Abstract
A study of 16 streams in eastern North America shows that riparian deforestation causes channel narrowing, which reduces the total amount of stream habitat and ecosystem per unit channel length and compromises in-stream processing of pollutants. Wide forest reaches had more macroinvertebrates, total ecosystem processing of organic matter, and nitrogen uptake per unit channel length than contiguous narrow deforested reaches. Stream narrowing nullified any potential advantages of deforestation regarding abundance of fish, quality of dissolved organic matter, and pesticide degradation. These findings show that forested stream channels have a wider and more natural configuration, which significantly affects the total in-stream amount and activity of the ecosystem, including the processing of pollutants. The results reinforce both current policy of the United States that endorses riparian forest buffers as best management practice and federal and state programs that subsidize riparian reforestation for stream restoration and water quality. Not only do forest buffers prevent nonpoint source pollutants from entering small streams, they also enhance the in-stream processing of both nonpoint and point source pollutants, thereby reducing their impact on downstream rivers and estuaries.
Deforestation, which annually averaged ≈14.6 million hectares (ha) worldwide between 1990 and 2000 (1), will continue as long as humans assign a higher value to wood products and agriculture than to “ecosystem services” (2) provided by the forest, such as watershed protection, wildlife conservation, and carbon sequestration (3). The deforestation of riparian areas not only reduces wildlife habitat and corridors but also directly impacts the stream itself by lowering water and habitat quality due to (i) loss of woody debris, leaf litter, and dissolved organic carbon inputs (4); (ii) lack of shade, which causes very high levels of photosynthetically active radiation (5), solar UV radiation (6), and temperature (7); and (iii) less buffering against nonpoint source pollutants (8). Although the deforestation that denuded most of eastern North America in the 19th century was reversed in upland areas decades ago, debate continues about whether riparian areas of that region and elsewhere should remain treeless (9, 10). Although recent U.S. legislation (11) has emphasized the use of forested buffers to keep nonpoint source pollutants out of streams (8), grass buffers can also intercept pollutants (12). Ultimately, the debate may turn on how buffers affect the structure and function of the stream itself and especially its ability to impede the downstream transport of pollutants to larger rivers, estuaries, and oceans. Here we test the hypothesis that the narrowing of small streams caused by riparian deforestation leads to a significant decline in (i) the amount and functional quality of stream ecosystem and (ii) the ability of that ecosystem to process water pollutants.
The conceptual basis of our hypothesis is that unnatural channel narrowing caused by riparian deforestation results in less wetted bottom (i.e., benthic) habitat per unit of channel length, increased water velocity, and lower bed roughness. By reducing the total amount of benthic stream ecosystem per unit of channel length, these physical changes compromise in-stream ecosystem function and the processing of pollutants. Our idea builds on an earlier hypothesis that riparian deforestation lowers water and habitat quality in streams (13) and on scientific research that has demonstrated more biological and biogeochemical activity on or in the bottoms of small streams than in their water columns (14). We show that, when averaged across many streams, important ecosystem services and both structural and functional ecosystem parameters (e.g., levels of nitrogen and phosphorus processing, dissolved organic matter processing, pesticide degradation, net stream metabolism, and the abundance of macroinvertebrates and fish) in forested reaches equaled or exceeded those in deforested reaches per unit of length of stream.
Methods
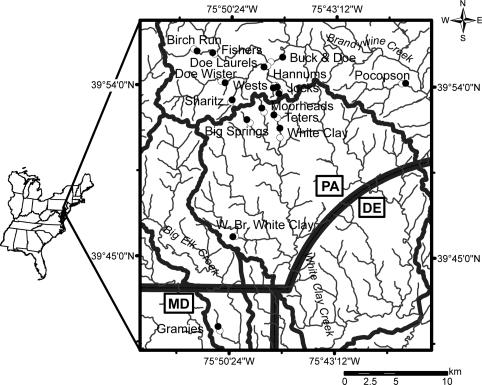
We studied contiguous (paired) forested and deforested reaches of 16 temperate streams in rural Piedmont watersheds in eastern North America (Fig. 1). Streams ranged from first to fifth order, with watershed areas of 0.1–123 km2. The forested reaches were upstream from the deforested reach at 11 sites and downstream at 5. Similar topographic gradients and riparian soils and lack of tributaries characterized most pairs of reaches. To avoid factors that might confound the primary study variable (presence or absence of forest), all deforested reaches lacked the typical anthropogenic disturbance common in the region (e.g., disturbance from equine, bovine, or row crop agriculture or urbanization). We studied geomorphology, biodegradable dissolved organic matter (BDOM), macroinvertebrates, and fish in all 16 streams. Because of time and budget constraints, we studied gross primary production (GPP), community respiration (CR), nutrient processing, and pesticide degradation in 8–14 streams. We sampled macroinvertebrates five times a year and BDOM, GPP, CR, nutrient processing, and pesticide degradation twice (summer and winter, with multiple measurements per season for GPP and CR). We measured all other parameters once and used quantitative methods (see below) to study most parameters.
Fig. 1.
