Abstract
O-mannosylation is an important protein modification in eukaryotes that is initiated by an evolutionarily conserved family of protein O-mannosyltransferases. The first mammalian protein O-mannosyltransferase gene described was the human POMT1. Mutations in the hPOMT1 gene are responsible for Walker–Warburg syndrome (WWS), a severe recessive congenital muscular dystrophy associated with defects in neuronal migration that produce complex brain and eye abnormalities. During embryogenesis, the murine Pomt1 gene is prominently expressed in the neural tube, the developing eye, and the mesenchyme. These sites of expression correlate with those in which the main tissue alterations are observed in WWS patients. We have inactivated a Pomt1 allele by gene targeting in embryonic stem cells and produced chimeras transmitting the defect allele to offspring. Although heterozygous mice were viable and fertile, the total absence of Pomt1–/– pups in the progeny of heterozygous intercrosses indicated that this genotype is embryonic lethal. An analysis of the mutant phenotype revealed that homozygous Pomt1–/– mice suffer developmental arrest around embryonic day (E) 7.5 and die between E7.5 and E9.5. The Pomt1–/– embryos present defects in the formation of Reichert's membrane, the first basement membrane to form in the embryo. The failure of this membrane to form appears to be the result of abnormal glycosylation and maturation of dystroglycan that may impair recruitment of laminin, a structural component required for the formation of Reichert's membrane in rodents. The targeted disruption of mPomt1 represents an example of an engineered deletion of a known glycosyltransferase involved in O-mannosyl glycan synthesis.
Protein O-mannosylation is an important protein modification in uni- and multicellular eukaryotes (1, 2). In mammals, all O-mannosyl glycans identified so far are variations of the tetrasaccharide NeuAcα2–3Galβ1–4GlcNAcβ1–2Man-Ser/Thr, although only a limited number of glycoproteins that carry this modification has been described (reviewed in ref. 2). The best-studied O-mannosylated glycoprotein is α-dystroglycan (α-DG), a component of the dystrophin glycoprotein complex that is implicated in the interaction between extracellular matrix proteins and the cytoskeleton of muscle and neurons (reviewed in refs. 3 and 4). The O-linked carbohydrate chains are involved in the binding of α-dystroglycan (α-DG) to its ligands, such as neuronal and muscle laminin/merosin. In recent years, it has become evident that a group of human neuromuscular diseases associated with hypoglycosylation of α-DG are the result of defects in known glycosyltransferases involved in O-mannosyl glycan synthesis (reviewed in refs. 2 and 5). These include congenital muscular dystrophies (CMDs) with neuronal migration defects, such as Walker–Warburg syndrome (WWS) (6), and muscle–eye–brain disease (MEB) (7). Other similar CMDs that feature abnormal α-DG glycosylation and mutations in putative glycosyltransferases are Fukuyama congenital muscular dystrophy (FCMD) (8), congenital muscular dystrophy 1C (MDC1C) (9), and congenital muscular dystrophy 1D (MDC1D) (10). Protein O-mannosylation is initiated in the endoplasmatic reticulum by the transfer of mannose from dolichyl phosphate-activated mannose to serine or threonine residues in secretory proteins (reviewed in ref. 1). This reaction is catalyzed by an evolutionarily conserved family of protein O-mannosyltransferases (PMTs) (11). Although these proteins have been most extensively characterized in yeast (1, 2), PMT homologues have been identified throughout the animal kingdom (with the exception of Caenorhabditis elegans). Moreover, in Drosophila melanogaster, mutations in the PMT family members rotated abdomen (rt) and twisted (tw) affect muscle development (12, 13). In human and mouse, two PMT family members are known to exist, namely POMT1 (14) and POMT2 (13). Human POMT1 and POMT2 catalyze protein O-mannosyl transfer to α-DG, which serves as a protein substrate (15). Mutations in the POMT1 gene result in WWS, a severe muscular dystrophy that also involves structural alterations in eye and brain malformations, such as cobblestone lissencephaly (6). Most WWS patients die within the first months of life, with a few surviving to 3 years of age. Patients with mutations in POMT1 show nuclear and extracellular matrix abnormalities in skeletal muscle, as well as defective glycosylation and the loss of laminin-binding activity in α-DG (16, 17).
Here, we show that during embryonic development, murine Pomt1 is expressed in the tissues affected in WWS patients. Targeted deletion of Pomt1 results in early embryonic lethality due to defects in the assembly of Reichert's membrane. Our results demonstrate the importance of O-mannosyl glycans not only in human disease, but also for critical processes during embryonic development such as the formation of early basement membranes.
Materials and Methods
Characterization and Mapping of the Mouse Pomt1 Gene. Rapid amplification of cDNA ends PCR was performed on a mouse 17-day embryo marathon-ready cDNA library (Clontech) to generate full-length Pomt1 cDNA. For chromosomal mapping, an HphI polymorphism in exon 20 between Mus musculus and Mus spretus was used in both panels of backcrosses: EUCIB (BSB) and The Jackson Institute (BSS).
