Mitogen-activated protein kinase phosphatases 1 and 6 promote ultraviolet B-induced stomatal closure by modulating hydrogen peroxide-induced nitric oxide production in Arabidopsis guard cells.
Abstract
Ultraviolet B (UV-B) radiation induces the activation of MITOGEN-ACTIVATED PROTEIN KINASE PHOSPHATASE1 (MKP1) and its targets MPK3 and MPK6, but whether they participate in UV-B guard cell signaling is not clear. Here, evidence shows that UV-B-induced stomatal closure in Arabidopsis (Arabidopsis thaliana) is antagonistically regulated by MKP1 and MPK6 via modulating hydrogen peroxide (H2O2)-induced nitric oxide (NO) production in guard cells. The mkp1 mutant was hypersensitive to UV-B-induced stomatal closure and NO production in guard cells but not to UV-B-induced H2O2 production, suggesting that MKP1 negatively regulates UV-B-induced stomatal closure via inhibiting NO generation in guard cells. Moreover, MPK3 and MPK6 were activated by UV-B in leaves of the wild type and hyperactivated in the mkp1 mutant, but the UV-B-induced activation of MPK3 and MPK6 was largely inhibited in mutants for ATRBOHD and ATRBOHF but not in mutants for NIA1 and NIA2. mpk6 mutants showed defects of UV-B-induced NO production and stomatal closure but were normal in UV-B-induced H2O2 production, while stomata of mpk3 mutants responded to UV-B just like those of the wild type. The defect of UV-B-induced stomatal closure in mpk6 mutants was rescued by exogenous NO but not by exogenous H2O2. Furthermore, double mutant mkp1/mpk6 and the single mutant mpk6 showed the same responses to UV-B in terms of either stomatal movement or H2O2 and NO production. These data indicate that MPK6, but not MPK3, positively regulates UV-B-induced stomatal closure via acting downstream of H2O2 and upstream of NO, while MKP1 functions negatively in UV-B guard cell signaling via down-regulation of MPK6.
UV-B radiation is a fraction of sunlight with high-energy shortwave photons (280–315 nm) that partially passes through the stratospheric ozone layer and reaches the earth’s surface. Plants, with a sessile growth habit, use the energy from sunlight for photosynthesis and inevitably are exposed to UV-B radiation. Thus, plants need highly efficient UV-B tolerance. In addition to protection provided by the UV RESISTANCE LOCUS8 (UVR8) photoreceptor-dependent UV-B-specific signaling pathway (Jenkins, 2014), plants contain UV-B-nonspecific signaling pathways (Jenkins, 2009; Nawkar et al., 2013). Signaling through mitogen-activated protein kinases (MPKs) plays pivotal roles in development and environmental interactions in plants (Colcombet and Hirt, 2008). In response to UV-B radiation, Arabidopsis (Arabidopsis thaliana) MPK3 and MPK6 as well as their orthologs in tomato (Lycopersicon peruvianum) are activated (Holley et al., 2003; González Besteiro et al., 2011). To counterbalance MPK activation, dual-specificity MPK phosphatases (MKPs) dephosphorylate both Thr and Tyr residues within the MPK activation loop and, thus, negatively regulate MPK signaling (Keyse, 2008; Bartels et al., 2010). In Arabidopsis, there are five MKPs, MKP1, MKP2, IBR5, DsPTP1, and PHS1 (Bartels et al., 2010), but only MKP1 has a specific role in the UV-B stress response (González Besteiro et al., 2011) and is phosphorylated and stabilized upon UV-B radiation (González Besteiro and Ulm, 2013). Previous work has shown that MKP1 interacts physically and genetically with MPK3 and MPK6 (Colcombet and Hirt, 2008; Bartels et al., 2009). Concomitantly, mkp1 hypersensitivity to UV-B stress is associated with hyperactive MPK3 and MPK6 (González Besteiro et al., 2011). Clearly, it is well known that the response to UV-B in plant cells involves the activation of either MKP1 or its targets MPK3 and MPK6, but much remains to be learned about their physiological roles and their interaction with other UV-B signaling components.
Stomata embedded in the epidermis of terrestrial plants are the major site of gas exchange, such as CO2, oxygen, and water vapor, and also are the possible entry gate for pathogens. Thus, the stomatal apertures need to be tightly controlled. With regard to the role of MPK signaling in stomatal movement, proteomic results revealed that several components of MPK signaling are preferentially present in guard cells (Zhao et al., 2008). In line with these results, the prevention of MPK activation by the MPK kinase (MEK) inhibitor PD98059 partially inhibited abscisic acid (ABA)-induced stomatal closure in Pisum sativum (Burnett et al., 2000), Vicia faba (Pei et al., 2000; Jiang et al., 2008), and Commelina communis (MacRobbie and Kurup, 2007). Furthermore, MPK9 and MPK12 localized preferentially in guard cells function redundantly and positively in ABA, methyl jasmonate, hydrogen peroxide (H2O2), and pathogen elicitor guard cell signaling (Jammes et al., 2009; Salam et al., 2012, 2013; Khokon et al., 2015). MPK12 and MPK4 also were shown to participate in stomatal CO2 signaling (Hõrak et al., 2016). Expressing guard cell-specific antisense AtMPK3 in Arabidopsis partially impaired ABA- and H2O2-inhibited stomatal opening as well as H2O2- and pathogen-induced stomatal closure (Gudesblat et al., 2007, 2009). The MEK1-MPK6 cascade also plays an important role in ABA-induced H2O2 production in guard cells (Xing et al., 2007, 2008). However, another study reported that MPK3 and MPK6 were not involved in stomatal ABA signaling but participated in flg22 guard cell signaling (Montillet et al., 2013). In tobacco (Nicotiana tabacum), silencing MPK4 impaired stomatal closure induced by CO2 and ozone but had normal ABA-induced closures (Gomi et al., 2005; Marten et al., 2008). However, knocking down the transcript levels of MPK4 in Nicotiana attenuata compromised stomatal closure induced by ABA, darkness, and H2O2 (Hettenhausen et al., 2012). Moreover, PHS1, PP2C5, PP2C1, and OsIBR5, three kinds of protein phosphatases that directly modulate MPK activity, also were found to be involved in ABA guard cell signaling (Quettier et al., 2006; Brock et al., 2010; Li et al., 2012). These findings indicate that the guard cell MPK signaling pathways respond to various stimuli and act as key regulators of stomatal movement via integration with related signals, such as H2O2. On exposure to UV-B radiation, many plant species exhibit decreases in stomatal aperture (Musil and Wand, 1993; Nogués et al., 1999; Jansen and Noort, 2000). However, in some species, UV-B was reported to induce either stomatal closure or opening, maybe depending on the physiological state of the guard cells (Jansen and Noort, 2000). With regard to the signal transduction mechanisms of UV-B-regulated stomatal movement, previous studies have shown that H2O2 and nitric oxide (NO) generation are required for UV-B-induced stomatal closure (He et al., 2005, 2013; Tossi et al., 2014). However, if and how UV-B-activated MKP1 and its targets MPK6 and MPK3 interact with H2O2 and NO in UV-B guard cell signaling remain unknown.
In this work, we focused our attention on the possible roles of UV-B-activated Arabidopsis MKP1 as well as its targets MPK3 and MPK6 in guard cell signaling. Our combined genetic and biochemical evidence not only shows that the UV-B-induced Arabidopsis stomatal closure is regulated negatively by MKP1 and positively by MPK6 but not by MPK3 but also indicates that MKP1 and MPK6 function in UV-B guard cell signaling via modulating NO production and work downstream of NADPH oxidase-dependent H2O2 generation.