Name of stream and location of paired study reaches in southeastern Pennsylvania (PA) and northern Maryland (MD). •, forested reach; ○, deforested reach.
Geomorphology. A global positioning system with differential correction was used to locate the top and bottom of each of the experimental reaches, which were ≈100–200 m in length. A laser level was used to quantify the longitudinal profile of each reach. Along this profile, we measured bankfull elevation, channel bottom and water surface elevations, width of the water surface, and width of bank at 10-m intervals as well as at important features, such as top of riffle, top of pool, and deepest point in pool. At every third equal interval (i.e., 30, 60, 90 m, etc., from the top of the reach), we used a laser level to survey a detailed channel cross-section orthogonal to the flow. Stream substratum particle size distribution was quantified by using a variation of the Wolman pebble count technique based on a sample of at least 200 particles. To better understand fluvial processes in forested versus deforested stream reaches, we computed bankfull channel roughness estimates by using a technique developed to provide a systematic approach for estimating Manning's n for natural channels (15).
Nutrient Uptake. The uptake of ammonium (NH3) and orthophosphate (PO4) was estimated as the longitudinal uptake rate, kl, or inverse of uptake length, by whole-stream nutrient amendments to 10 of the paired forested and deforested sites. All sites were studied in the cold season (late fall through early spring) and 6 of the 10 sites were also studied in the warm season (spring through early fall). The median background concentration in the study streams for ammonium was 15 μg/liter (range: 3–44 μg/liter) and for phosphate (as soluble reactive phosphorus) was 12 μg/liter (range: 4–35 μg/liter). Experiments involved metering dissolved ammonium and phosphate (together with bromide as a conservative tracer) into the stream at the upstream limit of each study reach at a rate sufficient to raise the concentration of each nutrient ≈30 μg/liter for 25–70 min. The actual median level of elevation for ammonium was 36 μg/liter (range: 17–83 μg/liter) and for phosphate was 28 μg/liter (range: 7–73 μg/liter). Forested and deforested reaches received separate injections. The longitudinal uptake rate, kl, was estimated by nonlinear regression from the relation r(x) = r0exp(–kl x), where r(x) is the background-corrected nutrient/bromide ratio, measured during the maximum, or plateau, concentration, at downstream distance x, and r0 is the estimated ratio at x = 0, the point of injection.
Pesticides. In-stream experiments regarding the transformation of herbicides (atrazine and linuron) and insecticides (dursban and methoxychlor) were performed in forested and deforested reaches of 10 streams ranging from first to fourth order. Stream water was collected from study reaches, filtered (Whatman GF/F), and dry-spiked (e.g., no solvent carrier) with pesticides before exposure. Experiments consisted of placing sealed quartz tubes (60-ml capacity) filled with the stream water/pesticide mixture into the stream by attaching them at various depths to frames inserted into the streambed. Tubes were exposed subsurface for 2–4 d. This method assured that degradation processes involving hydrolytic, microbial, and photooxidation processes occurred under ambient conditions of temperature and light. Dark and sterile controls were subtracted from total exposure results to determine contributions from photolysis and microbial transformation processes, respectively. The remainder was taken as chemical degradation (predominantly hydrolysis). Triplicate samples, blanks, and freshly spiked laboratory controls were extracted by using liquid–liquid extraction and three aliquots of methylene chloride. Concentrated extracts were analyzed by gas chromatography/MS by using either a Hewlett Packard model 5890 GC/5988MS or an Agilent model 6890GC/5973MS. For analysis, degradation per unit of channel length was calculated as follows: DL = 1/k [v · 3.6] –1 with k = ln [Ai/Af]/t where DL is the degradation length (km), v is velocity (m·s–1), k is degradation rate (h–1), Ai is the initial pesticide concentration, Af is the final pesticide concentration, and t is time (h).
Dissolved Organic Matter (DOM). In-stream DOM dynamics were studied by direct measurements of the baseflow concentrations of dissolved organic carbon (DOC) in both forested and deforested reaches. Stream samples collected for DOC were filtered (Whatman GF/F), preserved with sodium azide (antimicrobial agent), and analyzed by platinum-catalyzed persulfate oxidation. Biodegradable DOC was used in this study as a surrogate for BDOM. For each reach, 10 subsamples (organic, C-free, 40-ml vials) of the filtered water were prepared. Five vials were fixed with sodium azide, and the DOC level was measured within 7 d. The five other vials were incubated in the dark at room temperature for 28 d to allow the bacterial inoculum contained in the filtered water to grow and metabolize the DOC. After 28 d, the five subsamples were refiltered and analyzed for DOC. Biodegradable DOC was calculated as the difference between the initial and final DOC concentrations. All samples were processed with either an OI 700 or OI 1010 analyzer (OI Analytical, College Station, TX). Analysis involved the ratio of biodegradable DOC to DOC, which is reported as the percentage of BDOM and interpreted as an index of DOM quality or biological lability.
