA-based degradation of the DELLA GAI protein.
In support of this, we found that exogenous GA3 treatment of the double rga-24 gai-t6 mutant plants does not enhance the frequency of enlarged uninuclear microspores compared with the untreated rga-24 gai-t6 plants or GA-treated wild-type Ler plants (Fig. 1B), indicating that exogenous GA3 treatment has no additive effect on diploid male gamete formation in the double rga-24 gai-t6 mutant background. Collectively, these data demonstrate that exogenous GA3 treatment induces diploid pollen formation in Arabidopsis by promoting DELLA protein degradation.
Diploid Pollen in rga-24 gai-t6 Mutant and GA3-Treated Wild-Type Arabidopsis Plants Result from a Restitution of Male Meiotic Cell Division
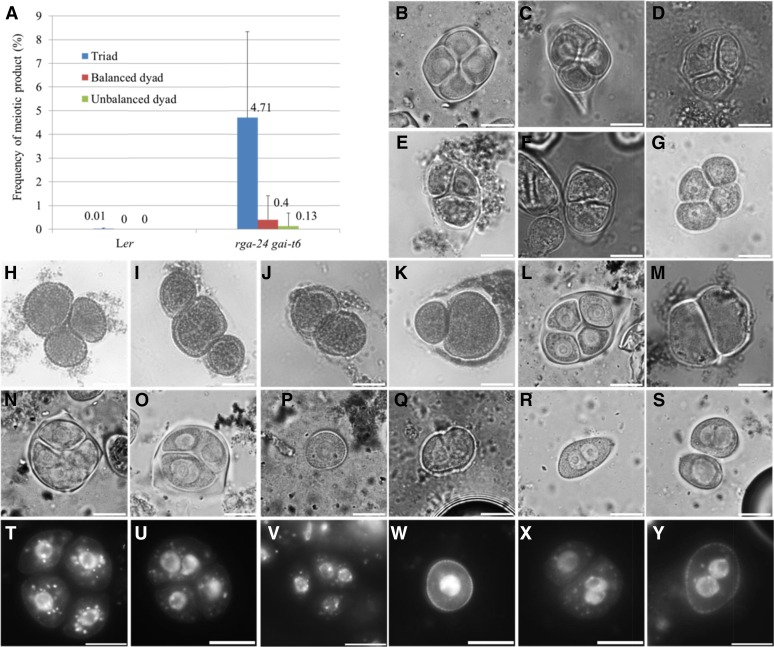
To determine whether GA3-induced formation of diploid pollen is caused by meiotic alterations, male meiocytes were examined at the tetrad stage using orcein staining (Fig. 3, B–F). Male meiosis in wild-type Arabidopsis plants typically results in balanced tetrads containing four haploid sporocytes (Fig. 3B). Similarly, upon GA3 treatment, the majority of tetrad-stage meiocytes appear normal, showing four equally sized microspores in a meiotic tetrad (Fig. 3C). However, at 36 to 48 h after GA3 treatment, a subset of meiotically restituted triad (Fig. 3, D and E) and dyad microspores (Fig. 3F) were observed, indicating that GA3 interferes with one or more processes during male meiotic cell division. Similarly, 3 or 4 days post GA3 treatment (100 µM), qrt1-2−/− plants (Fig. 3, G–K) were also found to produce a subpopulation of triad (Fig. 3, H and I) and dyad microspore configurations (balanced dyad, Figure 3J and unbalanced dyad, Figure 3K) in agreement with GA3-induced male meiotic restitution. The rga-24 gai-t6 double mutant also revealed a subset of triads (4.71%; Figure 3, N and O) and dyads (Fig. 3M; balanced dyad 0.4% and unbalanced dyad 0.13%), indicating for meiotic nonreduction similar as in GA3-treated plants.
Figure 3.
Male meiotic restitution in GA3-treated plants and the rga-24 gai-t6 double mutant. A, Histograms showing the frequency of meiotic nonreduction events in the rga-24 gai-t6 double mutant. Orcein staining of tetrad stage male meiocytes in control (B) and GA3-treated plants (C–F). Orcein staining of unicellular stage microspores in control (G) and in GA3-treated qrt1-2−/− plants (H–K). Orcein staining of tetrad stage meiocytes in wild-type Ler plants (L) and the rga-24 gai-t6 double mutant (M–O). Orcein staining of unicellular stage microspores in control (P), GA3-treated plants (Q) and in the rga-24 gai-t6 double mutant (R and S). DAPI staining of tetrad-stage male meiocytes and unicellular microspores in control (T and W), GA3-treated plants (U and X), and in the rga-24 gai-t6 double mutant (V and Y). Scale bars = 10 µm.
Further nuclear analysis of orcein stained tetrad-stage meiocytes additionally revealed that enlarged sporocytes in restituted rga-24 gai-t6 meiocytes either carry two equally sized nuclei (Fig. 3N) or alternatively harbor one single enlarged nucleus (Fig. 3O), closely according with diploid microspores either showing two distinct groups of five GFP foci (Fig. 2M) or exhibiting one fused nucleus with 10 GFP dots (Fig. 2N), respectively. Further confirmation of binuclear and higher ploidy mononuclear microspores in GA3-treated plants and untreated rga-24 gai-t6 mutant was obtained by DAPI staining of tetrad-stage meiocytes and unicellular-stage microspores (Fig. 3, T–Y). Both the rga-24 gai-t6 mutant and GA3-treated plants show syncytial colocalization of two or more nuclei in tetrad-stage male meiocytes (Fig. 3, U and V) and the unicellular microspore stage (Fig. 3, X and Y).
Collectively, these data suggest that diploid pollen in GA3-treated wild-type plants and in the rga-24 gai-t6 Arabidopsis mutant result from a similar defect in meiotic cell division, with ectopic events of meiotic nonreduction leading to the syncytial colocalization of two or more haploid nuclei in one microsporocyte.
The rga-24 gai-t6 Mutant Displays Normal Male Meiotic Chromosome Segregation
Meiotically restituted dyads and triads generally result from defective meiotic chromosome segregation, omission of either meiosis I or II, or alterations in spindle orientation. To determine which of these cellular processes is affected, male meiotic chromosome spreads of both the rga-24 gai-t6 double mutant and wild-type Ler plants were examined (Supplemental Fig. S4, A–L). In both wild-type and rga-24 gai-t6 mutant plants, four distinct sets of five chromosomes (haploid nuclei) were consistently observed in all telophase II meiocytes, indicating that male meiotic chromosome segregation in the rga-24 gai-t6 double mutant is not affected (Supplemental Fig. S4J). Following nucleation at the tetrad stage, haploid nuclei in rga-24 gai-t6 male meiocytes occasionally display an irregular spatial organization with two or more nuclei in proximity to each other. Under normal conditions, tetrad-stage, wild-type male meiocytes always exhibit two distinct perpendicularly oriented internuclear organelle bands, which physically separate the resulting nuclei in four distinct cytoplasmic domains and additionally determine the spatial positioning of the future cell walls (Supplemental Fig. S4K). In contrast, in the rga-24 gai-t6 double mutant, some tetrad-stage meiocytes showed a clear absence of the internuclear organelle band between two or more haploid nuclei, typically resulting in the physical clustering of two or more nuclei in one single cytoplasmic domain (Supplemental Fig. S4L). Hence, since the rga-24 gai-t6 mutant does not show any defect in chromosome segregation during male MI or MII, the cellular defect causing meiotic restitution and diploid pollen formation occurs in a later stage of sporogenesis, that is, after telophase II.