RESULTS
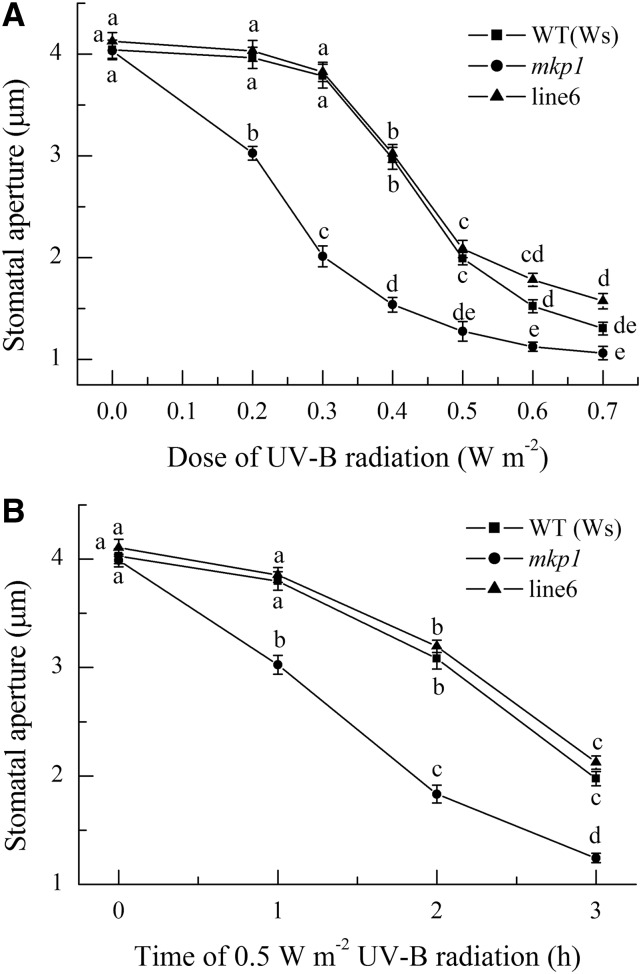
The mkp1 Mutant Is Hypersensitive to UV-B-Induced Stomatal Closure
A previous study has shown that MKP1 has a specific role in the UV-B stress response (González Besteiro et al., 2011), but whether MKP1 participates in UV-B guard cell signaling is unknown. To determine the role of MKP1 in UV-B guard cell signaling, freshly prepared leaves of wild-type Wassilewskija (Ws), null mutant mkp1 in the Ws background, and gMKP1:MKP1-complemented mkp1 (Ws; line 6; Ulm et al., 2001; Anderson et al., 2011) with open stomata were first exposed to 0.2 to 0.7 W m−2 UV-B radiation for 3 h. Stomatal analysis revealed that UV-B induced stomatal closure of all tested plants in a dose-dependent manner, but the mkp1 mutant showed greater stomatal closure than wild-type Ws and line 6 at all tested doses of UV-B radiation, even at doses lower than 0.4 W m−2 UV-B, which could not induce stomatal closure of wild-type Ws and line 6 but significantly induced the closure of mkp1 (Fig. 1A). These results indicate that mkp1 is hypersensitive to UV-B-induced stomatal closure. To further confirm this conclusion, the time courses of stomatal closure induced by 0.5 W m−2 UV-B radiation were next compared among Ws, mkp1, and line 6. Clearly, the stomata of mkp1 showed not only greater closure but also faster closure than those of Ws and line 6 (Fig. 1B). More importantly, the complemented line 6 showed stomatal closure similar to that found in wild-type Ws plants (Fig. 1), demonstrating that the hypersensitivity of mkp1 was caused by the loss of MKP1. Together, our data demonstrate that MKP1 has a negative regulatory role in UV-B-induced stomatal closure. As the dynamics of stomatal closure induced by 0.5 W m−2 UV-B showed clear differences between the wild type and mkp1 (Fig. 1), 0.5 W m−2 UV-B irradiation was chosen for the following experiments, which corresponds to 3.45 µmol m−2 s−1 and is close to the fluence rate of UV-B in sunlight (Brown and Jenkins, 2008; He et al., 2013).
Figure 1.
The mkp1 mutant is hypersensitive to UV-B-induced stomatal closure. Leaves of wild-type (WT) Ws, the mkp1 mutant, and line 6 (a representative line of mkp1 mutant complemented by the expression of wild-type MKP1) with open stomata were exposed to different doses of UV-B for 3 h (A) or to 0.5 W m−2 UV-B for different times (B), then stomatal apertures were measured in epidermal strips from abaxial surfaces of the treated leaves. The data shown are means ± se of at least three independent experiments, each with 50 stomata. Means with different letters are significantly different at P < 0.01.
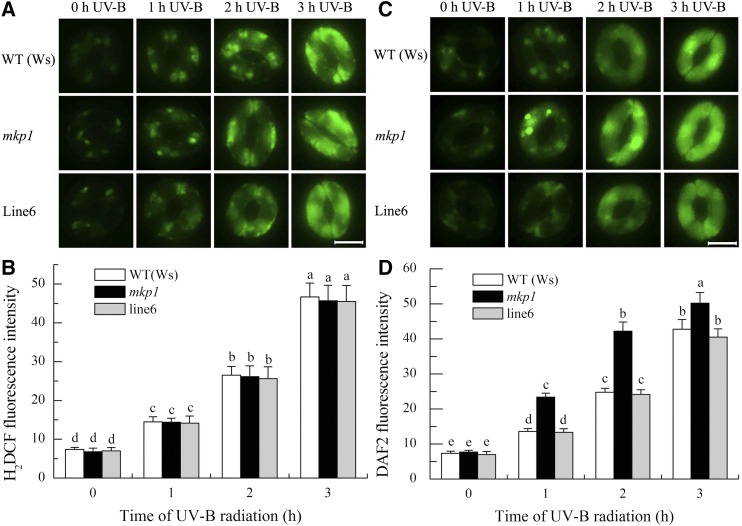
Hypersensitivity of the mkp1 Mutant to UV-B-Induced Stomatal Closure Is Associated with the Higher Production of NO in Guard Cells
Previous evidence has shown that both H2O2 and NO act as positive regulators in UV-B-induced stomatal closure (He et al., 2005, 2013; Tossi et al., 2014). To determine how MKP1 negatively regulates UV-B-induced stomatal closure, we further measured both H2O2 and NO production in guard cells of wild-type Ws, the mkp1 mutant, and line 6 under 0.5 W m−2 UV-B radiation using the H2O2 fluorescent dye 2′,7′-dichlorofluorescin diacetate (H2DCFDA) and the NO fluorescent dye 4,5-diaminofluorescein diacetate (DAF-2DA). Consistent with our previous results (He et al., 2005, 2013), UV-B had cumulative effects over time on both H2O2 and NO production in guard cells of the wild type, mkp1, and line 6 (Fig. 2). Furthermore, there were no significant differences among the H2O2 levels in guard cells of the wild type, mkp1, and line 6 in response to UV-B radiation (Fig. 2, A and B). However, NO levels in the guard cells of mkp1 were always higher than those of the wild type and line 6 during the UV-B radiation (Fig. 2, C and D), which were consistent with the faster and greater response of mkp1 stomata to UV-B (Fig. 1B). Thus, MKP1 seems to negatively regulate UV-B-induced stomatal closure by controlling NO production in guard cells.
Figure 2.
The mkp1 mutant is hypersensitive to UV-B-induced NO production but not to UV-B-induced H2O2 production in guard cells. Leaves of wild-type (WT) Ws, the mkp1 mutant, and line 6 (a representative line of mkp1 complemented by the expression of wild-type MKP1) with open stomata were exposed to 0.5 W m−2 UV-B for the indicated times, then epidermal strips were peeled from abaxial surfaces of the treated leaves and fluorescence images and pixel intensities in guard cells preloaded with 50 µm H2DCFDA (A and B) or 10 µM DCF-2DA (C and D) were recorded. Data of fluorescence pixel intensities (B and D) are displayed as means ± se of three replicates, each replicate with 20 stomata. Means with different letters are significantly different at P < 0.05. Bars in A and C = 10 μm.