Stream Metabolism. At most sites, metabolism was studied twice during both cold and warm seasons by making open-system measurements of dissolved O2 concentration over diel periods for 3–7 days. Sondes (model 600XL, Yellow Springs Instruments) equipped with dissolved O2, temperature, and conductivity probes, were deployed at the top and bottom of each 150- to 275-m-long study reach. Photosynthetically active radiation was measured every 10 sec above water near the sondes by using quantum sensors (Li-Cor, Lincoln, NE). Reaeration coefficients were determined from a propane evasion experiment performed once during each measurement period, in which concentrations of the gas and conservative tracer (bromide) were determined at 50-m intervals over the downstream distance. Propane was analyzed by using capillary gas chromatography with He as a carrier, and bromide was analyzed by using ion chromatography (Dionex DX-500). The mean propane/bromide ratio at each sampling station was plotted against downstream distance, and a curve was fit by using nonlinear regression. The proportion of propane lost per meter was multiplied by water velocity and 1.39 (to correct for molecular size) and 60 (s·min–1) to generate a KO2 for the reach. Stream width and depth were measured every 20 m at the time metabolism measures were made to account for seasonal variability. Rate of change curves of dissolved O2 concentrations were developed for each diel period. Depending on experimental and site conditions, data were analyzed by using (i) the two-station approach with reaeration from propane evasion (71% of the reach/season combinations); (ii) the two-station approach with reaeration estimated by using either the surface renewal model of Owens or the energy dissipation model of Tsivoglou and Neal (i.e., hydraulic-geomorphic approach; 6% combinations); (iii) the single station approach with reaeration from propane (13% combinations); or (iv) the single station with hydraulic-geomorphic approach for reaeration (10% combinations).
Macroinvertebrates. Surber samples (0.092 m2; 250-μm mesh) were collected at each of the 16 study streams (i.e., 32 forested and deforested reaches) five times during the year (March, May, July, September, and November). Riffle and pool habitats were sampled separately in each reach. For each habitat, a total of eight samples were taken at random. The samples were then combined at random into two groups of four samples each. Each of these groups was then subsampled by one-fourth to provide the equivalent of one composited Surber sample. Each composited sample was preserved in the field in 5% buffered formalin. Thus, two such composited samples were processed for riffle and pool habitats in each reach. Each preserved, composited macroinvertebrate sample was split into four (or factors of four, e.g., 1/4, 1/16, 1/64, or 1/256) subsamples for final processing. The actual size of the subsample depended on the number of individuals in the sample, with a target of 100–300 macroinvertebrates to be processed per subsample. Macroinvertebrates were identified to the species level or to the lowest taxonomic level possible (usually at least genus). For analysis, the macroinvertebrate abundance data for riffles and pools were weighted according to the relative abundance of each of these habitats in the study streams.
Fish. Sampling for fish occurred in late fall so that collections represent standing crop at the end of the main period of productivity. Fish were sampled by the depletion (removal) method. The length of stream sampled was at least 11 times the width of the stream for most sites. For each reach, block seines were used to keep fish from migrating into or out of the reach during the study. Each reach was electroshocked from bank to bank in a downstream to upstream direction (three to four passes per reach) by using a single backpack electroshocker. An exception was the largest site where a shore-based 5,000-W generator was used in addition to the backpack shocker, and only two passes were made per reach. For each pass, all fish were collected live and placed immediately in buckets for subsequent analysis of species identification, length, and wet mass. Maximum likelihood estimates of the abundance of size classes of species were made. Fish were weighed either individually (as needed to obtain a length–weight relationship) or in groups. Data were standardized to the area sampled (n/100 m2; using stream widths measured at the time of sampling) and length of shoreline sampled (n/100 m). The total number of pieces of woody debris >0.1 m in diameter and 1 m in length were counted within the bankfull channel in each study reach.
Unless otherwise stated, the statistical comparison of all parameters was done with two-way (stream × reach) ANOVA or a paired t test. Data for each parameter are presented graphically as a ratio of the mean forest reach value divided by the mean deforested reach value, with values <1 transformed by the negative inverse to keep graphs symmetrical.
Results
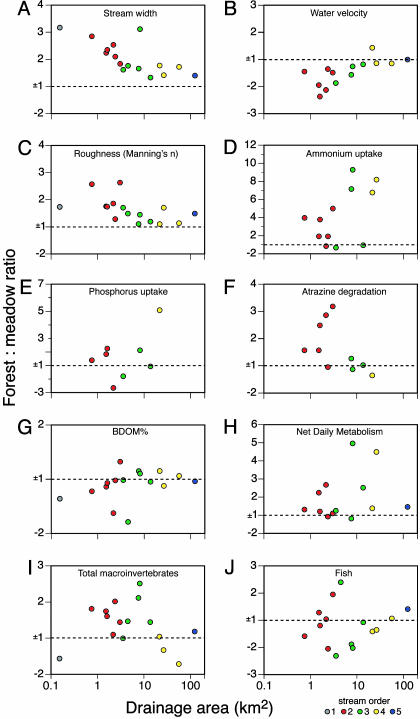
Forested stream channels were wider (Fig. 2A) and had lower average water velocity (Fig. 2B) and higher bed roughness (Fig. 2C) than adjacent deforested channels (P < 0.05 for each comparison). Based on these results, we tested all remaining study parameters on a per-unit-stream-length basis (i.e., lineal rather than areal density or functional output per unit of length rather than per unit of area). This approach enabled our analysis to account for differences in channel width between forested and deforested reaches as we evaluated ecosystem structure and function.