Pomt1 Expression. Membranes containing RNA from mouse and human tissues (OriGene Technologies, Rockville, MD) and total mouse embryo RNA (Seegene, Del Mar, CA) were hybridized with 32P-labeled Pomt1 (base pairs 449–1,193), Pomt2 (base pairs 659–1,727), or POMT1 (base pairs 1,398–2,184) cDNA probes. Normalization was performed by using human β-actin and chicken GAPDH.
Whole-Mount in Situ Hybridization and Histological Analysis. Embryos were obtained from timed pregnancies, dissected in PBS (pH 7.3), and fixed overnight at 4°C in 4% paraformaldehyde/PBS. Sense and antisense Pomt1 (base pairs 2,052–2,611) and brachyury (base pairs 1–1,765) probes were generated by using the digoxigenin RNA labeling kit (Roche Diagnostics). Whole-mount in situ hybridization was carried out as described (18). Stained embryos were cryoprotected overnight at 4°C in 30% sucrose/PBS, embedded in Cryoblock (Medite Medizintechnik, Burgdorf, Germany), and sectioned (35 μm) at –25°C. Processed sections were mounted under coverslips in Mowiol (Calbiochem).
Gene Targeting. A 15-kb region of the mouse Pomt1 gene (intron 2 to exon 20) was isolated from a 129/SvJ genomic library (Mobi-Tec, Göttingen, Germany), by using mouse Pomt1 cDNA as a probe. To construct the Pomt1 targeting vector, the plasmid pPNT was used (19). A 4.5-kb XhoI fragment (introns 2–9) was cloned into the XhoI site of pPNT as the long arm. A 2.3-kb KpnI-XbaI fragment, from the intergenic sequence 5′ upstream to intron 1 of Pomt1, was amplified by PCR from 129/SvJ genomic DNA by using primers a and b (see Supporting Text, which is published as supporting information on the PNAS web site). This fragment was cloned as the short arm into the XbaI-KpnI sites of pPNT between the neo and hsv tk cassettes in an opposite orientation.
The targeting construct was linearized and electroporated into embryonic day (E)14.1 embryonic stem (ES) cells. Homologous recombinants were identified by PCR by using primers 1 and 2 (see Supporting Text) and confirmed by Southern blot. Two independent targeted ES clones were obtained and injected into BALB/c blastocysts. Both clones yielded chimeras that transmitted the mutant Pomt1 allele to their offspring. Heterozygous Pomt1+/– progeny were mated, and the offspring was genotyped by Southern blotting and PCR by using primers 3–5 (see Supporting Text). Similarly, embryos from timed pregnancies were genotyped by PCR.
Antibodies and Immunohistochemistry. Rabbit anti-laminin (Sigma) and rat monoclonal anti-entactin (ELM1; Abcam, Cambridge, U.K.) antibodies were used at 1:200 dilutions. Monoclonal anti-α-dystroglycan antibodies (VIA4–1, 1:100; and IIH6, 1:200), polyclonal anti-β-dystroglycan (AP83, 1:50), and anti-α-dystroglycan antibodies (GT20ADG, 1:15) were kindly provided by Kevin Campbell (University of Iowa, Iowa City).
Decidual sacs from wild-type and heterozygous crosses were collected at E6.5 and E7.5, fixed overnight at 4°C in 4% paraformaldehyde/PBS, and cryoprotected or embedded in paraffin. Staining was performed on 7-μm sections according to the standard protocol for the M.O.M. Basic kit (Vector Laboratories). Primary antibodies were applied in PBS/mouse blocking solution for 1 h at room temperature, and anti-rabbit-, anti-rat-Alexa488 (Molecular Probes, 1:1,000), anti-goat-Cy3 (Jackson ImmunoResearch, 1:100), and anti-mouse-biotin (Vector Laboratories, 1:200) secondary antibodies were applied in PBS/mouse blocking solution for 30 min at room temperature. If necessary, a 15-min incubation with streptavidin-Cy3 conjugate (Jackson ImmunoResearch, 1:1,000) followed. Sections were mounted with ProLong Antifade kit (Molecular Probes) and viewed with an epifluorescence Zeiss Axioskop microscope.
Laser-Capture Microdissection for Genotyping. Tissues from paraffin-embedded embryos were isolated by using a Zeiss Axiovert microscope equipped with a P.A.L.M. microbeam unit (P.A.L.M. Microlaser Technologies, Bernried, Germany). Microdissected material was lysed for 3 h at 55°C in catapult buffer containing 0.5 M EDTA, pH 8.0; 1 M Tris, pH 8.0; 0.5% Igepal CA-630; and 0.2 mg/ml Proteinase K. The heat-inactivated lysate was used as template for PCR genotyping (see above).
