Abstract
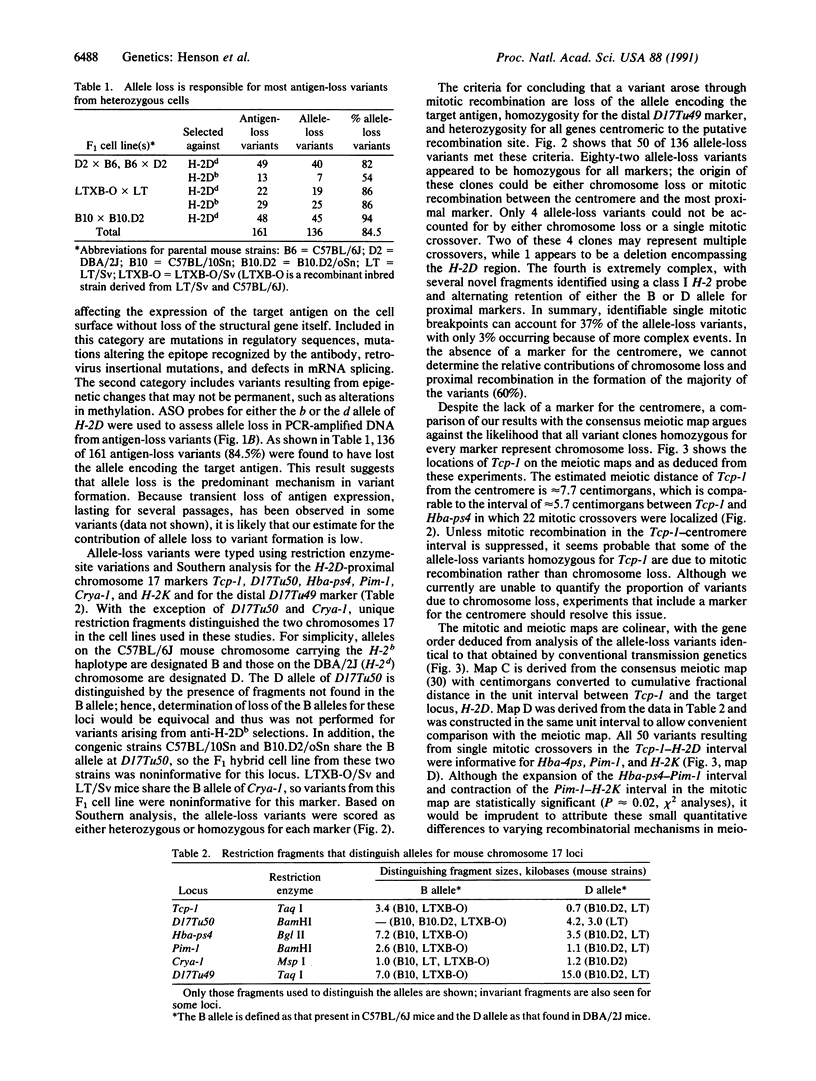
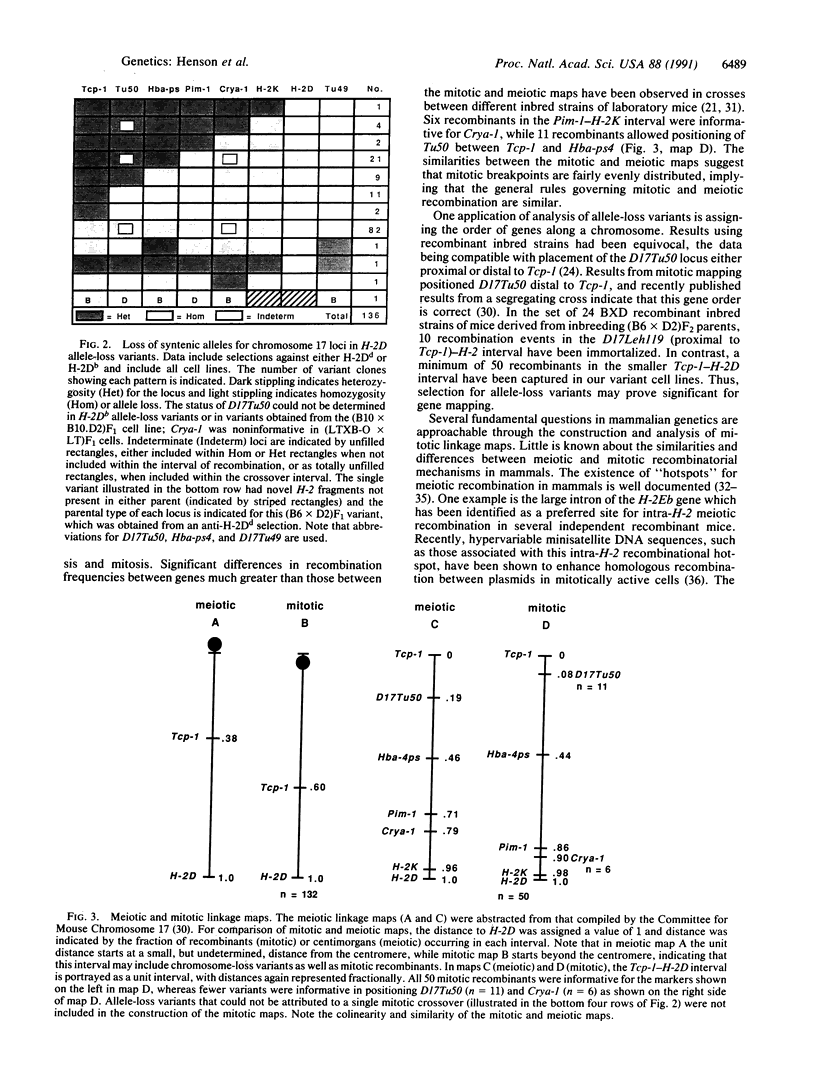
Loss of heterozygosity is a significant oncogenetic mechanism and can involve a variety of mechanisms including chromosome loss, deletion, and homologous interchromosomal mitotic recombination. Analysis of H-2 antigen-loss variants from heterozygous murine cell lines provides an experimental system to estimate the relative contributions of different mechanisms for allele loss and to compare the chromosomal patterns of mitotic and meiotic recombination. Cytotoxic anti-H-2D antibodies and complement were used to isolate 161 independent target antigen-negative clones from H-2d/H-2b heterozygous cell lines; of these, 131 (84.5%) lost the allele encoding the target antigen. Allele-loss variants were typed and scored as either heterozygous or homozygous for six H-2D-proximal chromosome 17 markers and for one distal marker by restriction enzyme-site variations and Southern analysis. A single mitotic crossover could account for 50 clones (37%), with heterozygosity for at least one proximal marker and loss of heterozygosity for all markers distal to the putative recombination site. Eighty-two allele-loss variants (60%) were homozygous for all markers; the origin of these clones could be either chromosome loss or mitotic recombination between the centromere and the most proximal marker. Only 4 clones (3%) arose through more complex events such as multiple crossovers or deletion. A mitotic linkage map for mouse chromosome 17 was constructed, and the gene order deduced from somatic recombination was identical to that obtained by conventional transmission genetics. These results demonstrate that mitotic recombination is a common event leading to allele loss, in spite of the lack of evidence for frequent somatic pairing of homologous chromosomes. Mitotic mapping provides a defined system for comparison of mitotic and meiotic recombination and may lead to practical advances for elucidating somatic mechanisms of oncogenesis and for gene therapy in targeting mutations to specific sites through homologous recombination.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bremner R., Balmain A. Genetic changes in skin tumor progression: correlation between presence of a mutant ras gene and loss of heterozygosity on mouse chromosome 7. Cell. 1990 May 4;61(3):407–417. doi: 10.1016/0092-8674(90)90523-h. [DOI] [PubMed] [Google Scholar]