Fig. 2.
The ratio of key physical, chemical, and biological measures obtained from adjacent forested and deforested reaches of Piedmont streams in eastern North America. Streams varied from first to fifth order, with watershed areas ranging from 0.1 to 123 km2. Ratios <1 were transformed by the negative inverse to keep the graphs symmetrical. (A) Wetted stream width (meters) at baseflow. (B) Water velocity (m·s–1). (C) Streambed roughness (Manning's n). (D) Ammonium uptake (per meter). (E) Phosphorus uptake (per meter). (F) Atrazine degradation (per kilometer). (G) BDOM (%). (H) NDM (gm O2·m–1·d–1). (I) Macroinvertebrates (n/meter). (J) Fish (n/meter).
Nitrogen (as ammonium) and phosphorus (as orthophosphate) uptake and degradation of pesticides were studied as representative and important measures of stream ecosystem services. To minimize the saturating effect of nutrient amendment, we kept nitrogen and phosphorus additions low relative to ambient levels and consistent across streams (16). There was no seasonal effect on ammonium uptake per unit length (P > 0.05), but the reach effect was highly significant (P = 0.004), with uptake in forested reaches dramatically exceeding that in deforested reaches (Fig. 2D). For phosphorus, there was no significant effect of either season or reach (Fig. 2E). Total water-column degradation (microbial, photolysis, and hydrolysis) of two herbicides (atrazine and linuron) and two insecticides (dursban and methoxychlor) was also measured. Because results were similar for all four compounds, we show data only for atrazine and give numerical results for the others below.¶ Although the degradation ratio was greater in the forested reach for the majority of streams (Fig. 2F), the amount of degradation per unit of channel length did not differ significantly between forested and deforested reaches, regardless of season.
We also evaluated the amount and quality of ecosystem parameters in the paired reaches. Although these parameters can and probably should also be viewed as measures of ecosystem services, we distinguish them here from nutrient and pesticide processing mainly in a regulatory sense (i.e., legal limits, total maximum daily loads, or specific guidelines do not exist for them). For BDOM, we focused on differences in quality rather than quantity (i.e., percentage BDOM). We found no significant season or reach effect for the percentage of BDOM (Fig. 2G). We measured net daily metabolism (NDM), which reflects the balance between autotrophic and heterotrophic processes in the ecosystem, as the difference between GPP and CR (i.e., NDM = GPP – CR). Results indicated no seasonal effect but significantly more negative (heterotrophic) rates of NDM per unit of length in the forested reaches (Fig. 2H).
For consumer organisms, we observed a significantly greater abundance of macroinvertebrates per unit of channel length in forested reaches than in adjacent deforested reaches when averaged across all seasons and sites (Fig. 2I). There was no significant difference in the total abundance of fish per unit of channel length between the study reaches (Fig. 2 J).
Discussion
Our study shows that most forested stream channels studied in the Piedmont region were wider and had lower average water velocity and higher bed roughness than adjacent deforested channels. Given that our geomorphological measurements were made at numerous points along each study reach, our conclusions are robust and apply to the entire reach and all in-stream habitats. For these streams, narrowing was caused by bank encroachment due to herbaceous plants (mostly grasses), which are shaded out under forest cover (13, 14). This same process has been described in urban watersheds in the region (17). Another study has also demonstrated that forested stream reaches exhibit 20–33% slower channel migration and lower floodplain accretion rates of sediment and thereby provide more stability than deforested channels (18). Thus, forested stream channels have significantly more benthic habitat and seem to be more stable than their deforested counterparts.
Our results show that the increased channel width in forested reaches (and, hence, more streambed area per unit length of stream) plays a critical role in the nutrient dynamics of these first-through fourth-order streams. It is known that dissolved nutrients are removed primarily by sorption onto bottom sediments or active uptake by microbial communities attached to bottom substrata (19–21). Although the ammonium uptake per unit of area did not differ between forested and deforested reaches, the amount taken up per unit of channel length was significantly greater (often by 2- to 10-fold) in the forested reaches of most streams. Although the phosphorus uptake per unit of area was actually higher in the deforested reaches (probably because of biotic and sorptive uptake because of greater amounts of algae and fine sediments), there was no significant difference in phosphorus uptake between forested and deforested reaches on a per-unit-of-channel-length basis. The nutrient uptake results seem to reflect a tradeoff, with greater benthic surface area in forested reaches more than compensating for equal (N) or higher (P) uptake rates per unit of area in deforested reaches. For nitrogen, the greater uptake in the forested reaches slows its downstream transport and significantly increases the possibility of its removal from the stream, either directly through denitrification (22) or indirectly through trophic links with terrestrial habitats (23). These findings suggest that restoring riparian forests can affect the transport of nitrogen to large rivers and estuaries. Hence, we propose amplifying a recent conclusion that “restoration and preservation of small stream ecosystems should be a central focus of management strategies to ensure maximum nitrogen processing in watersheds” (19) to include small stream ecosystems and their riparian forests.