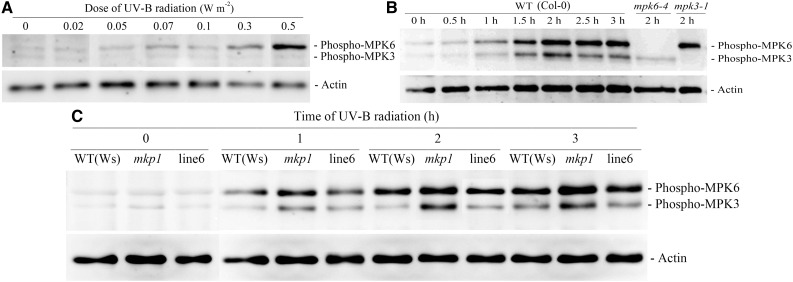
MPK3 and MPK6 Are Activated by UV-B and Hyperactivated in the mkp1 Mutant
To identify the UV-B-activated MPKs in Arabidopsis leaves under our experimental conditions, an immunoblot analysis was performed using an anti-phospho-p44/42 antibody that recognizes only the phosphorylated, active MPK forms. When leaves of wild-type Columbia-0 (Col-0) were exposed to 0.02 to 0.5 W m−2 UV-B radiation for 3 h, two major MPK forms were activated in a UV-B dose-dependent manner that were evident at 0.3 W m−2 and substantial at 0.5 W m−2 (Fig. 3A), which was consistent with the effects of different doses of UV-B on stomatal apertures in the wild type (Fig. 1A). When leaves of wild-type Col-0 and Ws were exposed to 0.5 W m−2 UV-B radiation for 0.5 to 3 h, two major MPK forms were gradually activated that were evident at 1 h, substantial at 1.5 h, and maximal at 2 h (Fig. 3, B and C), indicating that UV-B-induced activation of MPKs preceded stomatal closure of the wild type (Fig. 1B). By using null mutants mpk3-1 and mpk6-4, the two major UV-B-activated MPKs in Arabidopsis leaves were unambiguously recognized as MPK3 and MPK6 (Fig. 3B). Furthermore, the activation levels of MPK3 and MPK6 in mkp1 were higher than that in the wild-type Ws and complemented line 6 during 0.5 W m−2 UV-B radiation (Fig. 3C), which is in agreement with the function of MKP1 as a negative regulator of MPK3 and MPK6 (Colcombet and Hirt, 2008; Keyse, 2008; Bartels et al., 2009) and consistent with the results of González Besteiro et al. (2011). These results suggest not only that the activated MPK3 and/or MPK6 might mediate UV-B-induced stomatal closure but also that hyperactivation of MPK3 and/or MPK6 might be the underlying genetic cause of the mkp1 hypersensitivity to UV-B-induced NO production in guard cells and subsequent stomatal closure.
Figure 3.
UV-B activates MPK3 and MPK6 in leaves of wild-type (WT) Col-0 and Ws and hyperactivates MPK3 and MPK6 in leaves of the mkp1 mutant. A, Leaves of wild-type Col-0 were exposed to different doses of UV-B for 2 h. B, Leaves of wild-type Col-0 were exposed to 0.5 W m−2 UV-B for the indicated times or leaves of mutants mpk3-1 and mpk6-4 were exposed to 0.5 W m−2 UV-B for 2 h. C, Leaves of wild-type Ws, mutant mkp1, and line 6 (a representative line of mkp1 complemented by the expression of wild-type MKP1) were exposed to 0.5 W m−2 UV-B for the indicated times. After treatment, total proteins were extracted from the treated leaves and immunoblot analysis was performed with anti-phospho-p44/42 MPK and anti-actin (loading control) antibodies.
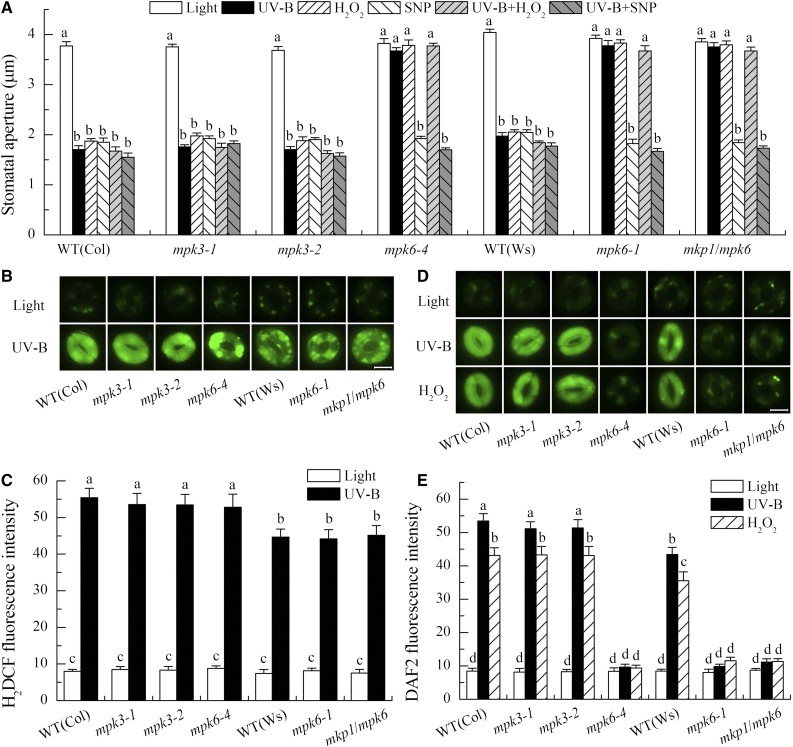
MPK6 Mediates UV-B-Induced Stomatal Closure and Works Downstream of H2O2 and Upstream of NO
To determine the roles of MPK3 and MPK6 and their relationship with H2O2 or NO in the UV-B-induced stomatal closure, we first examined the stomatal responses of null mutants for MPK3 and MPK6 to either 0.5 W m−2 UV-B radiation or exogenous H2O2 and NO. Interestingly, UV-B-induced stomatal closure was defective in the mutants for MPK6, and this defect could be rescued by application of the NO donor sodium nitroprusside (SNP) but not by H2O2 (Fig. 4A). Consistently, the stomata of mutants for MPK6 under white light alone also closed in response to SNP but did not close in response to H2O2 (Fig. 4A). In contrast, the stomata of mutants for MPK3 behaved just like those of the wild type in terms of responses to either UV-B or H2O2 and SNP (Fig. 4A). These results indicate that MPK6, but not MPK3, functions as a positive regulator in UV-B-induced stomatal closure and that MPK6 works downstream of H2O2 and upstream of NO in UV-B guard cell signaling.
Figure 4.
MPK6 mediates UV-B-induced stomatal closure via inducing NO generation in guard cells, and mkp1 hypersensitivity to UV-B-induced NO production and stomatal closure depend on MPK6 activation. A, Leaves of wild-type (WT) Col-0 and Ws, single mutants mpk3-1, mpk3-2, mpk6-1, and mpk6-4, and double mutant mkp1-1/mpk6-1 (mkp1/mpk6) with open stomata were incubated in MES buffer in the absence or presence of 100 µm H2O2 or 100 µm SNP under light alone or with 0.5 W m−2 UV-B for 3 h, then stomatal apertures were measured in epidermal strips from the abaxial surfaces of the treated leaves. Data presented are means ± se of at least three independent experiments, and means with different letters are significantly different at P < 0.01. B to E, Leaves of wild-type Col-0 and Ws and mutants mpk3-1, mpk3-2, mpk6-4, mpk6-1, and mkp1/mpk6 were incubated in MES buffer in the absence or presence of 100 µm H2O2 under light alone or with 0.5 W m−2 UV-B for 3 h, then fluorescence images (B and D) and pixel intensities (C and E) in guard cells preloaded with 50 µm H2DCFDA (B and C) or 10 µm DAF-2DA (D and E) were recorded. Data of fluorescence intensities are shown as means ± se of three independent experiments, and means with different letters are significantly different at P < 0.05. Bars in B and D = 10 µm.
To further confirm the relationship between MPK6 and H2O2 or MPK6 and NO in UV-B guard cell signaling, we measured UV-B-induced H2O2 and NO production in guard cells of wild-type Col-0 and Ws as well as mutants for MPK3 and MPK6. Consistent with the stomatal phenotypes, stomata of mutants for MPK6 showed defects of UV-B- and H2O2-induced NO production (Fig. 4, D and E) but no defect in UV-B-induced H2O2 production in guard cells (Fig. 4, B and C), while UV-B-induced H2O2 and NO production as well as H2O2-induced NO production in guard cells of mutants for MPK3 were similar to that of the wild type (Fig. 4, B–E). Taken together, our evidence clearly demonstrates that MPK6 mediates UV-B-induced stomatal closure via functioning downstream of H2O2 and upstream of NO.
mkp1 Hypersensitivity to UV-B-Induced NO Production and Stomatal Closure Depends on MPK6 Activation
Our results above clearly show that MKP1 and its target MPK6 have opposite roles in UV-B-induced NO production in guard cells and subsequent stomatal closure (Figs. 1, 2, and 4), suggesting that MKP1 regulation of the UV-B-induced stomatal closure depends on MPK6 activity. To further confirm this suggestion, we examined the responses of double mutant mkp1/mpk6 crossed by mkp1 and mpk6-1 (Anderson et al., 2011) to UV-B as well as H2O2 and NO. Consistently, the hypersensitivities of single mutant mkp1 to UV-B-induced NO production and stomatal closure were completely abolished in this double mutant, and the double mutant behaved just like the single mutants for MPK6 in terms of either stomatal movement or H2O2 and NO production in guard cells under either UV-B radiation or H2O2 and NO treatment (Fig. 4). These data confirm that MKP1 negatively regulates UV-B-induced NO generation in guard cells and the subsequent stomatal closure by controlling MPK6 activation.