- Carlson G. A., Goodman P. A., Lovett M., Taylor B. A., Marshall S. T., Peterson-Torchia M., Westaway D., Prusiner S. B. Genetics and polymorphism of the mouse prion gene complex: control of scrapie incubation time. Mol Cell Biol. 1988 Dec;8(12):5528–5540. doi: 10.1128/mcb.8.12.5528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavenee W. K., Dryja T. P., Phillips R. A., Benedict W. F., Godbout R., Gallie B. L., Murphree A. L., Strong L. C., White R. L. Expression of recessive alleles by chromosomal mechanisms in retinoblastoma. 1983 Oct 27-Nov 2Nature. 305(5937):779–784. doi: 10.1038/305779a0. [DOI] [PubMed] [Google Scholar]
- Cuypers H. T., Selten G., Quint W., Zijlstra M., Maandag E. R., Boelens W., van Wezenbeek P., Melief C., Berns A. Murine leukemia virus-induced T-cell lymphomagenesis: integration of proviruses in a distinct chromosomal region. Cell. 1984 May;37(1):141–150. doi: 10.1016/0092-8674(84)90309-x. [DOI] [PubMed] [Google Scholar]
- Edelmann W., Kröger B., Goller M., Horak I. A recombination hotspot in the LTR of a mouse retrotransposon identified in an in vitro system. Cell. 1989 Jun 16;57(6):937–946. doi: 10.1016/0092-8674(89)90332-2. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fulop G. M., Phillips R. A. The scid mutation in mice causes a general defect in DNA repair. Nature. 1990 Oct 4;347(6292):479–482. doi: 10.1038/347479a0. [DOI] [PubMed] [Google Scholar]
- Hasenkrug K. J., Cory J. M., Stimpfling J. H. Monoclonal antibodies defining mouse tissue antigens encoded by the H-2 region. Immunogenetics. 1987;25(2):136–139. doi: 10.1007/BF00364282. [DOI] [PubMed] [Google Scholar]
- Jackson J. A., Fink G. R. Gene conversion between duplicated genetic elements in yeast. Nature. 1981 Jul 23;292(5821):306–311. doi: 10.1038/292306a0. [DOI] [PubMed] [Google Scholar]
- James C. D., Carlbom E., Nordenskjold M., Collins V. P., Cavenee W. K. Mitotic recombination of chromosome 17 in astrocytomas. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2858–2862. doi: 10.1073/pnas.86.8.2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLEIN E., KLEIN G., HELLSTROM K. E. Further studies on isoantigenic variation in mouse carcinomas and sarcomas. J Natl Cancer Inst. 1960 Aug;25:271–294. [PubMed] [Google Scholar]
- KLEIN E., KLEIN G. STUDIES ON THE MECHANISM OF ISOANTIGENIC VARIANT FORMATION IN HETEROZYGOUS MOUSE TUMORS. III. BEHAVIOR OF H-2 ANTIGENS D AND K WHEN LOCATED IN THE TRANS POSITION. J Natl Cancer Inst. 1964 Mar;32:569–578. [PubMed] [Google Scholar]
- KLEIN E. Studies on the mechanism of isoantigenic variant formation in heterozygous mouse tumors. I. Behavior of H-2 antigens D and K: quantitative absorption tests on mouse sarcomas. J Natl Cancer Inst. 1961 Nov;27:1069–1093. [PubMed] [Google Scholar]