Results
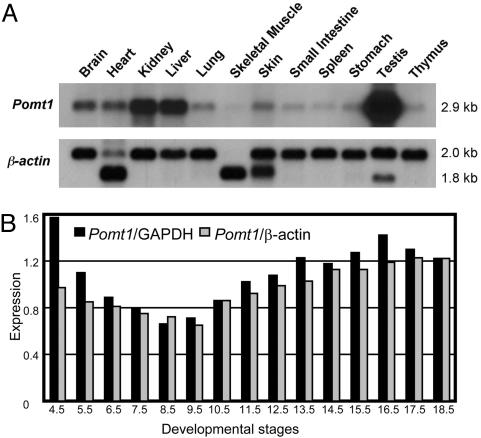
Characterization of the Mouse Pomt1 Gene. We isolated the full-length Pomt1 cDNA (2,885 bp; GenBank accession no. AY494857) that corresponded to a predicted protein of 724 aa from a mouse embryo cDNA library. The Pomt1 gene extends over 18,416 bp of genomic DNA, and its organization into 20 exons is conserved between human and mouse (data not shown). The Pomt1 gene was localized to centimorgan (cM) 18 of mouse chromosome 2 in the EUCIB backcross (BSB), between the D2Mit120 and D2Mit152 markers, and to cM 20 between the Abl and Pbx3 genes in the BSS backcross panel from The Jackson Laboratory. This 2B chromosomal region had a conserved synteny with human chromosome 9q34.1 where the POMT1 gene maps. Northern blot analysis identified a single 2.9-kb transcript in all tissues analyzed. However, the expression levels varied from the lowest levels observed in skeletal muscle to high levels of expression in testis (Fig. 1A). The same blot hybridized with Pomt2 revealed a similar expression pattern (Fig. 6A, which is published as supporting information on the PNAS web site). A similar pattern of human POMT1 expression was found in adult tissues (Fig. 6B). Northern blot analysis with mRNA from different developmental stages revealed that Pomt1 is also expressed throughout embryogenesis (Figs. 1B and 6C). High levels of Pomt1 mRNA were seen at very early stages of development, but one must take care in interpreting these results because this RNA may be the product of contamination with maternal tissues (supplier's note, Seegene, Del Mar, CA). However, the expression of Pomt1 appeared to decrease slightly during gastrulation and to later increase at the onset of organogenesis at E9.5, suggesting an important role for Pomt1 throughout embryogenesis.
Fig. 1.
Expression of mouse Pomt1 gene. (A) Northern blot analysis of adult tissues hybridized with cDNA probes: Pomt1 (2.9 kb) and β-actin (2.0 and 1.8 kb). (B) Pomt1 expression during mouse embryogenesis. Original Northern blot is shown in Fig. 6C. Northern blot was normalized by using GAPDH (black columns) and β-actin (gray columns).
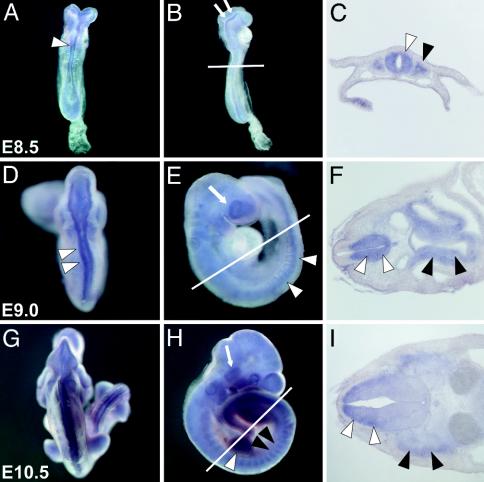
Mouse Pomt1 Is Expressed in WWS-Related Tissues During Early Embryogenesis. To examine the expression of Pomt1 during mouse embryogenesis in more detail, we performed whole-mount in situ hybridizations on embryos from different developmental stages (E7.5–10.5). At E7.5, Pomt1 transcripts could not be detected by in situ hybridization (data not shown). At later stages, low-level expression of Pomt1 mRNA was relatively ubiquitous, whereas higher levels of Pomt1 expression were observed in a specific and dynamic pattern. At E8.5, intense Pomt1 expression was detected in the neuronal tissues. Transcripts were found throughout the neural tube (Fig. 2 A and C) and in the dorsal aspects of the neural folds of the future midbrain region (Fig. 2B). In addition, Pomt1 was expressed in the somites (Fig. 2C). At E9.0, pronounced expression of Pomt1 transcripts was seen along the neural tube (Fig. 2 D and E) and in the developing eye (Fig. 2E). In cross sections of E9.0 embryos, Pomt1 transcripts were predominantly detected in the ventral domain of the neural tube, including the region from which motoneurons emerge. Furthermore, Pomt1 was expressed in the floor plate, notochord, and gut endoderm (Fig. 2F). At E10.5, Pomt1 transcripts were detected in the somites, the limb-bud mesenchyme, and the developing trigeminal ganglion (Fig. 2 G and H). In cross sections anterior to the forelimb bud, Pomt1 expression was observed in the mantle layer of the dorsal neural tube and the dermomyotome of the somites (Fig. 2I). This precise expression pattern suggests that Pomt1 is involved in the development of the muscles, the nervous system, and the eye, which is consistent with the neuronal, muscle, and eye abnormalities found in WWS patients (6).
Fig. 2.