H2O2 But Not NO Mediates UV-B-Induced Activation of MPK6 and MPK3
The results described above indicate that MPK6 works downstream of H2O2 and upstream of NO in UV-B guard cell signaling (Fig. 4), suggesting that UV-B-induced H2O2 production activates MPK6. To confirm this suggestion, the UV-B-induced activation levels of MPK6 as well as MPK3 were determined in Arabidopsis single and double mutants for ATRBOHD and ATRBOHF, which were shown to be responsible for UV-B-induced H2O2 generation and subsequent stomatal closure (He et al., 2013). Compared with the wild type, the UV-B-induced activation levels of MPK6 and MPK3 were reduced significantly in either signal mutants AtrbohD and AtrbohF or double mutant AtrbohD/F (Fig. 5A). Consistent with these results, application of 100 µm H2O2 also activated MPK6 and MPK3 in the wild-type leaves under light alone and even hyperactivated MPK3 in the mpk6-4 mutant leaves under light alone (Fig. 5B). In contrast, compared with the wild type, the UV-B-induced activation levels of MPK6 and MPK3 were not impaired in the Arabidopsis single and double mutants for NIA1 and NIA2 (Fig. 5C), which are required for the NO generation and stomatal closure induced by UV-B radiation (He et al., 2013). Together, these results indicate that UV-B-induced activation of MPK6 and MPK3 depend on the generation of H2O2 but not NO, further confirming that MPK6 functions downstream of H2O2 and upstream of NO in UV-B guard cell signaling.
Figure 5.
UV-B activation of MPK3 and MPK6 depends on H2O2 production but not NO production. A, Leaves of wild-type (WT) Col-0 and mutants AtrbohD, AtrbohF, and AtrbohD/F were exposed to light alone or with 0.5 W m−2 UV-B for 2 h. B, Leaves of wild-type Col-0 and mutant mpk6-4 were incubated in MES buffer containing 100 µm H2O2 for the indicated times. C, Leaves of wild-type Col-0 and Landsberg erecta (Ler) and mutants nia1-2 (nia1), nia2-1 (nia2), and nia1-1/nia2-5 (nia1/2) were exposed to light alone or with 0.5 W m−2 UV-B for 2 h. After treatments, total proteins were extracted from the treated leaves, and immunoblot analysis was performed with anti-phospho-p44/42 MPK and anti-actin (loading control) antibodies. The data shown are representative of up to three independent experiments.
DISCUSSION
Although previous work has shown that MPKs function in guard cell signaling and that UV-B radiation activates MKP1 and its targets MPK3 and MPK6 in Arabidopsis, it remained unknown whether MKP1 and its targets MPK3 and/or MPK6 are involved in UV-B guard cell signaling. Here, our combined genetic and biochemical analyses show that Arabidopsis MKP1 and its target MPK6 antagonistically regulate UV-B-induced stomatal closure via modulating NO generation in guard cells. Furthermore, we found that NADPH oxidase-dependent H2O2 production mediates the UV-B-triggered activation of MPK3 and MPK6. Therefore, our results reveal the important roles of MKP1 and MPK6 and their relationships with H2O2 and NO in UV-B guard cell signaling.
Arabidopsis MKP1 and Its Target MPK6 Antagonistically Regulate UV-B-Induced Stomatal Closure
MPKs play important roles in signaling plant responses to various stimuli. On exposure to UV-B radiation, previous studies and our data here showed that Arabidopsis MPK3 and MPK6 as well as their orthologs in tomato were mainly activated (Fig. 3; Holley et al., 2003; González Besteiro et al., 2011). Moreover, our data here further showed that the activation of MPK3 and MPK6 was only caused by the higher doses of UV-B radiation (Fig. 3A), further confirming that MPK activation is a UV-B stress response and belongs to the UV-B-nonspecific signaling pathways (González Besteiro et al., 2011). To counterbalance MPK activation, the Arabidopsis genome encodes five MKPs, and of those, MKP1 and MKP2 were shown to target MPK3 and MPK6 (Colcombet and Hirt, 2008; Bartels et al., 2009). Furthermore, in response to UV-B radiation, AtMKP1 was shown to be phosphorylated and stabilized and to negatively regulate the UV-B-activated AtMPK3 and AtMPK6 (González Besteiro et al., 2011; González Besteiro and Ulm, 2013; Fig. 3C). These results suggest that MKP1 and its targets MPK3 and MPK6 may play important roles in signaling plant responses to UV-B radiation. In accordance with this suggestion, the Arabidopsis mkp1 mutant affected UV-B-induced expression of PR and PDF1.2 (Kalbina and Strid, 2006); inhibition of MPK activation suppressed the UV-B-induced accumulation of catharanthine in Catharanthus roseus cell suspension cultures (Ramani and Chelliah, 2007); moreover, Arabidopsis MKP1 and its targets MPK3 and MPK6 antagonistically determined UV-B stress tolerance (González Besteiro et al., 2011). Clearly, the physiological roles of UV-B-activated MPK signaling pathways in plants are known little.
Here, by genetic analysis, we provide convincing evidence not only showing the negative role of AtMKP1 and the positive role of AtMPK6 in the regulation of UV-B-induced stomatal closure (Figs. 1 and 4) but also indicating that AtMKP1 negatively regulates UV-B-induced stomatal closure via the down-regulation of AtMPK6 activity (Figs. 3 and 4), in accordance with their antagonistic roles in UV-B stress tolerance and their interactions (Colcombet and Hirt, 2008; Bartels et al., 2009; González Besteiro et al., 2011). Moreover, although previous studies and our data here showed that AtMPK3 was also activated by UV-B and exogenous H2O2 in wild-type leaves and even hyperactivated by exogenous H2O2 in mpk6 mutant leaves (Figs. 3 and 5; Wang et al., 2010; González Besteiro et al., 2011), the stomatal analysis revealed that stomata of the loss-of-function mutants for MPK3 behaved just like those of the wild type in response to either UV-B or exogenous H2O2, but stomata of the mpk6 mutants showed defects in H2O2- or UV-B-induced closure (Fig. 4), suggesting that MPK3 might not be involved in UV-B and H2O2 guard cell signaling. These results are consistent with the normal stomatal responses of mpk3 knockout mutants to ABA (Montillet et al., 2013) but different from those of the MPK3 antisense lines in Arabidopsis partially impaired in H2O2- and pathogen-induced stomatal closure as well as H2O2- and ABA-inhibited stomatal opening (Gudesblat et al., 2007, 2009). At this point, the reasons for the differences between the stomatal responses of the loss-of-function mutant and the antisense lines for MPK3 are not clear. However, the off-target effects of antisense approaches could not be ruled out (Kok et al., 2015; Rossi et al., 2015).
Plants have at least two distinct UV-B signaling pathways, the UVR8-dependent (UV-B-specific) and UVR8-independent (UV-B-nonspecific) pathways (Jenkins, 2009). Despite the fact that the two UV-B pathways are all activated at high UV-B fluence rates or normal ambient UV-B conditions (Brown and Jenkins, 2008), their potential interplay is little understood. Previous work has shown that stomatal closure is induced only by high UV-B fluence rates (He et al., 2013; Tossi et al., 2014) and that the UV-B-induced activation of MPK signaling is not regulated by the UVR8-dependent pathway (González Besteiro et al., 2011), which, combined with the data presented here, indicates that the UVR8-independent pathway, like the MPK6-dependent pathway, mediates UV-B-induced stomatal closure. However, Tossi et al. (2014) have shown that UV-B-induced stomatal closure also is partially regulated by the UVR8-dependent pathway. Together, it is possible that both the UVR8-dependent and UVR8-independent pathways coordinately regulate UV-B-induced stomatal closure, suggesting that the two UV-B signaling pathways activated at high UV-B fluence rates or normal ambient levels of UV-B radiation interact to determine plant cell responses to UV-B radiation.