Small streams are also sites where toxic organic chemicals can degrade before being exported from watersheds. Although we expected a priori greater degradation in the deforested reaches because of the higher light∥ and photolysis, the amount of degradation per unit of channel length did not differ significantly between forested and deforested reaches, regardless of season, for all four pesticides evaluated. Moreover, photooxidation was less important than microbial and hydrolytic processes with regard to degradation of the pesticides in this study (L.J.S., unpublished data). This unexpected outcome is likely due to the fact that (i) stream narrowing reduces the amount of bottom habitat (and associated microbial biofilm) available per unit length of stream for degradative processes and (ii) high water velocities associated with channel narrowing cause pesticide molecules to travel significantly faster through deforested reaches, which reduces exposure time for microbial, hydrolytic, and photolytic processes. These same phenomena may also partially explain the lack of significant differences in levels of BDOM between forested and deforested reaches despite the fact that one would have expected a priori that the DOM in deforested reaches would be more labile or biodegradable because of more exudates from higher standing stocks of algae and higher photolytic conversion of nonlabile DOM compounds (24). These data suggest that both the additional contact area and time associated with the wider forested channels play an important role in the in-stream processing of both nutrients and other substances (natural and toxic) in small streams.
We also evaluated the amount and quality of the organic components of the ecosystem itself in the paired reaches. The natural food base of small Piedmont streams consists largely of coarse particulate organic matter (largely wood, leaf litter, seeds, and fruits), fine particulate organic matter (leaf fragments and soil organic matter), DOM, and algae and bacteria (14). We did not measure coarse particulate organic matter as a primary study variable because previous studies in the region (13), including one at our sites, demonstrated that the standing stock of coarse particulate organic matter per unit channel area or length was orders of magnitude greater in forested reaches than in adjacent deforested reaches, even when the deforested reach was downstream from the forested reach. However, we did measure the number of pieces of large woody coarse particulate organic matter as an ancillary habitat variable in this project and found the number to be significantly higher in the forested reaches [average (SE): 24.6 (3.0) versus 3.6 (0.9)]. We also measured NDM as an integrated assessment of total metabolic activity of the ecosystem in the study reaches. Results show that forested streams usually had significantly greater rates of heterotrophy (i.e., NDM was more negative per unit of length than deforested reaches). In fact, of the 13 streams studied for this parameter, annual mean NDM was 200–500% more negative in forested reaches in five streams and 20–200% times more negative in another five. Thus, forested reaches seem to process significantly greater amounts of organic matter per unit channel length than deforested reaches, a service whose value seems underestimated and often overlooked.
In our quantitative assessment of primary (macroinvertebrates) and secondary (fish) consumer organisms, we observed a greater abundance of macroinvertebrates per unit channel length in forested reaches than in adjacent deforested reaches when averaged across all seasons and sites. This increase included the abundance of disturbance-intolerant groups like mayflies and stoneflies, which suggests a more natural ecosystem. Because deforestation often increases macroinvertebrate abundance per unit of area (ref. 25 and this study), the greater abundance of macroinvertebrates in forested reaches reflects the fact that they are almost exclusively benthic, and forested channels have significantly more (and perhaps more natural) benthic habitat per unit of channel length. In contrast, the lack of a significant difference in the total abundance of fish per unit of channel length likely reflects their mobility and their pelagic (rather than benthic) nature and the increased cover provided in pools. We have shown elsewhere that fish species richness also did not differ between reaches (26). Similar fish responses have also been observed in urban watersheds (18). In addition, we know that our deforested study reaches were short, lessening their overall impact on the fish [e.g., in another study (27), fish abundance and community structure was insensitive to deforestation <1 km in extent].
Unlike conventional stream ecosystem studies, which compare activity on a per-unit-area basis, we analyzed the amount of stream ecosystem and its activity per unit of channel length. We did so because (i) stream width responds significantly to riparian vegetation, and (ii) stream ecosystem services (processing of organic matter, nutrients, and pollutants) are delivered on a per-unit-of-stream-length basis. Moreover, the structure and function of downstream ecosystems are affected more by the total amount of inputs of dissolved or particulate materials from upstream than their generation rate per unit area upstream. The previous lack of emphasis on reach analysis accrues because we currently lack a scaling factor for stream size that would allow direct and equitable comparison of data per unit of reach analogous to comparisons made between streams on a per-unit-of-area basis. Nevertheless, our data show that forested streams had significantly higher levels than their deforested counterparts of key ecosystem components (macroinvertebrates), ecosystem processing of organic matter (NDM), and delivery of an important ecosystem service (nitrogen processing) known to be related to ecosystem activities and processes. Moreover, forested streams had an equivalent amount of all other important ecosystem components (BDOM and fish) and a greater-than-expected delivery of an ecosystem service (pesticide degradation) that is closely tied to physical (water velocity and benthic surface area), chemical (hydrolysis), and biological (microbial) factors. Because the Piedmont was historically forested, we assume that forested stream channels and their ecosystems are closer to a natural state than their deforested counterparts. Our study suggests that approaching that natural state may translate into a higher delivery of certain services needed by humans, which supports an earlier contention that “natural is best” with regard to streambank vegetation (10).