Meiotic Restitution in the rga-24 gai-t6 Mutant and GA3-Treated Arabidopsis Plants Results from Defects in Male Meiotic Cytokinesis
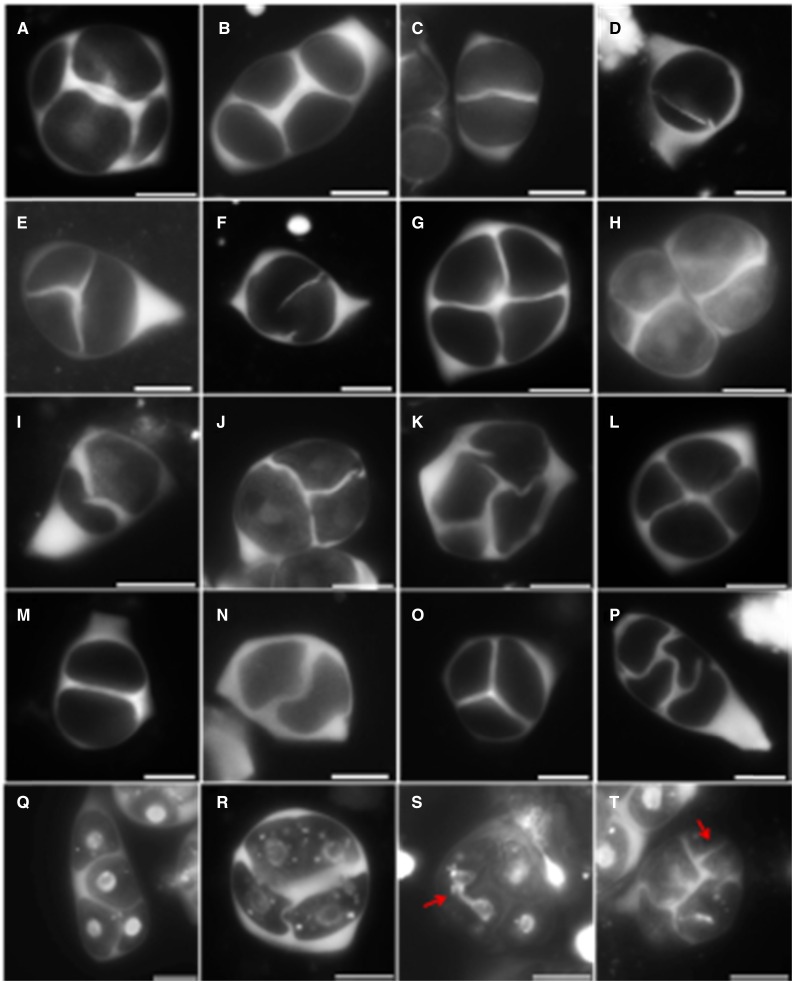
Both the normal meiotic chromosome dynamics and the eventual colocalization of haploid nuclei at the tetrad stage suggest that meiotically restituted dyads and triads in the rga-24 gai-t6 double mutant result from defects in male meiotic cytokinesis. In Arabidopsis male meiosis, the accumulation of intermediate callosic material at the end of MII reflects proper cell wall formation and cytokinesis. Aniline blue staining of GA3-treated wild-type Ler plants and DELLA double rga-24 gai-t6 and quadruple rga-t2 gai-t6 rgl1-1 rgl2-1 mutants revealed alterations in the deposition of callosic cell walls (Fig. 4). In most cases, GA3-treated plants (Fig. 4B) and DELLA mutants (Fig. 4, G and L) produce normal tetrads, similar to those seen in wild-type Ler, typically showing the presence of a distinct cross-shaped callosic cell wall that separates all four haploid spores (Fig. 4A). However, in GA3-treated plants, a subset of tetrad-stage meiocytes was found to produce partial or incomplete callosic cell walls at the end of MII, leading to the formation of balanced and unbalanced dyads and triads (Fig. 4, C–E). In this pool of altered meiotic products, a great variation in defect severity was observed, with some cells not forming any callosic wall whereas others produced cell wall stubs or cell plates with small gaps (Fig. 4F). Callose staining revealed similar defects in male meiotic cell wall formation in the DELLA double and quadruple mutants (Fig. 4, H–K and M–P).
Figure 4.
Aniline blue staining reveals male meiotic cytokinesis defects in GA3-treated plants and DELLA double and quadruple Arabidopsis mutants. Aniline blue staining of callosic cell walls in tetrad stage meiocytes of wild-type Ler plants (A), wild-type Ler plants sprayed with 100 µM GA3 (B–F), the rga-24 gai-t6 double mutant (G–K), and the rga-t2 gai-t6 rgl1-1 rgl2-1 quadruple mutant (L–P). Combined aniline blue and DAPI staining of tetrad-stage male meiocytes in wild-type Ler (Q) and rga-24 gai-t6 plants (R–T). Scale bars = 10 µm.
Combined DAPI and aniline blue staining on tetrad-stage male meiocytes additionally revealed that aberrations in rga-24 gai-t6 MII cell wall positioning lead to the colocalization and occasional clustering of two or more nuclei in one single microsporocyte (Fig. 4, R and S). Moreover, in a few rare events, tetrad-stage rga-24 gai-t6 meiocytes were found to form ectopic callosic cell walls (Fig. 4T), suggesting that one or more processes determining the spatial positioning of cytokinesis and the associated deposition of cell wall components is affected. Collectively, these data demonstrate that meiotic restitution in both GA3-treated plants and DELLA mutants is caused by defects in male meiotic cytokinesis.
As both the DELLA RGA and GAI genes are actively transcribed ubiquitously (Silverstone et al., 1998), the question arises whether cell wall formation in other tissues is also affected. To monitor for putative defects in somatic cell wall formation, epidermal cells and associated cell wall patterning of mature rga-24 gai-t6 petals was assessed using bright field microscopy. Somatic nuclei were concomitantly visualized using DAPI staining. A thorough scanning revealed that rga-24 gai-t6 petals exhibit normal epidermal cell wall morphology (Supplemental Fig. S5, E–G) and consistently show nuclei of equal size (Supplemental Fig. S5H), similar to wild-type Ler plants (Supplemental Fig. S5, A–D), indicating that somatic cell wall formation is not affected. Furthermore, propidium iodide staining of root tips did not reveal altered or incomplete cell walls in both wild-type Ler and rga-24 gai-t6 mutant plants (Supplemental Fig. S5, I and J). Hence, both the observations in root and petal tissues indicate that rga-24 gai-t6 somatic tissues do not show any defect in cell wall formation, suggesting that the DELLA proteins RGA and GAI specifically mediate cytokinesis in male meiotic cell division.
Cell Wall Defects in rga-24 gai-t6 Male Meiotic Tetrads Are Caused by Structural Alterations in the RMA
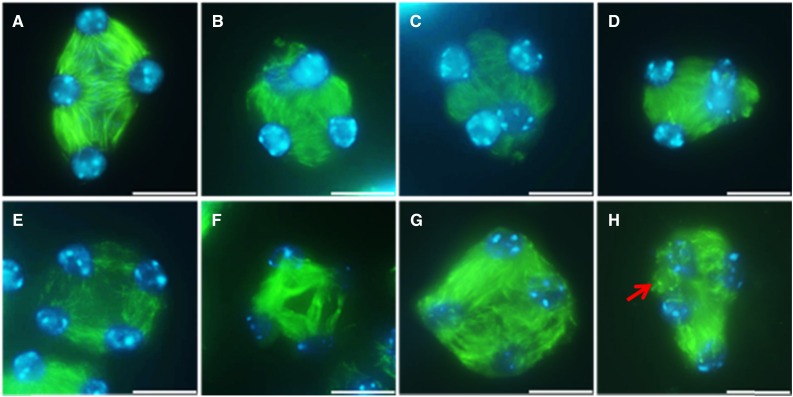
The positioning of the cell wall and associated deposition of cell wall material, such as intermediate callose, in somatic plant cells is guided by the phragmoplast (De Storme and Geelen, 2013a). In male meiosis, cytokinesis also depends on microtubular phragmoplast-like structures. However, due to their specific spatial intercellular organization, that is, formed at the intersection of microtubules (MTs) emanating from syncytial telophase II nuclei, they are referred to as RMAs (Peirson et al., 1997; Brown and Lemmon, 2001). Since RMAs are crucial for the formation and positioning of the meiotic cell wall (Otegui and Staehelin, 2004), we next performed immunocytological analysis of the MT subunit tubulin-α in male meiocytes of the rga-24 gai-t6 double mutant (Fig. 5). In wild-type Ler plants, TII male meiocytes show a microtubular structure that consists of six RMAs that are organized between the four haploid nuclei (Fig. 5A). Although most rga-24 gai-t6 male meiocytes show a similar TII cytoskeletal configuration, with distinct RMAs between each of the four haploid nuclei, a subset of TII male meiocytes in the rga-24 gai-t6 double mutant was found to exhibit clear alterations in the biogenesis and organization of the RMAs (Fig. 5, B–H). In some instances, adjacent nuclei in a single TII meiocyte were found to completely lack the presence of internuclear microtubules, hence causing a physical clustering of haploid nuclei in a triad figure (Fig. 5, B–D). Moreover, besides TII meiocytes that show partial or complete loss of RMA formation, some of the rga-24 gai-t6 tetrads with regularly separated nuclei display a reduced level of internuclear MT labeling, indicating minor aberrations in RMA biogenesis or MT stability (Fig. 5, E and F). In these slightly altered TII meiocytes, a subset of MTs of the RMA appears bent and exhibits a randomly organized pattern (Fig. 5, G and H). Moreover, fluorescent dots in some cells suggest that microtubules did not elongate properly or were depolymerized (Fig. 5H, arrow), indicating defects in RMA microtubule dynamics.