Whole-mount expression of Pomt1 in mouse embryos. Whole-mount in situ hybridizations (A, B, D, E, G, and H) and representative cryosections (C, F, and I) through the anterior neural tube at the level of the forelimb bud (indicated by a white line in B, E, and H). (A–C) At E8.5, strong Pomt1 expression is found along the neural tube (white arrowhead) and in the dorsal aspects of the neural fold (arrows). Expression was also detected in the somites (black arrowhead). (D–F) At E9.0, strong expression was seen in the ventral part of the neural tube (white arrowheads), in the developing eye (white arrow), and in the gut endoderm (black arrowheads). (G and H) At E10.5, high levels of Pomt1 mRNA were detected in the somites (black arrowheads), limb buds (white arrowhead), and trigeminal ganglion (white arrow). (I) Pronounced Pomt1 expression in the mantle layer of the dorsal neural tube (white arrowheads), as well as in the dermomyotome (black arrowheads), was verified in the E10.5 section.
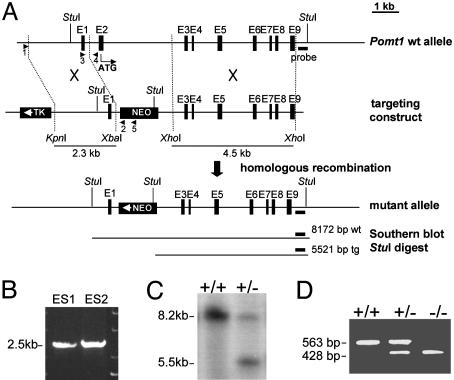
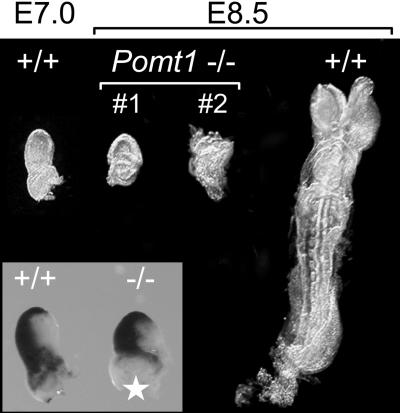
Loss of Pomt1 Results in Early Embryonic Lethality. The disruption of the Pomt1 gene was achieved by replacing exon 2, containing the start codon conserved among all vertebrates instead of the putative start codon located in exon 1, with a neomycin resistance gene (Fig. 3A). Successfully targeted clones were identified by PCR and Southern blotting (Fig. 3 B and C and data not shown). Two independent embryonic stem cell clones were used to generate chimeric mice that transmitted the mutant Pomt1 allele to their offspring. Pomt1+/– heterozygous mice developed normally and were fertile. The progeny of heterozygous intercrosses were 31% wild-type and 69% heterozygous Pomt1 (Table 1), an ≈1:2 ratio that is indicative of the Mendelian inheritance for a recessive embryonic lethal trait. To determine when homozygous mutant embryos die, we isolated embryos at different stages and genotyped them by PCR (Fig. 3D). Blastocysts at E3.5 were flushed from the uterus and genotyped directly or after in vitro culture. Homozygous mutant blastocysts with normal trophoblast outgrowth were indistinguishable from wild-type and heterozygous littermates (data not shown) and were identified in the expected Mendelian ratio of 1:2:1 (Table 1). Genotyping of embryos from E7.5 to E9.5 also revealed a 1:2:1 Mendelian ratio (Table 1), although Pomt1–/– embryos were significantly smaller and became increasingly disorganized with age (Fig. 4). Pomt1–/– embryos isolated at E8.5 displayed a variable degree of morphological abnormalities. Some mutant embryos appeared to remain at the egg-cylinder stage reminiscent of E6.5 embryos; others developed beyond that stage but appeared to suffer a later developmental block (Fig. 4, #1 and #2). Gastrulation did not seem to be impaired, because whole-mount in situ hybridization with brachyury revealed no obvious abnormalities (Fig. 4 Inset). Finally, Pomt1–/– embryos were resorbed during pregnancy, such that at E10.5, no tissue from homozygous mutant embryos could be recovered (Table 1). Taken together, these results suggest that Pomt1 fulfils a crucial function during early embryogenesis.
Fig. 3.
Targeted disruption of the Pomt1 gene. (A) Schematic representation of the targeting strategy: the genomic locus, the targeting construct, and the expected mutant Pomt1 allele after homologous recombination. Selectable markers: herpes simplex virus thymidine kinase gene (TK) and neomycin gene (NEO). The short (2.3-kb) and long (4.5-kb) arms for homologous recombination are represented. PCR primers are represented by arrows. (B) Primers 1 and 2 were used to identify two targeted embryonic stem clones after homologous recombination. (C) Southern blot analysis of genomic DNA from mouse tail tissue. Endogenous (8.2-kb) and targeted (5.5-kb) Pomt1 alleles. (D) PCR genotyping of embryos from timed matings. Primers 3 and 4 identify the endogenous allele, whereas primers 3 and 5 identify the targeted allele.
