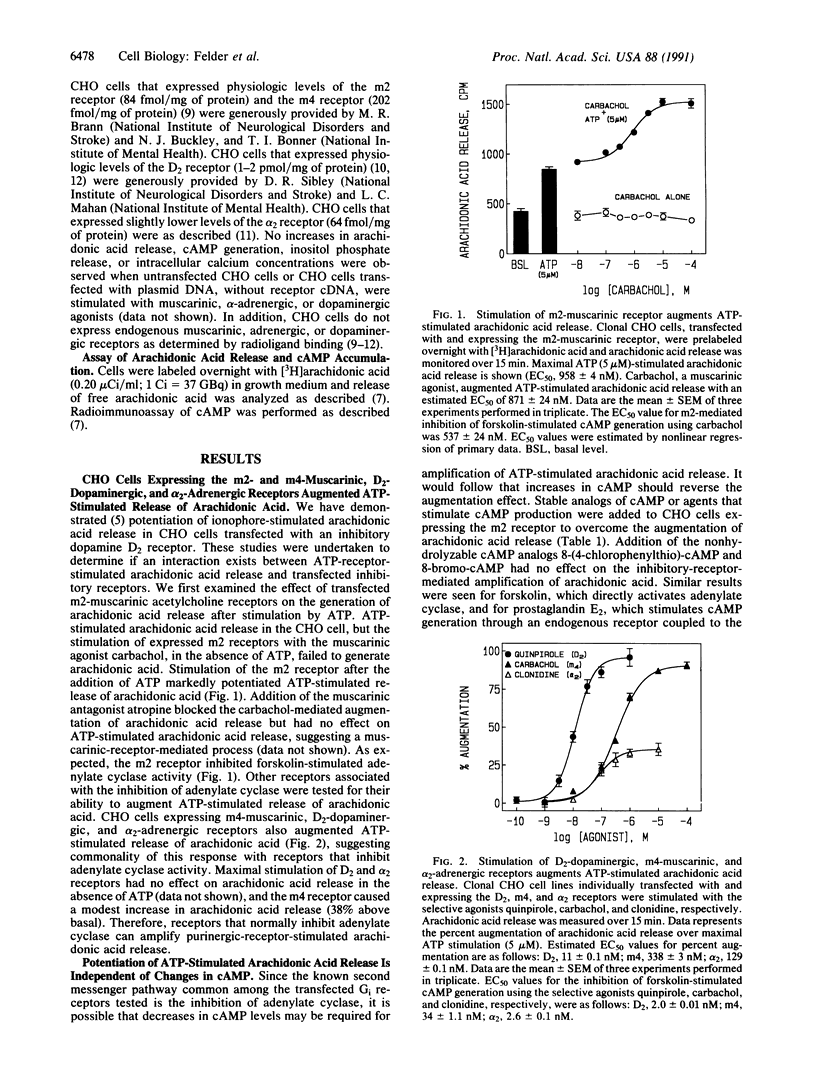
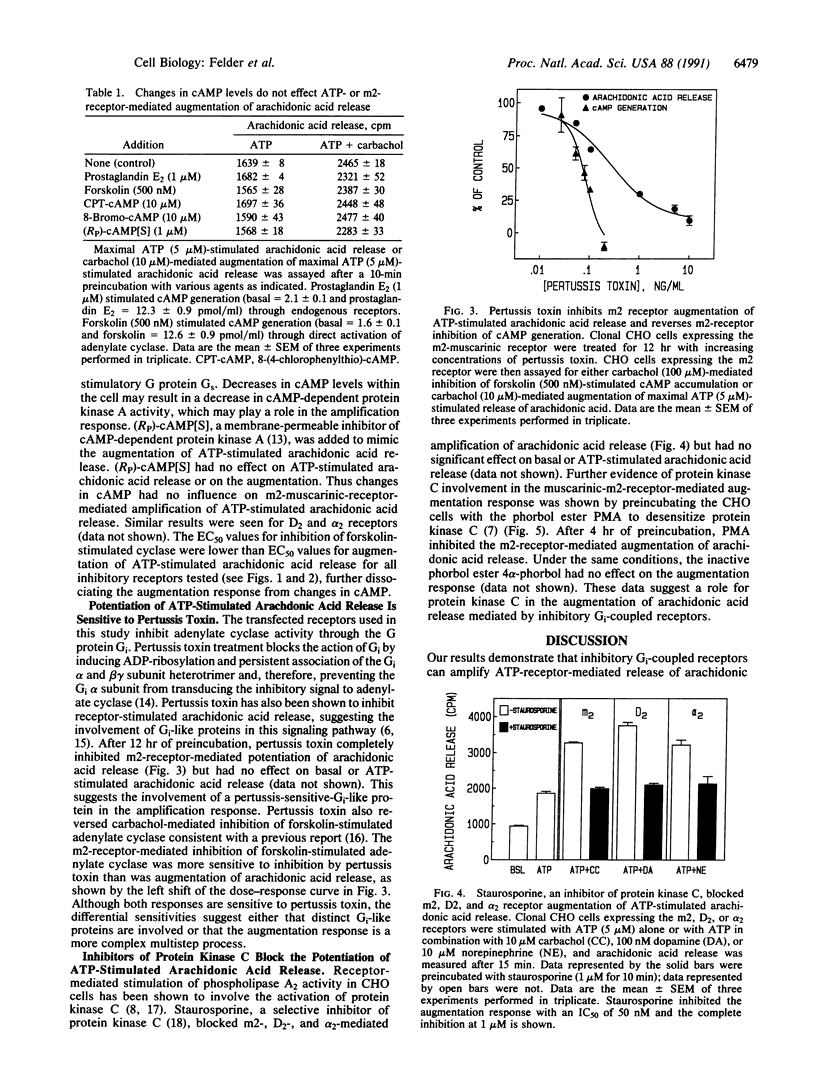
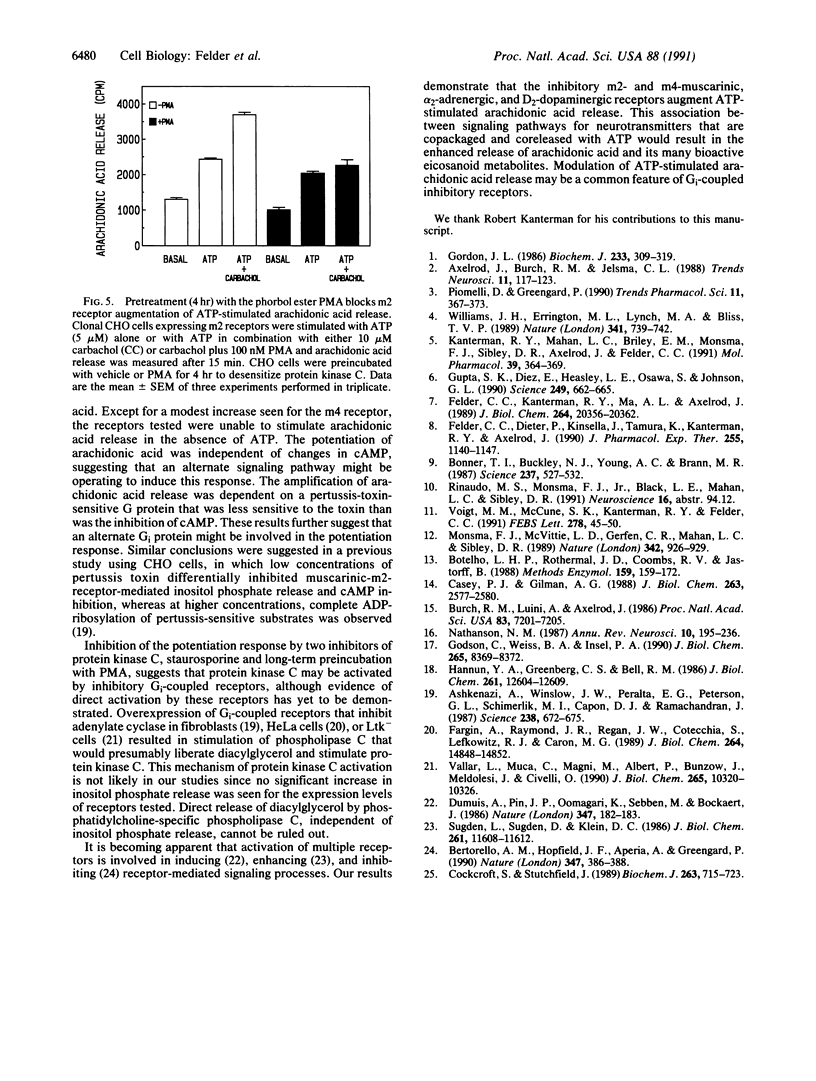
Abstract
ATP is copackaged and coreleased with adrenergic, serotonergic, and cholinergic neurotransmitters, suggesting a possible interaction between the signaling pathways for ATP and these coreleased neurotransmitters. Muscarinic m2 and m4, alpha 2-adrenergic, and D2-dopaminergic neurotransmitter receptors, which have in common their ability to inhibit adenylate cyclase through the inhibitory guanine nucleotide binding protein Gi, were transfected and expressed in Chinese hamster ovary (CHO) cells that contain endogenous ATP receptors coupled to the release of arachidonic acid. Normal functional coupling of m2, m4, alpha 2, and D2 receptors was demonstrated by their ability to inhibit forskolin-stimulated cAMP accumulation with dose-response activities consistent with previous reports for these Gi-coupled receptors. Stimulation of m2, m4, alpha 2, and D2 receptors resulted in an augmentation of ATP-stimulated arachidonic acid release. With the exception of the m4 receptor, none of the receptors tested was able to stimulate arachidonic acid release in the absence of ATP. Potentiation of ATP-stimulated arachidonic acid release was independent of changes in cAMP. The augmentation of ATP-stimulated arachidonic acid release and the inhibition of cAMP accumulation were both blocked by pertussis toxin, an inhibitor of Gi, but with different dose-response characteristics. Inhibition of protein kinase C with staurosporine or long-term pretreatment of the cells with the phorbol ester phorbol 12-myristate 13-acetate blocked the augmentation response. This demonstrates that Gi-coupled inhibitory receptors can amplify ATP-receptor-stimulated arachidonic acid release through a pertussis-toxin-sensitive G protein, independent of their ability to inhibit adenylate cyclase activity.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashkenazi A., Winslow J. W., Peralta E. G., Peterson G. L., Schimerlik M. I., Capon D. J., Ramachandran J. An M2 muscarinic receptor subtype coupled to both adenylyl cyclase and phosphoinositide turnover. Science. 1987 Oct 30;238(4827):672–675. doi: 10.1126/science.2823384. [DOI] [PubMed] [Google Scholar]