Figure 5.
Tubulin-α immunolocalization in TII male meiocytes of wild-type Ler and rga-24 gai-t6 double mutant plants. Structure of the RMAs in TII male meiocytes of wild-type Ler plants (A) and the rga-24 gai-t6 double mutant (B–H). Green: α-tubulin, cyan: DAPI. Scale bars = 10 µm.
Overall, the RMA defects observed in rga-24 gai-t6 TII meiocytes are consistent with the aberrations in male meiotic cell wall formation and form the structural basis for events of male meiotic restitution and the associated production of higher ploidy male gametes.
DELLA Downstream Factors MYB33 and MYB65 Are Redundantly Required for Male Meiotic Cytokinesis in Arabidopsis
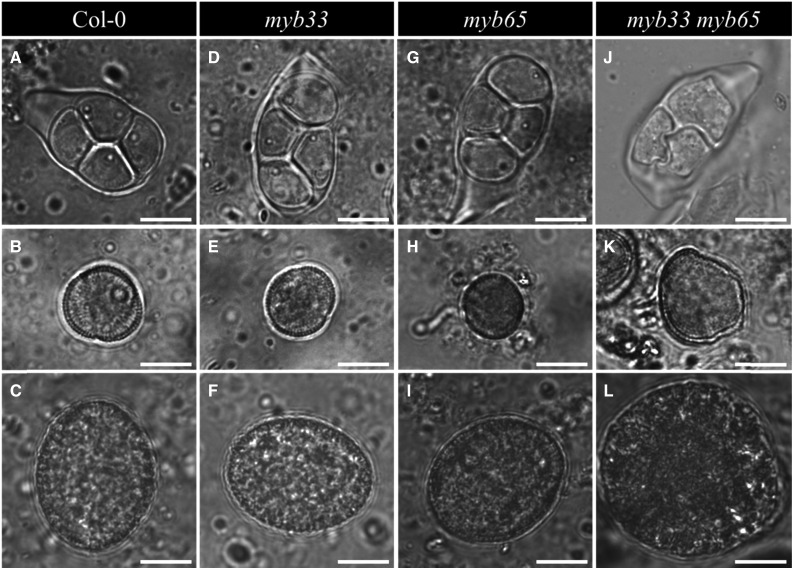
MYB33 and MYB65 are downstream factors of DELLA in regulating GA responsive processes (Gubler et al., 2002; Kaneko et al., 2003) and are well known in regulating anther development (Millar and Gubler, 2005). To investigate whether these two MYB factors participate in DELLA-mediated male meiotic cytokinesis in Arabidopsis, we monitored both sporogenesis and gametogenesis in the single and double myb33 myb65 mutant plants. Both single myb33 and myb65 mutant plants produce normal tetrads, haploid microspores, and haploid pollen grains, similar to wild-type Col-0 plants (Fig. 6, A–C, D–F, and G–I). In contrast, double myb33 myb65 mutant plants were found to produce a subset of meiotically restituted triads together with the associated formation of enlarged spores and mature pollen grains (Fig. 6, J–L). Oversized microspores at the unicellular stage (Fig. 6K) and larger pollen grains (Fig. 6L) occurred at a frequency of 4.35% and 4.64%, respectively. Similar to GA-treated wild-type plants and double rga-24 gai-t6 mutant plants, mature pollen from the double myb33 myb65 mutant also shows aberrant pollen wall morphology with lack of the thick protein-rich outer cell wall (Fig. 6L). These data indicate that MYB33 and MYB65 operate redundantly in regulating male sporogenesis in Arabidopsis, with a loss of function of both genes resulting in male meiotic restitution and the associated formation of larger, higher ploidy male gametes.
Figure 6.
Sporogenesis and gametogenesis in the single and double myb33 myb65 mutant. Tetrad (A), haploid unicellular stage microspore (B), and haploid pollen grain (C) in wild-type Col-0 plant. Tetrad (D), haploid unicellular stage microspore (E), and haploid pollen grain (F) in single myb33 mutant plant. Tetrad (G), haploid unicellular stage microspore (H), and haploid pollen grain (I) in single myb65 mutant plant. Triad (J), enlarged unicellular stage microspore (K), and enlarged pollen grain (L) in double myb33 myb65 mutant plant. Scale bars = 10 µm.
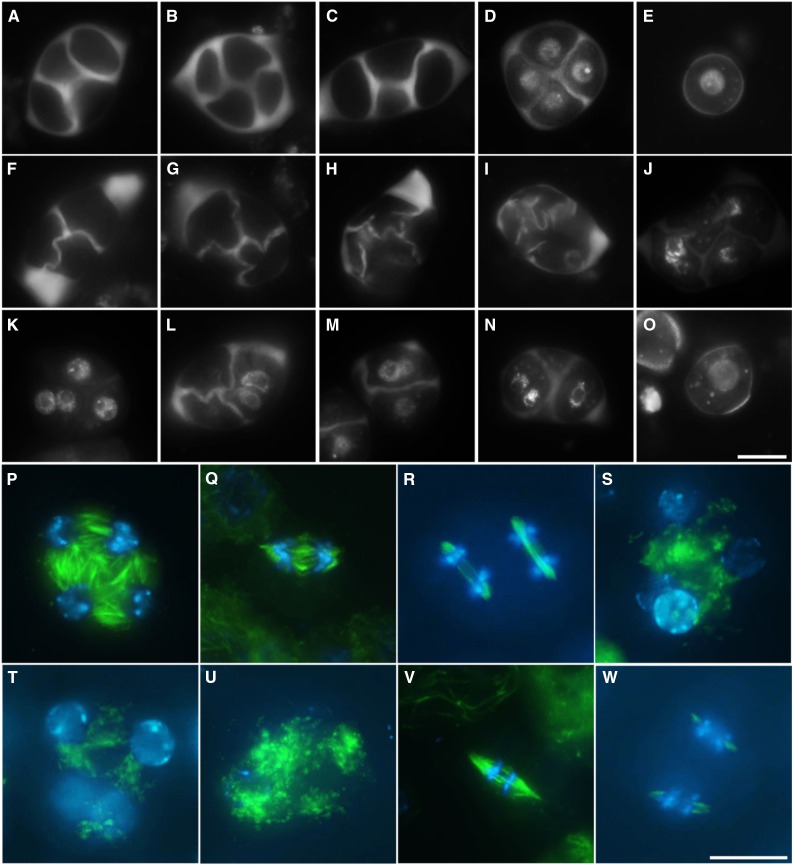
Aniline blue staining revealed that single myb33 and myb65 mutants produce tetrad-stage male meiocytes with regular callosic cell walls (Fig. 7, B and C), whereas male meiotic products in the myb33 myb65 double mutant display defects in cytokinesis with formation of incomplete or misshapen callosic cell walls (Fig. 7, F–I). Combined aniline blue and DAPI staining further revealed a colocalization of nuclei in the enlarged spore that results from lack of cell wall formation (Fig. 7, J–N). Tubulin-α immunolocalization of myb33 myb65 TII male meiocytes revealed a complete loss of RMA formation between colocalized nuclei (Fig. 7, S and T) and additionally displayed fluorescent dots in some male meiocytes, suggesting defects in microtubule polymerization or stability (Fig. 7U). MI and MII spindle organization in myb33 myb65 male meiotic cells appeared normal (Fig. 7, V and W), similar to those in wild-type Col-0 cells (Fig. 7, Q and R) and indicating that other microtubular structures in earlier stages of male meiosis are not affected, allowing normal chromosome segregation. Collectively, these findings indicate that the RMA biosynthesis and/or polymerization stability are affected in the double myb33 myb65 mutant, leading to defects in male meiotic cell wall formation.