Table 1. Offspring and embryo genotypes from different heterozygous matings.
| Genotypes
|
||||
|---|---|---|---|---|
| Developmental stage | Total | +/+ | +/- | -/- |
| Adult mice | 81 | 25 (31%) | 56 (69%) | 0 |
| E10.5 | 38 | 15 | 23 | 0 |
| E9.5 | 48 | 8 | 32 | 8* |
| E8.5 | 44 | 9 | 24 | 11* |
| E7.5 | 26 | 7 | 12 | 7* |
| E3.5 (blastocysts) | 109 | 23 | 69 | 17 |
As development proceeded, Pomt1 null mutants showed progressive growth retardation and degradation.
Fig. 4.
Morphology of wild-type and Pomt1–/– embryos. Representative littermates from an E8.5 Pomt1 heterozygous intercross are shown. PCR genotyping confirmed Pomt1–/– null mutants. Pomt1-deficient embryos display severe growth retardation presumably due to a developmental block in E6–7. For size comparison, an additional E7.0 wild-type embryo is presented. (Inset) Whole-mount in situ hybridization with the gastrulation marker brachyury. The asterisk indicates a missing part of the extraembryonic ectoderm used for PCR genotypization.
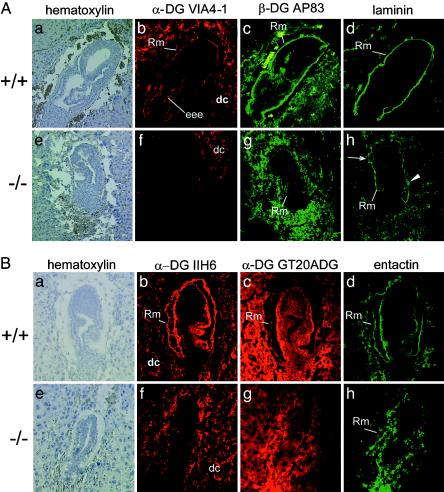
Loss of Pomt1 Results in the Disruption of Reichert's Membrane. One known protein substrate of POMT1 is α-DG. The posttranslational glycosylation of the α-DG protein, including the addition of O-mannosyl glycan chains, is critical in determining its ligand-binding properties (4, 17, 20). In mice, α-DG is a component of Reichert's membrane, one of the first basement membranes to form in the embryo (21, 22). We determined whether glycosylation of α-DG might be affected in Pomt1–/– embryos in genotyped sections from E7.5 homozygous embryos. In wild-type and heterozygous embryos, α-DG glyco-specific antibodies VIA4–1 and IIH6 mainly recognized Reichert's membrane and the maternal decidual cells surrounding the embryo (Fig. 5 Ab and Bb, and data not shown). In contrast, the α-DG glycoepitope was detected neither in Reichert's membrane nor in the embryonic tissue in sections of Pomt1–/– embryos (Fig. 5 Af and Bf), although the expression of this α-DG epitope could be observed in the surrounding maternal decidual cells.
Fig. 5.
Immunohistochemical characterization of extracellular matrix components in Pomt1–/– mutant embryos. Sagittal sections of paraffin-embedded E7.5 (A) or frozen E6.5 (B) embryos were stained with hematoxylin and analyzed with anti-α-DG antibodies directed against a glycoepitope (VIA4–1, IIH6) or the protein core (GT20ADG) and anti-β-DG (AP83), anti-laminin, and anti-nidogen/entactin antibodies, as indicated. Wild-type embryos (A and B a–d) and independent Pomt1 null mutants (A and Be–h) are shown. In Pomt1–/– embryos, the glycoepitope is missing in all embryo-derived cellular structures, although it is still present in the decidual cells. Discontinuous (arrows) and patchy (arrowhead) laminin and nidogen/entactin staining of the mutant embryos indicates a defect in the formation of Reichert's membrane. Genotypes were determined by PCR genotyping of laser-captured material. Rm, Reichert's membrane; eee, extraembryonic ectoderm; dc, maternal decidual cells.
These data suggested that, in vivo, POMT1 contributes to the major mannosyltransferase activity required for the incorporation of O-mannosyl glycans into α-DG. Immunofluorescence with antibodies directed against the protein core of the α- and β-subunit of dystroglycan, respectively, revealed a reduction of both DG proteins in Reichert's membrane in Pomt1 null mutants (Fig. 5 Ac, Ag, Bc, and Bg), indicating incorrect maturation or targeting due to hypoglycosylation.
The absence of O-mannosyl glycans and the reduced level of DG proteins might affect the distribution of laminin and thus the formation of basement membranes such as Reichert's membrane. Laminin, as well as the laminin-binding protein nidogen/entactin (23), was readily detected in the Reichert's membrane of wild-type embryos (Fig. 5 Ad and Bd). In Pomt1–/– embryos, the levels of laminin and nidogen were significantly reduced, and the distribution was disrupted, becoming discontinuous and in parts patchy (Fig. 5 Ah and Bh).
In summary, the targeted deletion of Pomt1 in mice impairs basement membrane formation, manifested by the disruption of Reichert's membrane. The failure of this membrane to protect the embryo is reflected by the fact that maternal red blood cells can be found in the yolk sacs of Pomt1 null mutant embryos (data not shown).