- Axelrod J., Burch R. M., Jelsema C. L. Receptor-mediated activation of phospholipase A2 via GTP-binding proteins: arachidonic acid and its metabolites as second messengers. Trends Neurosci. 1988 Mar;11(3):117–123. doi: 10.1016/0166-2236(88)90157-9. [DOI] [PubMed] [Google Scholar]
- Bertorello A. M., Hopfield J. F., Aperia A., Greengard P. Inhibition by dopamine of (Na(+)+K+)ATPase activity in neostriatal neurons through D1 and D2 dopamine receptor synergism. Nature. 1990 Sep 27;347(6291):386–388. doi: 10.1038/347386a0. [DOI] [PubMed] [Google Scholar]
- Bonner T. I., Buckley N. J., Young A. C., Brann M. R. Identification of a family of muscarinic acetylcholine receptor genes. Science. 1987 Jul 31;237(4814):527–532. doi: 10.1126/science.3037705. [DOI] [PubMed] [Google Scholar]
- Botelho L. H., Rothermel J. D., Coombs R. V., Jastorff B. cAMP analog antagonists of cAMP action. Methods Enzymol. 1988;159:159–172. doi: 10.1016/0076-6879(88)59017-1. [DOI] [PubMed] [Google Scholar]
- Burch R. M., Luini A., Axelrod J. Phospholipase A2 and phospholipase C are activated by distinct GTP-binding proteins in response to alpha 1-adrenergic stimulation in FRTL5 thyroid cells. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7201–7205. doi: 10.1073/pnas.83.19.7201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey P. J., Gilman A. G. G protein involvement in receptor-effector coupling. J Biol Chem. 1988 Feb 25;263(6):2577–2580. [PubMed] [Google Scholar]
- Cockcroft S., Stutchfield J. The receptors for ATP and fMetLeuPhe are independently coupled to phospholipases C and A2 via G-protein(s). Relationship between phospholipase C and A2 activation and exocytosis in HL60 cells and human neutrophils. Biochem J. 1989 Nov 1;263(3):715–723. doi: 10.1042/bj2630715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumuis A., Pin J. P., Oomagari K., Sebben M., Bockaert J. Arachidonic acid released from striatal neurons by joint stimulation of ionotropic and metabotropic quisqualate receptors. Nature. 