Our results undoubtedly underestimate the degree to which deforested streams in the study region deviate from those in a natural or quasi-natural state with regard to the integrity of the ecosystem and its ability to deliver ecosystem services. This is because (i) to avoid factors confounding the main study variables (presence or absence of forest), we chose deforested reaches lacking activities commonly associated with them that cause stream degradation (i.e., in-stream and stream-bank disturbance from watering or grazing activity of animal herds and chemical and sediment inputs associated with streamside farming and urbanization); (ii) we know that our deforested study reaches were short (<1.5 km), lessening their overall impact (e.g., fish); and (iii) we did not measure or report a number of important parameters that have consistently proved damaging to deforested streams [namely, impacts of increased diel, seasonal, and annual temperature (28), increased exposure to UV radiation (29), and less input of leaf litter and large woody debris (14) and terrestrial invertebrates (30)].
For White Clay Creek, which is one of our study watersheds and part of the U.S. National Wild and Scenic River System, total riparian deforestation would result in at least 50% less benthic stream habitat (due to channel narrowing), compared with total reforestation (13). Based on our data here, the uptake of nitrogen, and hence its potential loss from White Clay Creek through denitrification (22) and/or export to the terrestrial food web (23), would be similarly reduced. To deliberately design such a consequence might be deemed illegal. Yet the impact of stream narrowing continues unnoticed and unregulated in many watersheds in a region where each linear meter of stream helps to reduce the transport of contaminants to the rivers that feed the Chesapeake Bay, the largest estuary in the U.S., and the Delaware Bay.
Additionally, if our results were scaled up to the watershed level, they could significantly affect economic analyses (31) that weigh the loss of nonmarketed services of riparian forests (e.g., water quality and quantity, flood regulation, erosion control, recreation, carbon stocks, endangered species, wildlife corridors, etc.) against the marketed benefits of deforestation (e.g., timber harvest and dairy, beef, and row-crop production). This is particularly important for watersheds within the Chesapeake Bay drainage area, for which there is a target of a 40% reduction in nitrogen inputs by the year 2010 (32). Recent analyses of tropical upland forests have shown that the losses of nonmarketed services consistently outweigh the benefits of deforestation (33). We predict a similar outcome for riparian forests.
Our findings suggest that the fragmented landscape (<50% forested) created by humans in the Piedmont and elsewhere has produced a correspondingly fragmented condition for the resident streams: Reaches that function naturally (or at least quasinaturally) are separated by unnatural and often dysfunctional reaches, with a net loss of in-stream ecosystem services. More work is clearly needed to determine, e.g., how the net functional response (additive) of two streams of equal length, one forested and one deforested, would scale relative to having both streams in a fragmented (forested-deforested patches) state. Regardless, our data clearly support the concept of preserving and restoring riparian forests along as many stream reaches as possible in the Piedmont and other landscapes, especially those that were historically forested. The results strengthen current U.S. policy that endorses riparian forest buffers as best management practice (32), as well as federal (11) and state (34) programs that subsidize riparian reforestation for stream restoration and water quality purposes. Not only do forest buffers prevent nonpoint source pollutants from entering small streams, they also enhance the in-stream processing of both nonpoint and point source pollutants, thereby reducing their impact on downstream rivers and estuaries. The concept that riparian forest restoration plays a significant role in helping to abate point source pollution in small streams is new and greatly expands the notion of riparian forest buffers as best management practice.
The link among riparian vegetation, channel geomorphology, ecosystem function, and stream ecosystem services had been neither intuitively obvious nor scientifically measured. Demonstrating the increased value of riparian forest “services” relative to forest “products” could significantly change economic analyses (33) and lead to a reduction of riparian deforestation for profit, an increase in landowner perceptions of the value of riparian forests, and a corresponding decrease in the need for external incentives for landowner cooperation (35). Our data should also enhance public appreciation of stream ecosystem services, which should “help promote connections between science and management” of aquatic ecosystems (36). Moreover, the potential application of our data are extensive because they are based on small to intermediate streams, which represent >90% of the total stream lengths in most watersheds and play a major role in collecting, processing, and exporting nutrients to estuaries and oceans (19).
Finally, because the phenomenon of stream narrowing in eastern North America has been observed in other landscapes [e.g., elsewhere in North America (37, 38), Australia (39), and New Zealand (40)], we hypothesize that the results of our study have significant global implications, both for cost-benefit analyses and best-management practices for riparian habitat and for public policies affecting water quality.