Discussion
We have generated a knockout mouse model of a known glycosyltransferase, Pomt1. Several glycosyltransferases are involved in CMDs in humans. Mutations in POMT1 are responsible for WWS, a severe recessive CMD that is combined with ocular and retinal abnormalities and brain defects that include type II lissencephaly (6). Children born with WWS generally do not survive past the age of 3 years. The expression of Pomt1 in the neural tube, dermomyotome, and developing eye during mouse embryonic development emphasizes the importance of Pomt1 in all of the tissues most affected in WWS patients. However, the early embryonic lethality observed in Pomt1–/– null mutants contrasts with the effects of the mutations found in WWS patients. The reason that the murine Pomt1–/– null mutation results in a notably more severe phenotype than the mutations seen in human WWS patients may well lie in the defects observed in Reichert's membrane. Although Reichert's membrane is a feature particular to rodent embryos (21, 22), α-DG glycosylation might be a general and crucial prerequisite for the correct development of basement membranes also in humans. This is supported by the glia limitans defects observed in WWS patients (6).
It is widely accepted that the carbohydrate moiety in general and O-mannosyl glycans in particular play a crucial role in determining the ligand-binding properties of α-DG. It has been reported that laminin binding to α-DG is reduced or almost completely lost in muscle tissue of WWS, muscle–eye–brain (MEB) disease, Fukuyama CMD (FCMD), and congenital muscular dystrophy 1C (MDC1C) patients (9, 17, 20). Furthermore, in vitro correct glycosylation of DG is crucial for protein processing and targeting to the cell surface (24). In vivo targeting defects of α- and β-DG due to hypoglycosylation are observed in nonmuscle tissue, such as brain of the spontaneous mouse mutant (myd), which represents a null mutation of the putative glycosyltransferase LARGE and shows a relevant CMD phenotype (20, 25).
Interestingly, we found a reduction of both dystroglycan subunits (α- and β-chains) in the Reichert's membrane of Pomt1–/– embryos, which obviously resulted in aberrant recruitment of laminin to Reichert's membrane. In the dystroglycan null mutant embryo, the lack of laminin leads to a loss of this membranes' integrity and causes embryonic lethality early in gastrulation (22). Thus, the lethal phenotype observed in the Pomt1 mutant embryos mimics the dystroglycan knockout model. Furthermore, by considering these data together, we assume that the early embryonic lethality observed in Pomt1 null mutant mice is due to the failure of Reichert's membrane to function correctly as a filter, allowing free access of nutrients to the embryo while excluding maternal cells (21).
Because Reichert's membrane is specific to rodents, it may be difficult to establish appropriate mouse models for glycosylation disorders associated with CMDs. One possible approach to circumvent this problem would be the use of tissue- and stage-specific gene disruption, as recently attempted for α-DG (26, 27). The conditional ablation of dystroglycan from the embryonic but not the extraembryonic tissues that synthesize the components of Reichert's membrane overcomes embryonic lethality. However, although live offspring are born, the pups are severely affected and poorly viable.** This provides further evidence that the formation of a functional Reichert's membrane at the egg cylinder stage is the bottleneck, which mutations in dystroglycan and/or in its modifying glycosyltransferases are unable to overcome. Indeed, another mouse mutant also suffers early embryonic lethality at around E6.5–7.5, in that the putative glycosyltransferase fukutin gene is disrupted (28). In humans, Fukutin mutations cause FCMD (8) and a more severe spectrum of WWS-like symptoms (29, 30).
It seems that POMT1 enzymatic activity is completely lost in Pomt1–/–-deficient embryos. In yeast, members of the PMT family form specific protein complexes (31), and it has been suggested that in mammals, both POMT1 and POMT2 have to be present to confer maximal activity (15). Our Northern blot data support this suggestion, because both Pomt1 and Pomt2 have similar expression patterns. This may explain why residual POMT2 activity cannot compensate for POMT1 deficiency. The binding of α-DG to its ligands (e.g., laminin α2) is mediated via its sialyl-terminated carbohydrate chains (3, 32). Approximately 70% of these chains are of the O-mannosyl type, whereas the rest are mucin-type sugars initiated at an N-acetylgalactosamine sugar residue (33). Our data from mouse embryos, in conjunction with a previous study on human WWS muscle (17), emphasize that in nonmuscle tissue, O-mannosyl glycans are crucial for DG maturation and/or targeting to the cell surface, whereas in muscle, they mainly affect binding of α-DG to laminin. Neither the residual α-DG sugar moiety nor other laminin receptors like β1 integrin can compensate for the loss of O-mannosyl glycan-mediated binding (4). The recruitment of laminin to α-DG is critical for initial self assembly and cluster formation during basement membrane formation (34, 35). Laminin 1 is the major laminin found at early stages of basement membranes. The absence of the laminin 1 chain results in early embryonic death by E7 (36). Subsequently, such laminin clusters mediate the deposition of other structural extracellular matrix molecules, for example, nidogen/entactin or collagen IV (23).