NADPH Oxidase-Dependent H2O2 Mediates the UV-B Activation of MPK3 and MPK6
H2O2 is well known to function as a signaling molecule in stomatal guard cells (Wang and Song, 2008). MPK cascades are shown to be key players in reactive oxygen species signaling. For example, several studies have shown that Arabidopsis MPK3 and/or MPK6 as well as their orthologs in other plants may be activated not only by exogenous H2O2 (Kovtun et al., 2000; Yuasa et al., 2001; Wang et al., 2010) but also by both abiotic and biotic stresses-induced H2O2 (Asai et al., 2002; Pitzschke and Hirt, 2009; Furuya et al., 2014; Jalmi and Sinha, 2015). Consistent with these previous reports, our data here also show that MPK6 acts downstream of H2O2 in UV-B guard cell signaling. However, there are reports suggesting that MPK signaling may work upstream of H2O2 generation in pathogen-infected plants (Yoshioka et al., 2003; Asai et al., 2008). Moreover, the P38-like MPK and the MEK1-MPK6 cascade work upstream of H2O2 generation in ABA-treated guard cells (Xing et al., 2007, 2008; Jiang et al., 2008). Thus, we further investigated whether UV-B-induced H2O2 production is abnormal in the mutant plants for MPK6, MPK3, or MKP1. Unfortunately, the above mutant plants showed responses of H2O2 synthesis to UV-B identical to that of wild-type plants (Figs. 2, A and B, and 4, B and C), which not only confirms that MPK6 acts downstream of H2O2 in UV-B guard cell signaling but also indicates that the role of MPK6 in UV-B guard cell signaling is different from its role in ABA guard cell signaling (Xing et al., 2007, 2008). Combined together, we suggest that MPK6 may have different roles in mediating responses to different stimuli such as UV-B and ABA. In addition, although this study clearly shows that H2O2 mediates the UV-B-induced activation of MPK3 and MPK6, it is still unclear by what mechanism H2O2 activates MPK3 and MPK6. In response to abiotic or biotic stresses, several MPK3 and/or MPK6 cascades, such as ANP1-MPK3/6, MEKK1-MEK4/5-MPK3/6, and MEKK1-MEK2-MPK4/6, have been shown to work downstream of exogenous H2O2 or stress-triggered H2O2 (Kovtun et al., 2000; Asai et al., 2002; Teige et al., 2004; Pitzschke and Hirt, 2009; Furuya et al., 2014; Jalmi and Sinha, 2015). Moreover, MPK3 and MPK6 also have been shown to be activated by H2O2 via Arabidopsis NDPK2 or the Ser/Thr kinase OXI1 (Moon et al., 2003; Rentel et al., 2004). Thus, the possibility that UV-B-induced H2O2 activates MPK3 and MPK6 by way of a kind of MPK cascade, NDPK2, or OXI1 should be addressed in the future.
H2O2 Regulation of NO Production in UV-B Guard Cell Signaling Depends on the Activation of MPK6
It has been well documented that both H2O2 and NO act as essential signaling molecules in guard cells (Wang and Song, 2008; Gayatri et al., 2013). Moreover, several studies also revealed that H2O2 induces NO synthesis in the guard cell signaling triggered by several stimuli, such as darkness, ABA, UV-B, extracellular calmodulin, and hydrogen gas (She et al., 2004; Bright et al., 2006; Li et al., 2009; He et al., 2013; Xie et al., 2014). However, by what mechanisms H2O2 induces NO generation in guard cells is still unclear. In this report, our results revealed that both UV-B- and exogenous H2O2-induced NO generation in guard cells were greatly impaired in mutants for MPK6 (Fig. 4); meanwhile, under UV-B radiation, the mkp1 mutant with higher activity of MPK6 (Fig. 3) had more NO production in guard cells (Fig. 2). These results imply that the activation of MPK6 is essential for both UV-B- and H2O2-induced NO generation in guard cells. These results, combined with the findings that the activation of MPK6 was induced by exogenous H2O2 and UV-B-triggered H2O2 (Fig. 5) and that H2O2 mediates the UV-B-induced NO generation in guard cells (He et al., 2013), make it clear that H2O2 regulation of NO production in UV-B and H2O2 guard cell signaling depends on the activation of MPK6. Consistent with our results, Wang et al. (2010) also reported that H2O2-activated MPK6 modulates NO biosynthesis during Arabidopsis lateral root development. However, of the two nitrate reductases (NRs) NIA1 and NIA2, only NIA2 is responsible for the H2O2-induced NO production in Arabidopsis root cells and is phosphorylated and thus activated by MPK6, since MPK6 was demonstrated to interact strongly with NIA2, but not NIA1, in vivo (Wang et al., 2010). In guard cells, although both NIA1 and NIA2 are expressed, NIA1 is responsible for UV-B- as well as H2O2- and ABA-induced NO production, as nia1 mutants were deficient in stimuli-induced stomatal closure and NO generation, whereas the nia2 mutants responded normally to these stimuli (Bright et al., 2006; He et al., 2013). Given that NIA1 and NIA2 are responsible for NO production in differential processes, and that MPK6 only interacts directly with NIA2, it is likely that MPK6 induces the activation of NIA1 and NIA2 via distinct mechanisms. However, how MPK6 mediates UV-B- and H2O2-induced activation of NIA1 in guard cells requires further investigation.
In guard cells, both NO synthase and NR, the two potential enzymatic sources of NO in plants, have been shown to be responsible for NO synthesis induced by many factors, but, so far, no genes or proteins with sequence homology to known mammalian-type NO synthase have been found in plants. In contrast, a growing body of evidence supports the NR-sourced NO production in guard cells (Gayatri et al., 2013). However, the ability of NR in NO production is measured to be only about 1% of its nitrate reduction capacity (Planchet et al., 2005). Whether the posttranscriptional modification of NR, including MPK6-induced NR activation, will enhance the capacity of NR in NO production should be studied further.
On the other hand, considering that the UVR8-dependent pathway also regulates UV-B-induced NO generation in guard cells (Tossi et al., 2014) but does not affect UV-B-activated MPK signaling (González Besteiro et al., 2011), it seems that the UVR8-dependent pathway and the UVR8-independent MPK6 pathway coordinately regulate UV-B-induced stomatal closure via NO-dependent means.
In summary, the data presented here show that UV-B-activated MKP1 and its target MPK6 function negatively and positively in UV-B-induced stomatal closure in Arabidopsis, respectively, which will make the stomatal apertures at appropriate levels under sunlight UV-B radiation. The data also indicate that the UV-B-induced activation of MPK6 and MPK3 depends on H2O2 production by both ATRBOHD and ATRBOHF and that MPK6 mediates the UV-B-induced stomatal closure by inducing NIA1-sourced NO production in guard cells. However, the mechanism by which the UV-B-triggered NO accumulation induces stomatal closure is still unclear. Calcium (Ca2+) oscillation, the important cellular messenger in stomatal movement that can control K+ and Cl− channels in guard cells, has been shown to be regulated by NO via the generation of cGMP and cADP ribose and/or through protein kinases (Garcia-Mata et al., 2003; Sokolovski et al., 2005; Jeandroz et al., 2013; Joudoi et al., 2013; Abdul-Awal et al., 2016). In this article, although we did not study the role of Ca2+ signal in UV-B-induced stomatal closure, our unpublished data showed that exogenous Ca2+ rescued the defects of mutants for NIA1, MPK6, and ATRBOHD/F in the UV-B-induced stomatal closure and that inhibitors of Ca2+ channels prevented UV-B-induced stomatal closure but did not affect the UV-B-induced production of NO and H2O2 and the activation of MPK6 and MPK3, suggesting that the Ca2+ signal also plays an important role in UV-B guard cell signaling and works downstream of NO as well as H2O2 and MPK6. Moreover, the data that NO rescued the defect of mutants for MPK6 in UV-B-induced stomatal closure (Fig. 5A) and that the UV-B-induced activation of MPK6 and MPK3 was not impaired in the mutants for NIA1 and NIA2 (Fig. 5C) also suggest that MPK6 does not mediate the regulation of Ca2+ channels by NO in UV-B guard cell signaling. Thus, whether the UV-B-triggered NO induces stomatal closure via cGMP, cADP ribose, or other MPKs is still an interesting question.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Seeds of Arabidopsis (Arabidopsis thaliana) wild types and mutants mpk3-1 (SALK_151594), mpk3-2 (CS466883), mpk6-1 (CS31099), mpk6-4 (SALK_062471C), AtrbohD (CS9555), AtrbohF (CS9557), AtrbohD/F (CS9558), nia2-1 (CS2355), nia1-2 (CS6936), and nia1-1/nia2-5 (CS2356) were obtained from the Nottingham Arabidopsis Stock Centre. Seeds of mutants mkp1/mpk6 and mkp1 and the complemented gMKP1:MKP1 mkp1 (line 6) were provided by Drs. S.C. Peck (University of Missouri, Columbia) and R. Ulm (University of Geneva). Mutants mpk3-1, mpk3-2, mpk6-4, AtrbohD, AtrbohF, AtrbohD/F, nia2-1, and nia1-1/nia2-5 are in the Col-0 ecotype, mpk6-1, mkp1/mpk6, mkp1, and the complemented line 6 are in the Ws ecotype, and nia1-2 is in the Landsberg erecta ecotype. The genotypes of all mutants were confirmed by PCR analysis.