Figure 7.
Male meiotic cytokinesis defects in myb33 myb65 Arabidopsis plants. Aniline blue staining of tetrads in wild-type Col-0 (A) and single myb33 (B) and myb65 (C) mutant plants. D, Combined aniline blue/DAPI staining of tetrad-stage male meiocytes in wild-type Col-0 plant. E, DAPI staining of haploid unicellular stage microspore in wild-type Col-0 plants. F to I, Aniline blue staining of defective tetrad stage male meiocytes in double myb33 myb65 mutant plants. J to N, Combined aniline blue/DAPI staining of defective tetrad-stage male meiocytes in double myb33 myb65 mutant plants. O, DAPI staining of an enlarged unicellular stage microspore isolated from myb33 myb65 mutant plant. RMA (P) and MI and MII spindles (Q and R) in wild-type Col-0 plants. Defective RMA (S–U) and normal MI and MII spindles (V and W) in the double myb33 myb65 mutant. Green: α-tubulin, cyan: DAPI. Scale bars = 10 µm.
GA-Induced Defects in Male Meiotic Cytokinesis Correlate with DELLA Signaling in the Somatic Anther Tissue
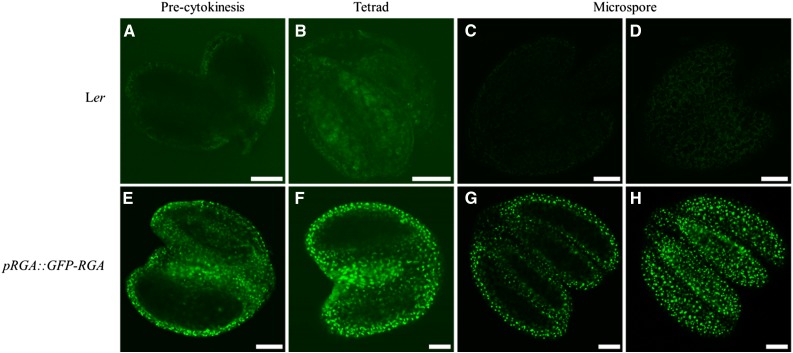
To assess the specific expression pattern of DELLAs during male sporogenesis, we analyzed expression of RGA in the Arabidopsis anther by using a pRGA::GFP-RGA marker in the Ler background. To stabilize the GFP-RGA signals in the developing flowers, we sprayed flowering pRGA::GFP-RGA plants with 1 mM paclobutrazol and monitored GFP-RGA expression/localization 6 hours post treatment using both confocal laser scanning (Fig. 8) and epi-fluorescence microscopy (Supplemental Fig. S6). No GFP signal was detected in the anthers isolated from wild-type Ler control plants (Fig. 8A–D; Supplemental Fig. S6, A–C). Con the contrary, in the plants harboring the pRGA::GFP-RGA construct, GFP-RGA expression was clearly visible in the anther, showing a distinct dotted pattern that is strictly confined to the somatic tissue surrounding the male meiocytes and/or microspores during anther development (Fig. 8E and Supplemental Fig. S6D, precytokinesis stage; Fig. 8F and Supplemental Fig. S6E, tetrad stage; Fig. 8, G and H and Supplemental Fig. S6F, microspore stage), indicating that RGA is not expressed in the male reproductive tract, that is, in the meiocytes and developing microspores but instead appears specifically expressed in the somatic cell layer, including the tapetum, that surrounds the meiotic and gametophytic cells.
Figure 8.
Confocal laser scanning microscopy of pRGA::GFP-RGA during different stages of Arabidopsis anther development. Anthers of wild-type Ler plants at during meiosis (A), tetrad stage (B), and early microspore development (C and D). Anthers of Ler plants harboring the pRGA::GFP-RGA constructs during meiosis (E), tetrad stage (F), and early microspore development (G and H). Scale bars = 50 µm.
To examine whether exogenous GA treatment reduces RGA expression in the tapetal cell layer during male sporogenesis, we next sprayed flowering pRGA::GFP-RGA plants with 100 µM GA3 (24 h post 1-mM PAC treatment, 0.06% Tween 20), and subsequently monitored the RGA-GFP signal 2 h post GA treatment. In the absence of exogenous GA treatment, a distinct pattern of RGA-GFP fluorescent signals was observed in the tapetal cell layer that surrounds the male meiocytes and young microspores during progressive anther development (Supplemental Fig. S7, A–C). However, 2 h post GA treatment, a significant decrease in RGA-GFP signal intensity in developing anthers was observed (Supplemental Fig. S7, D–F), indicating that exogenous GA treatment suppresses the expression of RGA in the tapetal cell layer during male meiosis and early gametogenesis.
DISCUSSION
GA-DELLA-MYB Signaling Module Regulates Male Meiotic Cytokinesis in Arabidopsis
In this study, we report that exogenous GA3 treatment induces 2n pollen formation in Arabidopsis by causing defects in male meiotic cytokinesis. Using genetic studies, we additionally demonstrate that combined loss-of-function of two downstream GA factors, i.e. the DELLA proteins RGA and GAI, causes a phenotype highly reminiscent to external GA treatment, suggesting that the transcriptional regulators RGA and GAI control the expression of proteins that either promote or interfere with regular meiotic cytokinesis. Altogether, our observations indicate that ectopic GA induces male meiotic restitution and 2n gamete formation in Arabidopsis by promoting the degradation of the DELLA RGA and GAI proteins. Cytological studies additionally revealed that combined loss of MYB33 and MYB65, two downstream DELLA targets, leads to similar male meiotic defects and associated formation of enlarged male gametes, suggesting a role of the GA-DELLA-MYB33/MYB65 regulatory pathway in controlling male meiotic cytokinesis in Arabidopsis. Moreover, as the double myb33 myb65 mutant displays similar RMA defects as the DELLA rga-24 gai-t6 mutant, we postulate that specific, yet unknown DELLA-MYB downstream factors directly control RMA biogenesis and associated microtubule dynamics in tetrad-stage male meiocytes. However, the identity of these downstream regulators and their specific mode of action in controlling male meiotic RMA formation remain largely elusive. Strikingly, in both GA3-treated wild-type plants as well as all DELLA mutants analyzed, cytokinesis defects occurred in only a minority of male meiocytes (around 4%), indicating that male meiotic RMA formation and cytokinesis is largely resilient to GA deregulation. Nevertheless, maintaining ploidy consistency is critical to regular sexual reproduction, with even small deviations in gametophytic ploidy level (e.g. diploid and aneuploid gametes) being highly relevant when considered in an ecophysiological perspective or on a larger evolutionary time scale.
A Role for GA in Integrating Environmental Stress and Reproductive Genome Instability?