Acknowledgments
We thank the following colleagues who over the years have contributed ideas and/or helped develop the original project: Janet Johnson, James Finley, Caren Glotfelty, Cecilia Ferreri, and Robin L. Vannote. We acknowledge the following technicians for invaluable field and laboratory assistance: Bernard Anderson, Martin Bernstein, Greg Coffman, David Funk, Linda Fuselier, Dave Gartner, Mike Gentile, Karen Jansson, Tom Johnson, William Kintzer, Dave Leib, Dave Montgomery, Maeve McBride, Claire Murray, John O'Brien, Paul Overbeck, Sally Peirson, Dave Rebuck, Sherman Roberts, Brad Thompson, Bruce Wallace, and Roberta Weber. The following student interns also helped implement the project: Michael Dadd, Kevin Fryberger, Noah Goodman, Sean Gorby, Denise Henry, Michael Hubbs, Lisa Johnson, Brent Kane, Susan Kenneally, Glynis Lough, Diane Macheski, Thomas K. O'Donnell, Megan Ritchie, Autumn Sabo, Jenny Schill, Rachael Sororuf, Susan Still, Aura Thompson, Elena Tkacz, Tom Trolley, Theresa Trovitch, Elizabeth Vogle, Vitaly Volfson, Matt Wolf, Sarah Wolf, Natalie Williams, and Susan Wychowanec. We especially thank David Funk and Charlie Dow for figure preparation and Jamie Blaine for final manuscript editing. This work was supported by National Science Foundation/Environmental Protection Agency Water and Watersheds Grant DEB-9613588, the Stroud Foundation, and the Pennswood Endowment Fund of the Stroud Water Research Center.
Abbreviations: ha, hectare; DOM, dissolved organic matter; BDOM, biodegradable DOM; GPP, gross primary production; DOC, dissolved organic carbon; CR, community respiration; NDM, net daily metabolism.
Footnotes
The forest/deforest ratio of pesticide degradation per kilometer of stream was as follows (listed in order of increasing watershed area): Dursban (1.3, 1.7, 2.2, 2.3, 1.1, 2.3, 1.2, –1.0, 1.3, –1.4); Linuron (1.4, 1.7, 2.3, 2.4, –1.0, 2.4, 1.3, –1.1, –1.1, –1.4), and Methoxychlor (1.2, 1.6, 2.2, 2.3, 1.1, 2.2, 1.4, 1.0, 1.2, –1.4).
In this study, UV light levels were not measured. Photosynthetically active radiation, which also contributes to photolysis, was significantly higher in deforested reaches than in forested reaches at most study streams during both the cold and warm seasons of the year. Mean (SD) daily photosynthetically active radiation (mol quanta m–2·day–1) values for the study sites were as follows: cold season, forest reach: 15.8 (7.8) and deforested reach: 8.3 (3.4); warm season, forest reach: 24.4 (8.6) and deforested reach: 4.1 (2.9).
References
- 1.Food and Agricultural Organization of the United Nations (2001) State of the World's Forests (Food and Agricultural Organization, Rome).
- 2.Daily, G. & Ellison, K. (2001) The New Economy of Nature (Island Press, Washington, DC).
- 3.Bonnie, R., Schwartzman, S., Oppenheimer, M. & Bloomfield, J. (2000) Science 288, 1763–1764. [DOI] [PubMed] [Google Scholar]
- 4.Castelle, A. J. & Johnson, A. W. (2000) Bulletin no. 799 (National Council for Air and Stream Improvement, Research Triangle Park, NC).
- 5.Rutherford, J. C., Davies-Colley, R. J., Quinn, J. M., Stroud, M. J. & Cooper, A. B. (1999) Stream Shade: Towards a Restoration Strategy (Department of Conservation Report, Wellington, New Zealand).
- 6.Kelly, D. J., Bothwell, M. L. & Schindler, D. W. (2003) Ecology 84, 2724–2740. [Google Scholar]
- 7.Johnson, S. L. & Jones, J. A. (2000) Can. J. Fish. Aquat. Sci. 57, 1–10. [Google Scholar]
- 8.Lowrance, R., Altier, L. S., Newbold, J. D., Schnabel, R. R., Groffman, P. M., Denver, J. M., Correll, D. L., Gilliam, J. W., Robinson, J. L., Brinsfield, R. B., et al. (1997) Environ. Manage. 21, 687–712. [DOI] [PubMed] [Google Scholar]
- 9.Lyons, J., Trimble, S. W. & Paine, L. K. (1997) J. Am. Water Resour. Assoc. 36, 919–930. [Google Scholar]
- 10.Montgomery, D. R. (1997) Nature 388, 328–329. [Google Scholar]
- 11.U.S. Dept. of Agriculture (2002) Farm Security and Rural Investment Act of 2002, Public Law 107-171 (107th Congress, 2nd session).
- 12.Lee, K. H., Isenhart, T. M. & Schultz, R. C. (2003) J. Soil Water Cons. 58, 1–7. [Google Scholar]
- 13.Sweeney, B. W. (1993) Proc. Acad. Nat. Sci. Phila. 144, 291–340. [Google Scholar]
- 14.Allan, J. D. (1995) Stream Ecology (Chapman & Hall, London).