Plants were grown on a mixture of soil:vermiculite:perlite (3:1:1, v/v/v) at a day/night temperature cycle of 18°C/22°C under a 16-h-light/8-h-dark photoperiod and a relative humidity of 80%. Photosynthetically active radiation during the day period was 0.1 mmol m−2 s−1 provided by QML YZ26RR16/G fluorescent tubes with no emission below 330 nm (Chaomei Lighting Electric Appliance) and measured with a Skye RS232 meter equipped with a Quantum sensor (Skye Instruments). Fully expanded leaves of 4- to 6-week-old plants were detached and used for experiments.
UV-B Treatments
UV-B irradiation was carried out in controlled-environment rooms at 22°C. The leaves were exposed to white light supplemented with UV-B. White light was provided by QML YZ26RR16/G fluorescent tubes as described above. UV-B was obtained from 40-W Q-panel UV 313 lamps (Largo; its maximum output is at 313 nm) covered with 0.13-mm-thick cellulose diacetate (West Design Products) to transmit radiation down to 290 nm. The lamps were suspended above and perpendicular to the abaxial surface of leaves. The desired fluence density was obtained by adjusting the distance between the lamps and the leaves, measured by an Optronics Laboratories spectroradiometer (model 742), and weighted with the generalized plant response action spectrum normalized to 300 nm.
Stomatal Bioassays
The stomatal experiments were performed with freshly detached leaves as described previously (He et al., 2013). Briefly, leaves were first floated on MES-KCl buffer (10 mm MES, 0.1 mm CaCl2, and 50 mm KCl, pH 6.15) with their abaxial surfaces facing up and under white light (0.1 mmol m−2 s−1) for 3 h to induce the stomata opening. Then, leaves were floated on the buffer alone or containing 100 µm H2O2 (Sigma-Aldrich) or 100 µm SNP (Sigma-Aldrich) under the same white light conditions with or without supplementary UV-B radiation for another 1 to 3 h. After treatments, the abaxial epidermis was stripped from the leaves and the pore size of stomata was measured with a calibrated light microscope. Aperture data are shown as means ± se of at least three independent experiments, each with 50 stomata.
NO and H2O2 Quantification
NO and H2O2 levels in guard cells were detected with fluorescent probes DAF-2DA and H2DCFDA, respectively, as described previously with slight modifications (Kojima et al., 1998; He et al., 2013). Briefly, the fresh epidermal strips from the treated leaves were incubated under dark in Tris-KCl buffer (10 mm Tris and 50 mm KCl, pH 7.2) with 10 µm DAF-2DA for 30 min or 50 µm H2DCFDA for 10 min and washed with fresh Tris-KCl buffer to remove residual probe. Samples were examined with an inverted fluorescence microscope (Eclipse TE 200; Nikon) equipped with a mercury lamp, an excitation filter (450–490 nm), a dichroic mirror (505 nm), and an emission filter (520–560 nm). For the quantification of fluorescence, the whole stomata areas of the micrographs were analyzed with Leica Image software. The presented images represented the same results from at least three independent experiments.
Protein Extraction and Immunoblot Analysis
Total protein of the treated leaves was extracted in extraction buffer (100 mm HEPES, pH 7.5, 5 mm EDTA, 5 mm EGTA, 10 mm dithiothreitol, 10 mm Na3VO4, 10 mm NaF, 1 mm phenylmethylsulfonyl fluoride, 5 µg mL−1 leupeptin, 5 µg mL−1 aprotinin, 5% glycerin, and 50 mm β-glycerophosphate), and the protein concentration was determined using the Quick Start Bradford Protein Assay (Bio-Rad). For immunoblot analysis, total cellular proteins (7.5 µg) were separated by electrophoresis on 12% SDS-polyacrylamide gels and electrophoretically transferred to a polyvinylidene fluoride membrane, according to the manufacturer’s instructions (Bio-Rad). We used polyclonal primary antibodies against phospho-p44/42 MPK (Cell Signaling Technologies) and monoclonal primary antibodies against actin (Sigma-Aldrich), with horseradish peroxidase-conjugated anti-rabbit or anti-mouse immunoglobulins (Abgent) as secondary antibodies, as required. Signal detection was performed using the Clarity Western ECL Substrate (Bio-Rad).
Statistical Analysis
Statistical analyses were performed using a one-way ANOVA followed by the lsd test.
Accession Numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers At3g55270 (MKP1), At3g45640 (MPK3), At2g43790 (MPK6), At5g47910 (ATRBOHD), At1g64060 (ATRBOHF), At1g77760 (NIA1), At1g37130 (NIA2), and At3g18780 (ACTIN2).
Acknowledgments
We thank Drs. Scott C. Peck and Roman Ulm and the Nottingham Arabidopsis Stock Centre for providing Arabidopsis seeds.
Glossary
- ABA
abscisic acid
- NO
nitric oxide
- Ws
Wassilewskija
- DAF-2DA
4,5-diaminofluorescein diacetate
- Col-0
Columbia-0
- SNP
sodium nitroprusside
Footnotes
This work was supported by the National Science Foundation of China (grant no. 31170370 to J.-M.H. and grant no. 31570397 to J.-M.H., F.-C.L., and W.J.) and the Fundamental Research Funds for the Central Universities (grant no. GK201401005 to J.-M.H.).