Because α-DG is the best-characterized O-mannosylated protein to date, our data on Pomt1 expression, together with the knockout phenotype, seem to be directly related to α-DG function and the observed loss of its O-mannosyl sugar moieties. Besides the known function of O-mannosyl glycans in laminin binding, our study provides in vivo evidence that O-mannosylation is important for proper maturation and/or targeting of the protein to the cell surface. Taken together, our study demonstrates the essential role of O-mannosyl glycans not only in disease but also for early rodent development and the formation of early basement membranes.
Supplementary Material
Acknowledgments
We thank L. A. Pérez Jurado and A. Cortés for assistance with initial Pomt1 studies and knockout vector construction, respectively. We thank C. Endres, M. Priesmeier-Gradl, N. Rieger, M. Neumann, and N. Zink for excellent technical assistance and P. Renner for expert care of the animals. We are grateful to Kevin Campbell for generously providing antibodies. We thank R. Barresi for helpful discussions and M. Sefton for critical reading of the manuscript. This work was supported by grants from Spanish Comisión Interministerial de Ciencia y Tecnología, Fondo de Investigaciones Sanitarias, the Comunidad de Madrid, the Deutsche Forschungsgemeinschaft (Grant SFB521), Fond der Chemischen Industrie, and the German National Genome Research Network. B.P. is a Ciencia y Tecnología and Fondo de Investigaciones Sanitarias fellow, and J.M.F. and M.C.V. were Comunidad de Madrid fellows.
Abbreviations: CMD, congenital muscular dystrophy; DG, dystroglycan; PMT, protein O-mannosyltransferase; WWS, Walker–Warburg syndrome; En, embryonic day n.
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AY494857)
Footnotes
Barresi, R., Satz, J. S., Sawatzki, S. M., Durbeej, M., Cohn, R. D., Henry, M. D., Moore, S. A., Tallquist, M. D., Soriano, P. & Campbell, K. P. (2003) Mol. Biol. Cell 14, 390a (abstr.).
References
- 1.Strahl-Bolsinger, S., Gentzsch, M. & Tanner, W. (1999) Biochim. Biophys. Acta 1426, 297–307. [DOI] [PubMed] [Google Scholar]
- 2.Willer, T., Valero, M. C., Tanner, W., Cruces, J. & Strahl, S. (2003) Curr. Opin. Struct. Biol. 13, 621–630. [DOI] [PubMed] [Google Scholar]
- 3.Endo, T. & Toda, T. (2003) Biol. Pharmacol. Bull. 26, 1641–1647. [DOI] [PubMed] [Google Scholar]
- 4.Michele, D. E. & Campbell, K. P. (2003) J. Biol. Chem. 278, 15457–15460. [DOI] [PubMed] [Google Scholar]
- 5.Muntoni, F., Brockington, M., Torelli, S. & Brown, S. C. (2004) Curr. Opin. Neurol. 17, 205–209. [DOI] [PubMed] [Google Scholar]
- 6.Beltrán-Valero de Bernabé, D., Currier, S., Steinbrecher, A., Celli, J., van Beusekom, E., van der Zwaag, B., Kayserili, H., Merlini, L., Chitayat, D., Dobyns, W. B., et al. (2002) Am. J. Hum. Genet. 71, 1033–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoshida, A., Kobayashi, K., Manya, H., Taniguchi, K., Kano, H., Mizuno, M., Inazu, T., Mitsuhashi, H., Takahashi, S., Takeuchi, M., et al. (2001) Dev. Cell 1, 717–724. [DOI] [PubMed] [Google Scholar]
- 8.Hayashi, Y. K., Ogawa, M., Tagawa, K., Noguchi, S., Ishihara, T., Nonaka, I. & Arahata, K. (2001) Neurology 57, 115–121. [DOI] [PubMed] [Google Scholar]
- 9.Brockington, M., Blake, D. J., Prandini, P., Brown, S. C., Torelli, S., Benson, M. A., Ponting, C. P., Estournet, B., Romero, N. B., Mercuri, E., et al. (2001) Am. J. Hum. Genet. 69, 1198–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Longman, C., Brockington, M., Torelli, S., Jimenez-Mallebrera, C., Kennedy, C., Khalil, N., Feng, L., Saran, R. K., Voit, T., Merlini, L., et al. (2003) Hum. Mol. Genet. 12, 2853–2861. [DOI] [PubMed] [Google Scholar]
- 11.Strahl-Bolsinger, S., Immervoll, T., Deutzmann, R. & Tanner, W. (1993) Proc. Natl. Acad. Sci. USA 90, 8164–8168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martín-Blanco, E. & García-Bellido, A. (1996) Proc. Natl. Acad. Sci. USA 93, 6048–6052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Willer, T., Amselgruber, W., Deutzmann, R. & Strahl, S. (2002) Glycobiology 12, 771–783. [DOI] [PubMed] [Google Scholar]