1990 Sep 13;347(6289):182–184. doi: 10.1038/347182a0. [DOI] [PubMed] [Google Scholar]
- Fargin A., Raymond J. R., Regan J. W., Cotecchia S., Lefkowitz R. J., Caron M. G. Effector coupling mechanisms of the cloned 5-HT1A receptor. J Biol Chem. 1989 Sep 5;264(25):14848–14852. [PubMed] [Google Scholar]
- Felder C. C., Dieter P., Kinsella J., Tamura K., Kanterman R. Y., Axelrod J. A transfected m5 muscarinic acetylcholine receptor stimulates phospholipase A2 by inducing both calcium influx and activation of protein kinase C. J Pharmacol Exp Ther. 1990 Dec;255(3):1140–1147. [PubMed] [Google Scholar]
- Felder C. C., Kanterman R. Y., Ma A. L., Axelrod J. A transfected m1 muscarinic acetylcholine receptor stimulates adenylate cyclase via phosphatidylinositol hydrolysis. J Biol Chem. 1989 Dec 5;264(34):20356–20362. [PubMed] [Google Scholar]
- Godson C., Weiss B. A., Insel P. A. Differential activation of protein kinase C alpha is associated with arachidonate release in Madin-Darby canine kidney cells. J Biol Chem. 1990 May 25;265(15):8369–8372. [PubMed] [Google Scholar]
- Gordon J. L. Extracellular ATP: effects, sources and fate. Biochem J. 1986 Jan 15;233(2):309–319. doi: 10.1042/bj2330309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S. K., Diez E., Heasley L. E., Osawa S., Johnson G. L. A G protein mutant that inhibits thrombin and purinergic receptor activation of phospholipase A2. Science. 1990 Aug 10;249(4969):662–666. doi: 10.1126/science.2166341. [DOI] [PubMed] [Google Scholar]
- Hannun Y. A., Loomis C. R., Merrill A. H., Jr, Bell R. M. Sphingosine inhibition of protein kinase C activity and of phorbol dibutyrate binding in vitro and in human platelets. J Biol Chem. 1986 Sep 25;261(27):12604–12609. [PubMed] [Google Scholar]