References
- Abdul-Awal SM, Hotta CT, Davey MP, Dodd AN, Smith AG, Webb AAR (2016) NO-mediated [Ca2+]cyt increases depend on ADP-ribosyl cyclase activity in Arabidopsis. Plant Physiol 171: 623–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JC, Bartels S, González Besteiro MA, Shahollari B, Ulm R, Peck SC (2011) Arabidopsis MAP Kinase Phosphatase 1 (AtMKP1) negatively regulates MPK6-mediated PAMP responses and resistance against bacteria. Plant J 67: 258–268 [DOI] [PubMed] [Google Scholar]
- Asai S, Ohta K, Yoshioka H (2008) MAPK signaling regulates nitric oxide and NADPH oxidase-dependent oxidative bursts in Nicotiana benthamiana. Plant Cell 20: 1390–1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asai T, Tena G, Plotnikova J, Willmann MR, Chiu WL, Gomez-Gomez L, Boller T, Ausubel FM, Sheen J (2002) MAP kinase signalling cascade in Arabidopsis innate immunity. Nature 415: 977–983 [DOI] [PubMed] [Google Scholar]
- Bartels S, Anderson JC, González Besteiro MA, Carreri A, Hirt H, Buchala A, Métraux JP, Peck SC, Ulm R (2009) MAP kinase phosphatase1 and protein tyrosine phosphatase1 are repressors of salicylic acid synthesis and SNC1-mediated responses in Arabidopsis. Plant Cell 21: 2884–2897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels S, González Besteiro MA, Lang D, Ulm R (2010) Emerging functions for plant MAP kinase phosphatases. Trends Plant Sci 15: 322–329 [DOI] [PubMed] [Google Scholar]
- Bright J, Desikan R, Hancock JT, Weir IS, Neill SJ (2006) ABA-induced NO generation and stomatal closure in Arabidopsis are dependent on H2O2 synthesis. Plant J 45: 113–122 [DOI] [PubMed] [Google Scholar]
- Brock AK, Willmann R, Kolb D, Grefen L, Lajunen HM, Bethke G, Lee J, Nürnberger T, Gust AA (2010) The Arabidopsis mitogen-activated protein kinase phosphatase PP2C5 affects seed germination, stomatal aperture, and abscisic acid-inducible gene expression. Plant Physiol 153: 1098–1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown BA, Jenkins GI (2008) UV-B signaling pathways with different fluence-rate response profiles are distinguished in mature Arabidopsis leaf tissue by requirement for UVR8, HY5, and HYH. Plant Physiol 146: 576–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett EC, Desikan R, Moser RC, Neill SJ (2000) ABA activation of an MBP kinase in Pisum sativum epidermal peels correlates with stomatal responses to ABA. J Exp Bot 51: 197–205 [DOI] [PubMed] [Google Scholar]
- Colcombet J, Hirt H (2008) Arabidopsis MAPKs: a complex signalling network involved in multiple biological processes. Biochem J 413: 217–226 [DOI] [PubMed] [Google Scholar]
- Furuya T, Matsuoka D, Nanmori T (2014) Membrane rigidification functions upstream of the MEKK1-MKK2-MPK4 cascade during cold acclimation in Arabidopsis thaliana. FEBS Lett 588: 2025–2030 [DOI] [PubMed] [Google Scholar]
- Garcia-Mata C, Gay R, Sokolovski S, Hills A, Lamattina L, Blatt MR (2003) Nitric oxide regulates K+ and Cl− channels in guard cells through a subset of abscisic acid-evoked signaling pathways. Proc Natl Acad Sci USA 100: 11116–11121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gayatri G, Agurla S, Raghavendra AS (2013) Nitric oxide in guard cells as an important secondary messenger during stomatal closure. Front Plant Sci 4: 425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomi K, Ogawa D, Katou S, Kamada H, Nakajima N, Saji H, Soyano T, Sasabe M, Machida Y, Mitsuhara I, et al. (2005) A mitogen-activated protein kinase NtMPK4 activated by SIPKK is required for jasmonic acid signaling and involved in ozone tolerance via stomatal movement in tobacco. Plant Cell Physiol 46: 1902–1914 [DOI] [PubMed] [Google Scholar]
- González Besteiro MA, Bartels S, Albert A, Ulm R (2011) Arabidopsis MAP kinase phosphatase 1 and its target MAP kinases 3 and 6 antagonistically determine UV-B stress tolerance, independent of the UVR8 photoreceptor pathway. Plant J 68: 727–737 [DOI] [PubMed] [Google Scholar]
- González Besteiro MA, Ulm R (2013) Phosphorylation and stabilization of Arabidopsis MAP kinase phosphatase 1 in response to UV-B stress. J Biol Chem 288: 480–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudesblat GE, Iusem ND, Morris PC (2007) Guard cell-specific inhibition of Arabidopsis MPK3 expression causes abnormal stomatal responses to abscisic acid and hydrogen peroxide. New Phytol 173: 713–721 [DOI] [PubMed] [Google Scholar]
- Gudesblat GE, Torres PS, Vojnov AA (2009) Xanthomonas campestris overcomes Arabidopsis stomatal innate immunity through a DSF cell-to-cell signal-regulated virulence factor. Plant Physiol 149: 1017–1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He JM, Ma XG, Zhang Y, Sun TF, Xu FF, Chen YP, Liu X, Yue M (2013) Role and interrelationship of Gα protein, hydrogen peroxide, and nitric oxide in ultraviolet B-induced stomatal closure in Arabidopsis leaves. Plant Physiol 161: 1570–1583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He JM, Xu H, She XP, Song XG, Zhao WM (2005) The role and the interrelationship of hydrogen peroxide and nitric oxide in the UV-B induced stomatal closure in broad bean. Funct Plant Biol 32: 237–247 [DOI] [PubMed] [Google Scholar]
- Hettenhausen C, Baldwin IT, Wu J (2012) Silencing MPK4 in Nicotiana attenuata enhances photosynthesis and seed production but compromises abscisic acid-induced stomatal closure and guard cell-mediated resistance to Pseudomonas syringae pv tomato DC3000. Plant Physiol 158: 759–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holley SR, Yalamanchili RD, Moura DS, Ryan CA, Stratmann JW (2003) Convergence of signaling pathways induced by systemin, oligosaccharide elicitors, and ultraviolet-B radiation at the level of mitogen-activated protein kinases in Lycopersicon peruvianum suspension-cultured cells. Plant Physiol 132: 1728–1738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hõrak H, Sierla M, Tõldsepp K, Wang C, Wang YS, Nuhkat M, Valk E, Pechter P, Merilo E, Salojärvi J, et al. (2016) A dominant mutation in the HT1 kinase uncovers roles of MAP kinases and GHR1 in CO2-induced stomatal closure. Plant Cell 28: 2493–2509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalmi SK, Sinha AK (2015) ROS mediated MAPK signaling in abiotic and biotic stress: striking similarities and differences. Front Plant Sci 6: 769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jammes F, Song C, Shin D, Munemasa S, Takeda K, Gu D, Cho D, Lee S, Giordo R, Sritubtim S, et al. (2009) MAP kinases MPK9 and MPK12 are preferentially expressed in guard cells and positively regulate ROS-mediated ABA signaling. Proc Natl Acad Sci USA 106: 20520–20525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen MAK, Noort REVD (2000) Ultraviolet-B radiation induces complex alterations in stomatal behaviour. Physiol Plant 110: 189–194 [Google Scholar]
- Jeandroz S, Lamotte O, Astier J, Rasul S, Trapet P, Besson-Bard A, Bourque S, Nicolas-Francès V, Ma W, Berkowitz GA, et al. (2013) There’s more to the picture than meets the eye: nitric oxide cross talk with Ca2+ signaling. Plant Physiol 163: 459–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins GI. (2009) Signal transduction in responses to UV-B radiation. Annu Rev Plant Biol 60: 407–431 [DOI] [PubMed] [Google Scholar]
- Jenkins GI. (2014) The UV-B photoreceptor UVR8: from structure to physiology. Plant Cell 26: 21–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J, Wang P, An G, Wang P, Song CP (2008) The involvement of a P38-like MAP kinase in ABA-induced and H2O2-mediated stomatal closure in Vicia faba L. Plant Cell Rep 27: 377–385 [DOI] [PubMed] [Google Scholar]
- Joudoi T, Shichiri Y, Kamizono N, Akaike T, Sawa T, Yoshitake J, Yamada N, Iwai S (2013) Nitrated cyclic GMP modulates guard cell signaling in Arabidopsis. Plant Cell 25: 558–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalbina I, Strid A (2006) The role of NADPH oxidase and MAP kinase phosphatase in UV-B-dependent gene expression in Arabidopsis. Plant Cell Environ 29: 1783–1793 [DOI] [PubMed] [Google Scholar]
- Keyse SM. (2008) The regulation of stress-activated MAP kinase signalling by protein phosphatases. Top Curr Genet 20: 33–49 [Google Scholar]