- 15.Arcement, G. J. & Schneider, V. R. (1989) USGS Survey Water Supply Paper 2339, 1–38. [Google Scholar]
- 16.Mulholland, P. J., Tank, J. L., Webster, J. R., Bowden, W. B., Dodds, W. K., Gregory, S. V., Grimm, N. B., Hamilton, S. K., Johnson, S. L., Marti, E., et al. (2002) J. North Am. Benthol. Soc. 21, 544–560. [Google Scholar]
- 17.Hession, W. C., Pizzutto, J. E., Johnson, T. E. & Horwitz, R. J. (2003) Geology 31, 147–150. [Google Scholar]
- 18.Hession, W. C., Johnson, T. E., Charles, D. F., Horwitz, R. J., Kreeger, D. A., Velinsky, D. J., Pizzuto, J. E., Marshall, B. D. & Newbold, J. D. (2003) in Protection and Restoration of Urban and Rural Streams, eds. Clar, M. L., Carpenter, D. D., Gracie, J. N. & Slate, L. O. (Am. Soc. Civil Eng., Reston, VA), pp. 373–382.
- 19.Peterson, B. J., Wollheim, W. M., Mulholland, P. J., Webster, J. R., Meyer, J. L., Tank, J. L., Marti, E., Bowden, W. B., Valett, H. M., Hershey, A. E., et al. (2001) Science 292, 86–90. [DOI] [PubMed] [Google Scholar]
- 20.Mulholland, P. J. & Hill, W. R. (1997) Water Resour. Res. 33, 1297–1306. [Google Scholar]
- 21.Mulholland, P. J., Elwood, J. W., Newbold, J. D., Ferren, L. A. & Webster, J. R. (1985) Ecology 66, 1012–1023. [Google Scholar]
- 22.Laursen, A. E. & Seitzinger, S. P. (2002) Hydrobiologia 485, 67–81. [Google Scholar]
- 23.Sabo, J. L. & Power, M. E. (2002) Ecology 83, 1860–1869. [Google Scholar]
- 24.Kaplan, L. A. & Newbold, J. D. (2003) in Aquatic Ecosystems: Interactivity of Dissolved Organic Matter, eds. Findlay, S. E. & Sinsabaugh, R. L. (Academic, New York), pp. 97–119.
- 25.Inoue, M. & Nakano, S. (2001) Ecol. Res. 16, 233–247. [Google Scholar]
- 26.Horwitz, R. J., Hession, W. C. & Sweeney, B. W. (2000) Proc. Amer. Water Resources Assoc. Int. Conf. on Riparian Ecology and Management in Multi-Land Use Watersheds (Am. Water Resources Assoc., Middleburg, VA), pp. 197–202.
- 27.Jones, E. B. D., III, Helfman, G. S., Harper, L. O. & Bolstad, P. V. (1999) Conserv. Biol. 13, 1454–1464. [Google Scholar]
- 28.Abell, R. & Allan, J. D. (2002) Verh. Int. Ver. Theor. Angew. Limnol. 28, 232–237. [Google Scholar]
- 29.Bothwell, M. L., Sherbot, D. M. & Pollock, C. M. (1994) Science 265, 97–100. [DOI] [PubMed] [Google Scholar]
- 30.Kawaguchi, Y. & Nakano, S. (2001) Freshwater Biol. 46, 303–316. [Google Scholar]
- 31.Loomis, J., Kent, P., Strange, L., Fausch, K. & Covich, A. (2000) Ecol. Econ. 33, 103–117. [Google Scholar]
- 32.Chesapeake Bay Commission (2002) Chesapeake 2000 Agreement U.S.E.P.A. (Chesapeake Bay Program, Annapolis, MD).
- 33.Balmford, A., Bruner, A., Cooper, P., Costanza, R., Farber, S., Green, R. E., Jenkins, M., Jefferiss, P., Jessamy, V., Madden, J., et al. (2002) Science 297, 950–953. [DOI] [PubMed] [Google Scholar]
- 34.Pennsylvania Conservation Reserve Enhancement Program (2003) Addendum Agreement: Commonwealth of PA, USDA, Commodity Credit Corp.
- 35.Kline, J. D., Alig, R. J. & Johnson, R. L. (2000) Ecol. Econ. 33, 29–43. [Google Scholar]
- 36.Palmer, M., Bernhardt, E., Chornesky, E., Collins, S., Dobson, S., Duke, C., Gold, B., Jacobson, R., Kingsland, S., Kranz, R., et al. (2004) Science 304, 1251–1252. [DOI] [PubMed] [Google Scholar]
- 37.Zimmerman, C. J., Goodlet, C. & Comer, G. H. (1967) in Symposium on River Morphology (Int. Assoc. Hydrol. Sci.), pp. 255–275.
- 38.Trimble, S. W. (1997) Geology 25, 467–469. [Google Scholar]
- 39.Campbell, I. C. (1993) in Ecology and Management of Riparian Zones in Australia (Land and Water Research and Development Corporation, Marcoola, Queensland, Australia), pp. 21–30.
- 40.Davies-Colley, R. J. (1997) N. Z. J. Mar. Freshwater Res. 31, 599–608. [Google Scholar]