- Kanterman R. Y., Mahan L. C., Briley E. M., Monsma F. J., Jr, Sibley D. R., Axelrod J., Felder C. C. Transfected D2 dopamine receptors mediate the potentiation of arachidonic acid release in Chinese hamster ovary cells. Mol Pharmacol. 1991 Mar;39(3):364–369. [PubMed] [Google Scholar]
- Monsma F. J., Jr, McVittie L. D., Gerfen C. R., Mahan L. C., Sibley D. R. Multiple D2 dopamine receptors produced by alternative RNA splicing. Nature. 1989 Dec 21;342(6252):926–929. doi: 10.1038/342926a0. [DOI] [PubMed] [Google Scholar]
- Nathanson N. M. Molecular properties of the muscarinic acetylcholine receptor. Annu Rev Neurosci. 1987;10:195–236. doi: 10.1146/annurev.ne.10.030187.001211. [DOI] [PubMed] [Google Scholar]
- Piomelli D., Greengard P. Lipoxygenase metabolites of arachidonic acid in neuronal transmembrane signalling. Trends Pharmacol Sci. 1990 Sep;11(9):367–373. doi: 10.1016/0165-6147(90)90182-8. [DOI] [PubMed] [Google Scholar]
- Sugden A. L., Sugden D., Klein D. C. Essential role of calcium influx in the adrenergic regulation of cAMP and cGMP in rat pinealocytes. J Biol Chem. 1986 Sep 5;261(25):11608–11612. [PubMed] [Google Scholar]
- Vallar L., Muca C., Magni M., Albert P., Bunzow J., Meldolesi J., Civelli O. Differential coupling of dopaminergic D2 receptors expressed in different cell types. Stimulation of phosphatidylinositol 4,5-bisphosphate hydrolysis in LtK- fibroblasts, hyperpolarization, and cytosolic-free Ca2+ concentration decrease in GH4C1 cells. J Biol Chem. 1990 Jun 25;265(18):10320–10326. [PubMed] [Google Scholar]
- Voigt M. M., McCune S. K., Kanterman R. Y., Felder C. C. The rat alpha 2-C4 adrenergic receptor gene encodes a novel pharmacological subtype. FEBS Lett. 1991 Jan 14;278(1):45–50. doi: 10.1016/0014-5793(91)80080-m. [DOI] [PubMed] [Google Scholar]
- Williams J. H., Errington M. L., Lynch M. A., Bliss T. V. Arachidonic acid induces a long-term activity-dependent enhancement of synaptic transmission in the hippocampus. Nature. 1989 Oct 26;341(6244):739–742. doi: 10.1038/341739a0. [DOI] [PubMed] [Google Scholar]