During growth and development, plants suffer from various stresses, and the reproductive system is particularly sensitive to adverse environmental conditions (De Storme and Geelen, 2014). Previously, we reported that a short period of severe cold (1–40 h at 4–5°C) induces male meiotic restitution and 2n gamete formation by causing defects in male meiotic cytokinesis. More specifically, low temperatures were found to specifically affect the RMA cytoskeletal network at the telophase II stage, consequently impairing male meiotic cell plate formation and cell wall deposition. In contrast, other microtubular structures in the developing meiocytes, such as MI and MII spindles and the cortical MT array, were not affected by low-temperature stress, as reflected by the regular chromosome segregation dynamics in MI and MII (De Storme et al., 2012). Based on these findings, it is suggested that cold-induced formation of 2n pollen is governed by specific regulators and signaling pathways that specifically interfere with RMA biogenesis and/or MT dynamics during male meiotic cytokinesis.
Here, in this study, we demonstrate that perturbation of endogenous GA levels mimics the cold-induced defects in Arabidopsis male sporogenesis, causing highly similar defects in male meiotic RMA biogenesis and cell wall formation. Based on this similarity, we hypothesize that GA may putatively play an important regulatory role in (cold) stress-induced restitution of male meiosis and associated production of 2n pollen. Previous studies have demonstrated that GA signaling plays a prominent role in the plants’ response to cold stress through transient activation of CBF1/DREB1b, a transcriptional regulator controlling downstream cold-responsive genes (Colebrook et al., 2014). However, the putative role for GA in mediating cold-induced 2n gamete formation is probably indirect, since cold has been found to reduce endogenous GA levels, which is incongruent with the observation that increased GA levels induce events of male meiotic restitution. For example, in Arabidopsis seedlings, cold reduces endogenous bioactive GA levels by repressing expression of the GA-biosynthetic GA20 oxidase and stimulating the expression of the GA-catabolizing GA2-oxidase, thereby enabling cellular accumulation of DELLAs and associated reduction of tissue growth (Achard et al., 2008). Similarly, in rice, endogenous GA levels in developing anthers were found to decrease upon exposure to low temperatures (Sakata et al., 2014), suggesting that cold generally reduces endogenous GA levels instead of accumulating it.
Whether low temperature-induced disorders in male meiotic RMA structure are mediated by increased GA levels and whether this occurs in a direct or indirect manner remains to be determined. In plants, microtubules are notoriously sensitive to cold shock, with each species having a specific critical temperature underneath which MTs become highly unstable and disassemble. However, despite numerous reports of cold-induced effects on MT stability, it is yet unknown how this is mechanistically controlled and whether and how this is regulated at the molecular level. A recent study by Locascio et al. (2013) provides preliminary evidence for mechanistic link of GA signaling and microtubule organization. These authors reported that cortical MT reorganization in elongating hypocotyls strongly depends on the availability of the prefoldin complex in the cytosol and that this availability is regulated by the nuclear-localized DELLA proteins RGA and GAI, which bind and inactivate prefoldin (Locascio et al., 2013). However, despite this mechanistic insight into the regulatory role of DELLAs in cortical MT stability, it is yet unknown whether male meiotic RMA formation is controlled by a similar regulatory mechanism and whether cold stress may interfere with this to cause defects in male meiotic cell wall formation. In both GA-treated plants and the double rga-24 gai-t6 mutant, we have not observed aberrant organization of cortical microtubules in somatic cells or defects in cell division that could be caused by faulty mitotic cytoskeletal configurations. Hence, the different response of MT structures in somatic and male meiotic cells suggests there may exist different mechanisms by which GA mediates microtubular dynamics in somatic and reproductive tissues, putatively reflecting the differences in the structural organization and molecular regulation of the MT structures in male meiosis (RMAs) and somatic cell division (phragmoplast and cortical MT arrays; De Storme and Geelen, 2013).
Different Roles of GA in Microsporogenesis and Gametogenesis
Several studies demonstrated that GA is required for floral development by repressing the activity of DELLAs (Cheng et al., 2004; Tyler et al., 2004). In the GA-deficient ga1-3 mutant, male meiocytes display normal MI and MII chromosome segregation and cell wall formation (Cheng et al., 2004), indicating that GA is not required for the meiotic cell division program. This is confirmed in our study by monitoring the sporogenesis of GA-insensitive gai mutant. On the other hand, ga1-3 plants show clear defects in microspore development and pollen maturation because of impaired tapetum development (Cheng et al., 2004; Aya et al., 2009; Mutasa-Göttgens and Hedden, 2009), indicating that GA is essential for microspore development and pollen maturation through its promotive role in tapetum development. Several studies have provided accumulating evidence that GA plays an important role in the developmental regulation of the tapetal cell layer; a nourishing tissue that promotes microspore development and the outer cell wall formation. In rice, for example, impaired GA signaling causes alterations in tapetal cell programmed cell death (Aya et al., 2009), a process that is vital for microspore development through the supplementation of nutrients, hence causing major gametophytic defects such as spore abortion and male sterility. A conserved family of transcription factors, GAMYBs, that are controlled by the GA signaling pathway have been linked with tapetum functioning and pollen outer cell wall formation (Aya et al., 2011). The GAMYB members MYB33 and MYB65 are redundantly required for the development and persistence of the tapetum cell layer during male reproductive development (Millar and Gubler, 2005; Plackett et al., 2011). In this report, we demonstrate that the combined loss of MYB33 and MYB65 in Arabidopsis not only affects male gametogenesis but also causes defects in male meiotic cell division, similar to the DELLA rga-24 gai-t6 double mutant, indicating that the GA-DELLA-MYB pathway plays an important role in the developmental control of both male sporogenesis and gametogenesis.
The regulatory role of GA signaling (e.g. DELLAs-MYBs) in male meiotic cytokinesis shown in this study, together with fact that GA plays an essential role in tapetum and microspore development, corroborates with a previous report by Chhun et al. (2007), who reported that GA biosynthesis genes are expressed at a relatively low level in early stages of anther development (i.e. before meiotic cytokinesis) compared with that of GA-signaling genes and that the situation reversely alters at later stages in anther development (Chhun et al., 2007). Although the role of GA in regulating male gametophyte development and maintaining plant male fertility has been widely investigated, our data further clarify that a restriction of GA signaling and associated maintenance of DELLA activity in Arabidopsis is critical for successful male meiotic cell division and gametophytic ploidy stability.
Male Meiotic Cytokinesis: Regulated by GA Signaling in the Surrounding Somatic Tissue?
The GAMYB transcription factor family is conserved in land plants and seems to regulate a range of reproductive processes (Aya et al., 2011). In agreement with GA being involved in the control of tapetum and microspore development, several members of the GAMYB transcription factor family (MYB33 and MYB65 in Arabidopsis, HvGAMYB in barley, and OSGAMYB in rice) have been shown to be expressed in young anthers prior to flower anthesis (Kaneko et al., 2003; Murray et al., 2003; Millar and Gubler, 2005). Although MYB33 transcripts were reported to occur in a wide range of tissues, translational fusions showed that expression of MYB33 is mainly confined to the somatic cell layers of young anthers, likely due to MIR159-controlled restriction of mRNA translation. Lower expression levels were detected in developing microspores (Millar and Gubler, 2005). In this study, RGA expression analysis using a GFP translational fusion indicated that basal RGA levels are very low in young flower buds. Upon treatment with paclobutrazol, however, GFP expression strongly increases in most anther tissues, including the four outer somatic cell layers, except for meiocytes and microspores. This therefore demonstrates that there is certain level of endogenous GA biosynthesis present in young anther tissues that restricts the expression of the DELLA RGA protein to a certain limit, but up to a level that still allows full completion of male meiotic cell wall formation.