- 14.Jurado, L. A., Coloma, A. & Cruces, J. (1999) Genomics 58, 171–180. [DOI] [PubMed] [Google Scholar]
- 15.Manya, H., Chiba, A., Yoshida, A., Wang, X., Chiba, Y., Jigami, Y., Margolis, R. U. & Endo, T. (2004) Proc. Natl. Acad. Sci. USA 101, 500–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sabatelli, P., Columbaro, M., Mura, I., Capanni, C., Lattanzi, G., Maraldi, N. M., Beltran-Valero de Barnabe, D., van Bokoven, H., Squarzoni, S. & Merlini, L. (2003) Biochim. Biophys. Acta 1638, 57–62. [DOI] [PubMed] [Google Scholar]
- 17.Kim, D. S., Hayashi, Y. K., Matsumoto, H., Ogawa, M., Noguchi, S., Murakami, N., Sakuta, R., Mochizuki, M., Michele, D. E., Campbell, K. P., et al. (2004) Neurology 62, 1009–1011. [DOI] [PubMed] [Google Scholar]
- 18.Sporle, R. & Schughart, K. (1998) Dev. Genet. 22, 359–373. [DOI] [PubMed] [Google Scholar]
- 19.Tybulewicz, V. L., Crawford, C. E., Jackson, P. K., Bronson, R. T. & Mulligan, R. C. (1991) Cell 65, 1153–1163. [DOI] [PubMed] [Google Scholar]
- 20.Michele, D. E., Barresi, R., Kanagawa, M., Saito, F., Cohn, R. D., Satz, J. S., Dollar, J., Nishino, I., Kelley, R. I., Somer, H., et al. (2002) Nature 418, 417–421. [DOI] [PubMed] [Google Scholar]
- 21.Salamat, M., Miosge, N. & Herken, R. (1995) Anat. Embryol. 192, 275–281. [DOI] [PubMed] [Google Scholar]
- 22.Williamson, R. A., Henry, M. D., Daniels, K. J., Hrstka, R. F., Lee, J. C., Sunada, Y., Ibraghimov-Beskrovnaya, O. & Campbell, K. P. (1997) Hum. Mol. Genet. 6, 831–841. [DOI] [PubMed] [Google Scholar]
- 23.Timpl, R. & Brown, J. C. (1996) BioEssays 18, 123–132. [DOI] [PubMed] [Google Scholar]
- 24.Esapa, C. T., Bentham, G. R. B., Schroeder, J. E., Kroeger, S. & Blake, D. J. (2003) FEBS Lett. 555, 209–216. [DOI] [PubMed] [Google Scholar]
- 25.Grewal, P. K. & Hewitt, J. E. (2002) Biochim. Biophys. Acta 1573, 216–224. [DOI] [PubMed] [Google Scholar]
- 26.Moore, S. A., Saito, F., Chen, J., Michele, D. E., Henry, M. D., Messing, A., Cohn, R. D., Ross-Barta, S. E., Westra, S., Williamson, R. A., et al. (2002) Nature 418, 422–425. [DOI] [PubMed] [Google Scholar]
- 27.Cohn, R. D., Henry, M. D., Michele, D. E., Barresi, R., Saito, F., Moore, S. A., Flanagan, J. D., Skwarchuk, M. W., Robbins, M. E., Mendell, J. R., et al. (2002) Cell 110, 639–648. [DOI] [PubMed] [Google Scholar]
- 28.Takeda, S., Kondo, M., Sasaki, J., Kurahashi, H., Kano, H., Arai, K., Misaki, K., Fukui, T., Kobayashi, K., Tachikawa, M., et al. (2003) Hum. Mol. Genet. 12, 1449–1459. [DOI] [PubMed] [Google Scholar]
- 29.Silan, F., Yoshioka, M., Kobayashi, K., Simsek, E., Tunc, M., Alper, M., Cam, M., Guven, A., Fukuda, Y., Kinoshita, M., et al. (2003) Ann. Neurol. 53, 392–396. [DOI] [PubMed] [Google Scholar]
- 30.de Bernabé, D. B., van Bokhoven, H., van Beusekom, E., Van den Akker, W., Kant, S., Dobyns, W. B., Cormand, B., Currier, S., Hamel, B., Talim, B., et al. (2003) J. Med. Genet. 40, 845–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Girrbach, V. & Strahl, S. (2003) J. Biol. Chem. 278, 12554–12562. [DOI] [PubMed] [Google Scholar]
- 32.Winder, S. J. (2001) Trends Biochem. Sci. 26, 118–124. [DOI] [PubMed] [Google Scholar]
- 33.Sasaki, T., Yamada, K., Matsumura, T., Shimizu, T., Kobata, A. & Endo, T. (1998) Biochim. Biophys. Acta 1425, 599–606. [DOI] [PubMed] [Google Scholar]
- 34.Henry, M. D. & Campbell, K. P. (1998) Cell 95, 859–870. [DOI] [PubMed] [Google Scholar]
- 35.Henry, M. D., Satz, J. S., Brakebusch, C., Costell, M., Gustafsson, E., Fassler, R. & Campbell, K. P. (2001) J. Cell Sci. 114, 1137–1144. [DOI] [PubMed] [Google Scholar]
- 36.Miner, J. H., Li, C., Mudd, J. L., Go, G. & Sutherland, A. E. (2004) Development (Cambridge, U.K.) 131, 2247–2256. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.