- Khokon MA, Salam MA, Jammes F, Ye W, Hossain MA, Uraji M, Nakamura Y, Mori IC, Kwak JM, Murata Y (2015) Two guard cell mitogen-activated protein kinases, MPK9 and MPK12, function in methyl jasmonate-induced stomatal closure in Arabidopsis thaliana. Plant Biol (Stuttg) 17: 946–952 [DOI] [PubMed] [Google Scholar]
- Kojima H, Nakatsubo N, Kikuchi K, Urano Y, Higuchi T, Tanaka J, Kudo Y, Nagano T (1998) Direct evidence of NO production in rat hippocampus and cortex using a new fluorescent indicator: DAF-2 DA. Neuroreport 9: 3345–3348 [DOI] [PubMed] [Google Scholar]
- Kok FO, Shin M, Ni CW, Gupta A, Grosse AS, van Impel A, Kirchmaier BC, Peterson-Maduro J, Kourkoulis G, Male I, et al. (2015) Reverse genetic screening reveals poor correlation between morpholino-induced and mutant phenotypes in zebrafish. Dev Cell 32: 97–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovtun Y, Chiu WL, Tena G, Sheen J (2000) Functional analysis of oxidative stress-activated mitogen-activated protein kinase cascade in plants. Proc Natl Acad Sci USA 97: 2940–2945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JH, Liu YQ, Lü P, Lin HF, Bai Y, Wang XC, Chen YL (2009) A signaling pathway linking nitric oxide production to heterotrimeric G protein and hydrogen peroxide regulates extracellular calmodulin induction of stomatal closure in Arabidopsis. Plant Physiol 150: 114–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Feng D, Zhang D, Su J, Zhang Y, Li Z, Mu P, Liu B, Wang H, Wang J (2012) Rice MAPK phosphatase IBR5 negatively regulates drought stress tolerance in transgenic Nicotiana tabacum. Plant Sci 188-189: 10–18 [DOI] [PubMed] [Google Scholar]
- MacRobbie EA, Kurup S (2007) Signalling mechanisms in the regulation of vacuolar ion release in guard cells. New Phytol 175: 630–640 [DOI] [PubMed] [Google Scholar]
- Marten H, Hyun T, Gomi K, Seo S, Hedrich R, Roelfsema MR (2008) Silencing of NtMPK4 impairs CO-induced stomatal closure, activation of anion channels and cytosolic Ca signals in Nicotiana tabacum guard cells. Plant J 55: 698–708 [DOI] [PubMed] [Google Scholar]
- Montillet JL, Leonhardt N, Mondy S, Tranchimand S, Rumeau D, Boudsocq M, Garcia AV, Douki T, Bigeard J, Laurière C, et al. (2013) An abscisic acid-independent oxylipin pathway controls stomatal closure and immune defense in Arabidopsis. PLoS Biol 11: e1001513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon H, Lee B, Choi G, Shin D, Prasad DT, Lee O, Kwak SS, Kim DH, Nam J, Bahk J, et al. (2003) NDP kinase 2 interacts with two oxidative stress-activated MAPKs to regulate cellular redox state and enhances multiple stress tolerance in transgenic plants. Proc Natl Acad Sci USA 100: 358–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musil CF, Wand SJE (1993) Responses of sclerophyllous Ericaceae to enhanced level of ultraviolet-B radiation. Environ Exp Bot 33: 233–242 [Google Scholar]
- Nawkar GM, Maibam P, Park JH, Sahi VP, Lee SY, Kang CH (2013) UV-Induced cell death in plants. Int J Mol Sci 14: 1608–1628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogués S, Allen DJ, Morison JI, Baker NR (1999) Characterization of stomatal closure caused by ultraviolet-B radiation. Plant Physiol 121: 489–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei ZM, Murata Y, Benning G, Thomine S, Klüsener B, Allen GJ, Grill E, Schroeder JI (2000) Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature 406: 731–734 [DOI] [PubMed] [Google Scholar]
- Pitzschke A, Hirt H (2009) Disentangling the complexity of mitogen-activated protein kinases and reactive oxygen species signaling. Plant Physiol 149: 606–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planchet E, Jagadis Gupta K, Sonoda M, Kaiser WM (2005) Nitric oxide emission from tobacco leaves and cell suspensions: rate limiting factors and evidence for the involvement of mitochondrial electron transport. Plant J 41: 732–743 [DOI] [PubMed] [Google Scholar]
- Quettier AL, Bertrand C, Habricot Y, Miginiac E, Agnes C, Jeannette E, Maldiney R (2006) The phs1-3 mutation in a putative dual-specificity protein tyrosine phosphatase gene provokes hypersensitive responses to abscisic acid in Arabidopsis thaliana. Plant J 47: 711–719 [DOI] [PubMed] [Google Scholar]
- Ramani S, Chelliah J (2007) UV-B-induced signaling events leading to enhanced-production of catharanthine in Catharanthus roseus cell suspension cultures. BMC Plant Biol 7: 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rentel MC, Lecourieux D, Ouaked F, Usher SL, Petersen L, Okamoto H, Knight H, Peck SC, Grierson CS, Hirt H, et al. (2004) OXI1 kinase is necessary for oxidative burst-mediated signalling in Arabidopsis. Nature 427: 858–861 [DOI] [PubMed] [Google Scholar]
- Rossi A, Kontarakis Z, Gerri C, Nolte H, Hölper S, Krüger M, Stainier DYR (2015) Genetic compensation induced by deleterious mutations but not gene knockdowns. Nature 524: 230–233 [DOI] [PubMed] [Google Scholar]
- Salam MA, Jammes F, Hossain MA, Ye W, Nakamura Y, Mori IC, Kwak JM, Murata Y (2012) MAP kinases, MPK9 and MPK12, regulate chitosan-induced stomatal closure. Biosci Biotechnol Biochem 76: 1785–1787 [DOI] [PubMed] [Google Scholar]
- Salam MA, Jammes F, Hossain MA, Ye W, Nakamura Y, Mori IC, Kwak JM, Murata Y (2013) Two guard cell-preferential MAPKs, MPK9 and MPK12, regulate YEL signalling in Arabidopsis guard cells. Plant Biol (Stuttg) 15: 436–442 [DOI] [PubMed] [Google Scholar]
- She XP, Song XG, He JM (2004) Role and relationship of nitric oxide and hydrogen peroxide in light/dark-regulated stomatal movement in Vicia faba. Acta Bot Sin 46: 1292–1300 [Google Scholar]
- Sokolovski S, Hills A, Gay R, Garcia-Mata C, Lamattina L, Blatt MR (2005) Protein phosphorylation is a prerequisite for intracellular Ca2+ release and ion channel control by nitric oxide and abscisic acid in guard cells. Plant J 43: 520–529 [DOI] [PubMed] [Google Scholar]
- Teige M, Scheikl E, Eulgem T, Dóczi R, Ichimura K, Shinozaki K, Dangl JL, Hirt H (2004) The MKK2 pathway mediates cold and salt stress signaling in Arabidopsis. Mol Cell 15: 141–152 [DOI] [PubMed] [Google Scholar]
- Tossi V, Lamattina L, Jenkins GI, Cassia RO (2014) Ultraviolet-B-induced stomatal closure in Arabidopsis is regulated by the UV RESISTANCE LOCUS8 photoreceptor in a nitric oxide-dependent mechanism. Plant Physiol 164: 2220–2230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulm R, Revenkova E, di Sansebastiano GP, Bechtold N, Paszkowski J (2001) Mitogen-activated protein kinase phosphatase is required for genotoxic stress relief in Arabidopsis. Genes Dev 15: 699–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Du Y, Li Y, Ren D, Song CP (2010) Hydrogen peroxide-mediated activation of MAP kinase 6 modulates nitric oxide biosynthesis and signal transduction in Arabidopsis. Plant Cell 22: 2981–2998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Song CP (2008) Guard-cell signalling for hydrogen peroxide and abscisic acid. New Phytol 178: 703–718 [DOI] [PubMed] [Google Scholar]
- Xie Y, Mao Y, Zhang W, Lai D, Wang Q, Shen W (2014) Reactive oxygen species-dependent nitric oxide production contributes to hydrogen-promoted stomatal closure in Arabidopsis. Plant Physiol 165: 759–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Y, Jia W, Zhang J (2007) AtMEK1 mediates stress-induced gene expression of CAT1 catalase by triggering H2O2 production in Arabidopsis. J Exp Bot 58: 2969–2981 [DOI] [PubMed] [Google Scholar]
- Xing Y, Jia W, Zhang J (2008) AtMKK1 mediates ABA-induced CAT1 expression and H2O2 production via AtMPK6-coupled signaling in Arabidopsis. Plant J 54: 440–451 [DOI] [PubMed] [Google Scholar]
- Yoshioka H, Numata N, Nakajima K, Katou S, Kawakita K, Rowland O, Jones JD, Doke N (2003) Nicotiana benthamiana gp91phox homologs NbrbohA and NbrbohB participate in H2O2 accumulation and resistance to Phytophthora infestans. Plant Cell 15: 706–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuasa T, Ichimura K, Mizoguchi T, Shinozaki K (2001) Oxidative stress activates ATMPK6, an Arabidopsis homologue of MAP kinase. Plant Cell Physiol 42: 1012–1016 [DOI] [PubMed] [Google Scholar]
- Zhao Z, Zhang W, Stanley BA, Assmann SM (2008) Functional proteomics of Arabidopsis thaliana guard cells uncovers new stomatal signaling pathways. Plant Cell 20: 3210–3226 [DOI] [PMC free article] [PubMed] [Google Scholar]