Based on these findings, we conclude that the DELLA-GAMYB module is predominantly expressed in the somatic tissue surrounding the developing meiocytes, playing a putative important role in the appropriate functioning of the tapetum, possibly in cooperation with the other anther outer cell layers. Since expression of DELLAs and GAMYBs in meiocytes and developing microspores is generally low, the observed defects in male meiotic cytokinesis and cell wall formation in corresponding mutants and GA-treated plants may indirectly result from tapetal dysfunctioning rather than from a cell-autonomous male meiotic deregulation of the DELLA-GAMYB module. Our data therefore provide preliminary evidence for molecular communication between the somatic anther cells and enclosed meiocytes/microspores to guide and generate not only pollen outer cell wall maturation but also MT cytoskeletal dynamics and cell wall formation during the final stage of meiosis.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Arabidopsis (Arabidopsis thaliana) Col-0 and Ler accessions were obtained from the Nottingham Arabidopsis Stock Centre. For GA3 treatment analysis, the qrt1-2−/− mutant (Preuss et al., 1994; Francis et al., 2006) was ordered from Nottingham Arabidopsis Stock Centre. The gai mutant was kindly provided by Patrick Achard. For the in vivo gametophytic ploidy determination, Arabidopsis plants containing pWOX2::CENH3-GFP (De Storme and Geelen, 2011) were used. The DELLA double mutant rga-24 gai-t6 and the quadruple mutant rga-t2 gai-t6 rgl1-1 rgl2-1 were previously described (Achard et al., 2006) and kindly provided by Patrick Achard and Nicholas Harberd. The rga-24 gai-t6 double mutant plants harboring pWOX2::CENH3-GFP were isolated in F2 population from the rga-24 gai-t6 double mutant intercrossing with pWOX2::CENH3-GFP transgenic line, according to the phenotype of pWOX2::CENH3-GFP transgenic line and genotyping of rga-24 and gai-t6 mutation. The rga-24 and gai-t6 single mutants were acquired from F2 population from the rga-24 gai-t6 double mutant intercrossing with pWOX2::CENH3-GFP transgenic line by genotyping rga-24 and gai-t6 mutation respectively. The pentuple rga-t2 gai-t6 rgl1-1 rgl2-1 rgl3-1 mutant was kindly shared by M. Höfte. The single and double myb33 myb65 mutants were kindly provided by Anthony Millar. The pRGA::GFP-RGA transgenic plants were obtained from Nicholas Harberd. Primers used for genotyping are listed in Supplemental Table SI.
Seeds were germinated on K1 medium for 6 to 8 d, and then seedlings were transferred to soil and cultivated in growth chambers at 12 h light/12 h night, 20°C, and <70% humidity. Upon flowering, the photoperiod was changed to a 16-h-day/8-h-night regime. For GA3 treatment, flowering plants were sprayed by water (+0.02% Tween) and 100 µM GA3 (+0.02% Tween).
GA3 and PAC Treatment Analysis
To determine the effect of GA3 treatment on male meiotic products, tetrads and microspores were observed at 36 to 48 h and 72 to 96 h later after GA3 and water spraying, respectively. To monitor the effect of GA3 treatment on larger pollen formation, mature pollen grains were analyzed in the 14 d following GA3 treatment. For pRGA::GFP-RGA expression analysis, to stabilize the GFP-RGA signals in Arabidopsis flowers, flowering pRGA::GFP-RGA plants were sprayed with paclobutrazol (1 mM, 0.06% Tween 20) and water. Anthers at different developmental stages were isolated to glass slides with a drop of diluted water and analyzed by microscopy.
Pollen Viability Examination
For performing FDA staining, mature pollen grains from newly opened flowers are released in a drop of FDA buffer (2 mg/mL in acetone) on a glass slide, and the fluorescence was observed after 10 min of staining. For pollen germination, mature pollen grains from newly opened flowers are soiled on pollen growth medium (5 mm CaCl2, 1 mm MgSO4, 5mM KCl, 0.01 mm H3BO3, 18% Suc, 1.5% agarose, pH at 7.5 modified by 0.1 m NaOH), and the pollen tube is observed after incubation in humid chamber under dark for 24 h.
Cytology
Pollen DAPI staining, callosic cell wall staining, and analysis of the male meiotic products (tetrad-stage analysis by orcein and DAPI staining) was performed as described previously (De Storme et al., 2012). Buds producing significant numbers of mature meiotic products were used for quantification and monitoring assays. DAPI and aniline blue-combined staining of male meiotic products was performed by releasing spores in a drop of DAPI solution (1 mg/ mL; Sigma) and aniline blue solution (0.1% [m/v] in 0.033% K3PO4 [m/v]) mixture. Visualization of pWOX2::CENH3-GFP constructs was performed by releasing spores in a 0.05 m NaPO4 (pH 7.0) and 0.5% Triton X-100 (v/v) solution. Male meiotic spreads were performed as described previously (De Storme and Geelen, 2011). Visual assessment of petal leaves was performed according to the method of Carland et al. (2002). For observation of the cell wall in root tip, roots were cut from 5-d-old seedlings and mounted on a glass slide with 10 μg/mL propidium iodide solution for 5 min, followed by washing three times using diluted water before observation.
Tubulin Immunolocalization
α-Tubulin immunolocalization analysis was performed as described previously (De Storme et al., 2012).
Microscopy
Both bright-field and fluorescence microscopy were performed using an Olympus IX81 inverted fluorescence microscope equipped with an X-Cite Series 120Q UV lamp and an Olympus XM10 camera. Fluorescence from pRGA::GFP-RGA transgenic line and propidium iodide staining was captured using Nikon A1r confocal laser scanning microscope equipped with Axiovision software (LiMiD). Bifluorescent images and Z-stacks were processed using ImageJ. Brightness and contrast settings were adjusted using Photoshop CS6.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Male meiotic restitution and enlarged male gametes in rga-t2 gai-t6 rgl1-1 rgl2-1 rgl3-1 mutant plants.
Supplemental Figure S2. Mature pollen grain in GA3-treated wild-type Ler plant.
Supplemental Figure S3. Viability determination of enlarged mature pollen grains in the double rga-24 gai-t6 mutant plants.
Supplemental Figure S4. The Arabidopsis rga-24 gai-t6 mutant displays regular chromosome dynamics during male meiosis I and II.
Supplemental Figure S5. Microscopy analysis of cell wall and nuclei in somatic tissues.
Supplemental Figure S6. Expression pattern of pRGA::GFP-RGA in Arabidopsis anther.
Supplemental Figure S7. Exogenous GA treatment induces decrease of pRGA::GFP-RGA signals in Arabidopsis anther.
Supplemental Table S1. Primers for genotyping.
Supplementary Material
Acknowledgments
We thank Patrick Achard for sharing the double rga-24 gai-t6 and quadruple rga-t2 gai-t6 rgl1-1 rgl2-1 mutant seeds as well as gai mutant seeds. We thank Nicholas Harberd for providing the double rga-24 gai-t6 and quadruple rga-t2 gai-t6 rgl1-1 rgl2-1 mutant seeds as well as the pRGA::GFP-RGA transgenic plants. We thank Monica Höfte for sharing the DELLA pentuple rga-t2 gai-t6 rgl1-1 rgl2-1 rgl3-1 mutant. Many thanks to Anthony Millar for providing the single and double myb33 myb65 mutants. We also thank G. Meesen for his support in confocal microscopy. We thank FWO PostDoc grant 1293014N (personal grant Nico De Storme) for supporting the research. We thank LiMiD-Hercules Foundation project AUGE/013 funding for confocal microscope. We thank the China Scholarship Council for providing partial funding (personal grant Bing Liu) for this research.
Glossary
- DAPI
4’,6-diamidino-2-phenylindole
- FDA
fluorescein diacetate
- GA
gibberellin
- MT
microtubule
- RMA
radial microtubule array
Footnotes
This work was supported by the FWO PostDoc grant 1293014N, by the LiMiD-Hercules Foundation project AUGE/013, and by China Scholarship Council.
Articles can be viewed without a subscription.
References
- Abdul-Baki AA. (1992) Determination of pollen viability in tomatoes. J Am Soc Hortic Sci 117: 473–476 [Google Scholar]
- Achard P, Cheng H, De Grauwe L, Decat J, Schoutteten H, Moritz T, Van Der Straeten D, Peng J, Harberd NP (2006) Integration of plant responses to environmentally activated phytohormonal signals. Science 311: 91–94 [DOI] [PubMed] [Google Scholar]
- Achard P, Gong F, Cheminant S, Alioua M, Hedden P, Genschik P (2008) The cold-inducible CBF1 factor-dependent signaling pathway modulates the accumulation of the growth-repressing DELLA proteins via its effect on gibberellin metabolism. Plant Cell 20: 2117–2129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achard P, Herr A, Baulcombe DC, Harberd NP (2004) Modulation of floral development by a gibberellin-regulated microRNA. Development 131: 3357–3365 [DOI] [PubMed] [Google Scholar]
- Adams KL, Wendel JF (2005) Polyploidy and genome evolution in plants. Curr Opin Plant Biol 8: 135–141 [DOI] [PubMed] [Google Scholar]
- Alonso-Peral MM, Li J, Li Y, Allen RS, Schnippenkoetter W, Ohms S, White RG, Millar AA (2010) The microRNA159-regulated GAMYB-like genes inhibit growth and promote programmed cell death in Arabidopsis. Plant Physiol 154: 757–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aya K, Hiwatashi Y, Kojima M, Sakakibara H, Ueguchi-Tanaka M, Hasebe M, Matsuoka M (2011) The gibberellin perception system evolved to regulate a pre-existing GAMYB-mediated system during land plant evolution. Nat Commun 2: 544. [DOI] [PubMed] [Google Scholar]
- Aya K, Ueguchi-Tanaka M, Kondo M, Hamada K, Yano K, Nishimura M, Matsuoka M (2009) Gibberellin modulates anther development in rice via the transcriptional regulation of GAMYB. Plant Cell 21: 1453–1472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretagnolle F, Thompson JD (1995) Gametes with the somatic chromosome number: mechanisms of their formation and role in the evolution of autopolyploid plants. New Phytol 129: 1–22 [DOI] [PubMed] [Google Scholar]
- Brown RC, Lemmon BE (2001) The cytoskeleton and spatial control of cytokinesis in the plant life cycle. Protoplasma 215: 35–49 [DOI] [PubMed] [Google Scholar]
- Carland FM, Fujioka S, Takatsuto S, Yoshida S, Nelson T (2002) The identification of CVP1 reveals a role for sterols in vascular patterning. Plant Cell 14: 2045–2058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H, Qin L, Lee S, Fu X, Richards DE, Cao D, Luo D, Harberd NP, Peng J (2004) Gibberellin regulates Arabidopsis floral development via suppression of DELLA protein function. Development 131: 1055–1064 [DOI] [PubMed] [Google Scholar]
- Cheng H, Song S, Xiao L, Soo HM, Cheng Z, Xie D, Peng J (2009) Gibberellin acts through jasmonate to control the expression of MYB21, MYB24, and MYB57 to promote stamen filament growth in Arabidopsis. PLoS Genet 5: e1000440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhun T, Aya K, Asano K, Yamamoto E, Morinaka Y, Watanabe M, Kitano H, Ashikari M, Matsuoka M, Ueguchi-Tanaka M (2007) Gibberellin regulates pollen viability and pollen tube growth in rice. Plant Cell 19: 3876–3888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claeys H, De Bodt S, Inzé D (2014) Gibberellins and DELLAs: central nodes in growth regulatory networks. Trends Plant Sci 19: 231–239 [DOI] [PubMed] [Google Scholar]
- Colebrook EH, Thomas SG, Phillips AL, Hedden P (2014) The role of gibberellin signalling in plant responses to abiotic stress. J Exp Biol 217: 67–75 [DOI] [PubMed] [Google Scholar]
- Davière JM, Achard P (2013) Gibberellin signaling in plants. Development 140: 1147–1151 [DOI] [PubMed] [Google Scholar]
- Davière JM, Achard P (2016) A pivotal role of DELLAs in regulating multiple hormone signals. Mol Plant 9: 10–20 [DOI] [PubMed] [Google Scholar]
- Debeaujon I, Koornneef M (2000) Gibberellin requirement for Arabidopsis seed germination is determined both by testa characteristics and embryonic abscisic acid. Plant Physiol 122: 415–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- d’Erfurth I, Cromer L, Jolivet S, Girard C, Horlow C, Sun Y, To JP, Berchowitz LE, Copenhaver GP, Mercier R (2010) The cyclin-A CYCA1;2/TAM is required for the meiosis I to meiosis II transition and cooperates with OSD1 for the prophase to first meiotic division transition. PLoS Genet 6: e1000989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d’Erfurth I, Jolivet S, Froger N, Catrice O, Novatchkova M, Mercier R (2009) Turning meiosis into mitosis. PLoS Biol 7: e1000124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d’Erfurth I, Jolivet S, Froger N, Catrice O, Novatchkova M, Simon M, Jenczewski E, Mercier R (2008) Mutations in AtPS1 (Arabidopsis thaliana parallel spindle 1) lead to the production of diploid pollen grains. PLoS Genet 4: e1000274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Storme N, Copenhaver GP, Geelen D (2012) Production of diploid male gametes in Arabidopsis by cold-induced destabilization of postmeiotic radial microtubule arrays. Plant Physiol 160: 1808–1826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Storme N, Geelen D (2011) The Arabidopsis mutant jason produces unreduced first division restitution male gametes through a parallel/fused spindle mechanism in meiosis II. Plant Physiol 155: 1403–1415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Storme N, Geelen D (2013a) Cytokinesis in plant male meiosis. Plant Signal Behav 8: e23394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Storme N, Geelen D (2013b) Pre-meiotic endomitosis in the cytokinesis-defective tomato mutant pmcd1 generates tetraploid meiocytes and diploid gametes. J Exp Bot 64: 2345–2358 [DOI] [PubMed] [Google Scholar]
- De Storme N, Geelen D (2013c) Sexual polyploidization in plants: cytological mechanisms and molecular regulation. New Phytol 198: 670–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Storme N, Geelen D (2014) The impact of environmental stress on male reproductive development in plants: biological processes and molecular mechanisms. Plant Cell Environ 37: 1–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Storme N, Keçeli BN, Zamariola L, Angenon G, Geelen D (2016) CENH3-GFP: a visual marker for gametophytic and somatic ploidy determination in Arabidopsis thaliana. BMC Plant Biol 16: 1–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Storme N, Mason A (2014) Plant speciation through chromosome instability and ploidy change: cellular mechanisms, molecular factors and evolutionary relevance. Curr Plant Biol 1: 10–33 [Google Scholar]
- De Storme N, Zamariola L, Mau M, Sharbel TF, Geelen D (2013) Volume-based pollen size analysis: an advanced method to assess somatic and gametophytic ploidy in flowering plants. Plant Reprod 26: 65–81 [DOI] [PubMed] [Google Scholar]
- Fleet CM, Sun TP (2005) A DELLAcate balance: the role of gibberellin in plant morphogenesis. Curr Opin Plant Biol 8: 77–85 [DOI] [PubMed] [Google Scholar]
- Francis KE, Lam SY, Copenhaver GP (2006) Separation of Arabidopsis pollen tetrads is regulated by QUARTET1, a pectin methylesterase gene. Plant Physiol 142: 1004–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gocal GFW, Sheldon CC, Gubler F, Moritz T, Bagnall DJ, MacMillan CP, Li SF, Parish RW, Dennis ES, Weigel D, et al. (2001) GAMYB-like genes, flowering, and gibberellin signaling in Arabidopsis. Plant Physiol 127: 1682–1693 [PMC free article] [PubMed] [Google Scholar]
- Gubler F, Chandler PM, White RG, Llewellyn DJ, Jacobsen JV (2002) Gibberellin signaling in barley aleurone cells. Control of SLN1 and GAMYB expression. Plant Physiol 129: 191–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heslop-Harrison J, Heslop-Harrison Y (1970) Evaluation of pollen viability by enzymatically induced fluorescence; intracellular hydrolysis of fluorescein diacetate. Stain Technol 45: 115–120 [DOI] [PubMed] [Google Scholar]
- Huang D, Wang S, Zhang B, Shang-Guan K, Shi Y, Zhang D, Liu X, Wu K, Xu Z, Fu X, et al. (2015) A gibberellin-mediated DELLA-NAC signaling cascade regulates cellulose synthesis in rice. Plant Cell 27: 1681–1696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H, Martin C (1999) Multifunctionality and diversity within the plant MYB-gene family. Plant Mol Biol 41: 577–585 [DOI] [PubMed] [Google Scholar]
- Kaneko M, Itoh H, Inukai Y, Sakamoto T, Ueguchi-Tanaka M, Ashikari M, Matsuoka M (2003) Where do gibberellin biosynthesis and gibberellin signaling occur in rice plants? Plant J 35: 104–115 [DOI] [PubMed] [Google Scholar]
- Kosetsu K, Matsunaga S, Nakagami H, Colcombet J, Sasabe M, Soyano T, Takahashi Y, Hirt H, Machida Y (2010) The MAP kinase MPK4 is required for cytokinesis in Arabidopsis thaliana. Plant Cell 22: 3778–3790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locascio A, Blázquez MA, Alabadí D (2013) Dynamic regulation of cortical microtubule organization through prefoldin-DELLA interaction. Curr Biol 23: 804–809 [DOI] [PubMed] [Google Scholar]
- Millar AA, Gubler F (2005) The Arabidopsis GAMYB-like genes, MYB33 and MYB65, are microRNA-regulated genes that redundantly facilitate anther development. Plant Cell 17: 705–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok DWS, Peloquin SJ (1975) THREE MECHANISMS OF 2n POLLEN FORMATION IN DIPLOID POTATOES. Can J Genet Cytol 17: 217–225 [Google Scholar]
- Murray F, Kalla R, Jacobsen J, Gubler F (2003) A role for HvGAMYB in anther development. Plant J 33: 481–491 [DOI] [PubMed] [Google Scholar]
- Mutasa-Göttgens E, Hedden P (2009) Gibberellin as a factor in floral regulatory networks. J Exp Bot 60: 1979–1989 [DOI] [PubMed] [Google Scholar]
- Otegui MS, Staehelin LA (2004) Electron tomographic analysis of post-meiotic cytokinesis during pollen development in Arabidopsis thaliana. Planta 218: 501–515 [DOI] [PubMed] [Google Scholar]
- Pécrix Y, Rallo G, Folzer H, Cigna M, Gudin S, Le Bris M (2011) Polyploidization mechanisms: temperature environment can induce diploid gamete formation in Rosa sp. J Exp Bot 62: 3587–3597 [DOI] [PubMed] [Google Scholar]
- Peirson BN, Bowling SE, Makaroff CA (1997) A defect in synapsis causes male sterility in a T-DNA-tagged Arabidopsis thaliana mutant. Plant J 11: 659–669 [DOI] [PubMed] [Google Scholar]
- Plackett AR, Ferguson AC, Powers SJ, Wanchoo-Kohli A, Phillips AL, Wilson ZA, Hedden P, Thomas SG (2014) DELLA activity is required for successful pollen development in the Columbia ecotype of Arabidopsis. New Phytol 201: 825–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plackett AR, Thomas SG, Wilson ZA, Hedden P (2011) Gibberellin control of stamen development: a fertile field. Trends Plant Sci 16: 568–578 [DOI] [PubMed] [Google Scholar]
- Preuss D, Rhee SY, Davis RW (1994) Tetrad analysis possible in Arabidopsis with mutation of the QUARTET (QRT) genes. Science 264: 1458–1460 [DOI] [PubMed] [Google Scholar]
- Ramanna MS, Jacobsen E (2003) Relevance of sexual polyploidization for crop improvement – A review. Euphytica 133: 3–8 [Google Scholar]
- Ramsey J, Schemske DW (1998) Pathways, mechanisms, and rates of polyploid formation in flowering plants. Annu Rev Ecol Syst 29: 467–501 [Google Scholar]
- Ravi M, Marimuthu MP, Siddiqi I (2008) Gamete formation without meiosis in Arabidopsis. Nature 451: 1121–1124 [DOI] [PubMed] [Google Scholar]
- Sakata T, Oda S, Tsunaga Y, Shomura H, Kawagishi-Kobayashi M, Aya K, Saeki K, Endo T, Nagano K, Kojima M, et al. (2014) Reduction of gibberellin by low temperature disrupts pollen development in rice. Plant Physiol 164: 2011–2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstone AL, Ciampaglio CN, Sun T (1998) The Arabidopsis RGA gene encodes a transcriptional regulator repressing the gibberellin signal transduction pathway. Plant Cell 10: 155–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstone AL, Jung H-S, Dill A, Kawaide H, Kamiya Y, Sun TP (2001) Repressing a repressor: gibberellin-induced rapid reduction of the RGA protein in Arabidopsis. Plant Cell 13: 1555–1566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielman M, Preuss D, Li FL, Browne WE, Scott RJ, Dickinson HG (1997) TETRASPORE is required for male meiotic cytokinesis in Arabidopsis thaliana. Development 124: 2645–2657 [DOI] [PubMed] [Google Scholar]
- Stracke R, Werber M, Weisshaar B (2001) The R2R3-MYB gene family in Arabidopsis thaliana. Curr Opin Plant Biol 4: 447–456 [DOI] [PubMed] [Google Scholar]
- Sun TP. (2010) Gibberellin-GID1-DELLA: a pivotal regulatory module for plant growth and development. Plant Physiol 154: 567–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun TP. (2011) The molecular mechanism and evolution of the GA-GID1-DELLA signaling module in plants. Curr Biol 21: R338–R345 [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Soyano T, Kosetsu K, Sasabe M, Machida Y (2010) HINKEL kinesin, ANP MAPKKKs and MKK6/ANQ MAPKK, which phosphorylates and activates MPK4 MAPK, constitute a pathway that is required for cytokinesis in Arabidopsis thaliana. Plant Cell Physiol 51: 1766–1776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y, Soyano T, Sasabe M, Machida Y (2004) A MAP kinase cascade that controls plant cytokinesis. J Biochem 136: 127–132 [DOI] [PubMed] [Google Scholar]
- Trognitz BR. (1991) Comparison of different pollen viability assays to evaluate pollen fertility of potato dihaploids. Euphytica 56: 143–148 [Google Scholar]
- Tyler L, Thomas SG, Hu J, Dill A, Alonso JM, Ecker JR, Sun TP (2004) Della proteins and gibberellin-regulated seed germination and floral development in Arabidopsis. Plant Physiol 135: 1008–1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willige BC, Ghosh S, Nill C, Zourelidou M, Dohmann EMN, Maier A, Schwechheimer C (2007) The DELLA domain of GA INSENSITIVE mediates the interaction with the GA INSENSITIVE DWARF1A gibberellin receptor of Arabidopsis. Plant Cell 19: 1209–1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RN, Somerville CR (1995) Phenotypic suppression of the gibberellin-insensitive mutant (gai) of Arabidopsis. Plant Physiol 108: 495–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Liu Q, Yao T, Fu X (2014) Shedding light on integrative GA signaling. Curr Opin Plant Biol 21: 89–95 [DOI] [PubMed] [Google Scholar]
- Zeng Q, Chen JG, Ellis BE (2011) AtMPK4 is required for male-specific meiotic cytokinesis in Arabidopsis. Plant J 67: 895–906 [DOI] [PubMed] [Google Scholar]
- Zhou X, Mo X, Gui M, Wu X, Jiang Y, Ma L, Shi Z, Luo Y, Tang W (2015) Cytological, molecular mechanisms and temperature stress regulating production of diploid male gametes in Dianthus caryophyllus L. Plant Physiol Biochem 97: 255–263 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.