- Keil R. L., Roeder G. S. Cis-acting, recombination-stimulating activity in a fragment of the ribosomal DNA of S. cerevisiae. Cell. 1984 Dec;39(2 Pt 1):377–386. doi: 10.1016/0092-8674(84)90016-3. [DOI] [PubMed] [Google Scholar]
- Klein J., Figueroa F., David C. S. H-2 haplotypes, genes and antigens: second listing. II. The H-2 complex. Immunogenetics. 1983;17(6):553–596. doi: 10.1007/BF00366126. [DOI] [PubMed] [Google Scholar]
- Kobori J. A., Strauss E., Minard K., Hood L. Molecular analysis of the hotspot of recombination in the murine major histocompatibility complex. Science. 1986 Oct 10;234(4773):173–179. doi: 10.1126/science.3018929. [DOI] [PubMed] [Google Scholar]
- Lew A. M., Lillehoj E. P., Cowan E. P., Maloy W. L., van Schravendijk M. R., Coligan J. E. Class I genes and molecules: an update. Immunology. 1986 Jan;57(1):3–18. [PMC free article] [PubMed] [Google Scholar]
- Lichter P., Cremer T., Borden J., Manuelidis L., Ward D. C. Delineation of individual human chromosomes in metaphase and interphase cells by in situ suppression hybridization using recombinant DNA libraries. Hum Genet. 1988 Nov;80(3):224–234. doi: 10.1007/BF01790090. [DOI] [PubMed] [Google Scholar]
- Luria S. E., Delbrück M. Mutations of Bacteria from Virus Sensitivity to Virus Resistance. Genetics. 1943 Nov;28(6):491–511. doi: 10.1093/genetics/28.6.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon M. F., Zenthon J., Evans E. P., Burtenshaw M. D., Willison K. R. Extent of the mouse t complex and its inversions shown by in situ hybridization. Immunogenetics. 1988;27(5):375–382. doi: 10.1007/BF00395134. [DOI] [PubMed] [Google Scholar]
- Morris R. E., David C. S., Manhart M. D. Tumor and histocompatibility antigen interaction: direct visualization by a double-label bridging technique for immuno-electron microscopy. Mol Immunol. 1982 Aug;19(8):973–982. doi: 10.1016/0161-5890(82)90305-4. [DOI] [PubMed] [Google Scholar]
- Nelson F. K., Frankel W., Rajan T. V. Mitotic recombination is responsible for the loss of heterozygosity in cultured murine cell lines. Mol Cell Biol. 1989 Mar;9(3):1284–1288. doi: 10.1128/mcb.9.3.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozato K., Mayer N., Sachs D. H. Hybridoma cell lines secreting monoclonal antibodies to mouse H-2 and Ia antigens. J Immunol. 1980 Feb;124(2):533–540. [PubMed] [Google Scholar]
- Panthier J. J., Guénet J. L., Condamine H., Jacob F. Evidence for mitotic recombination in Wei/+ heterozygous mice. Genetics. 1990 May;125(1):175–182. doi: 10.1093/genetics/125.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter T. A., Zeff R. A., Frankel W., Rajan T. V. Mitotic recombination between homologous chromosomes generates H-2 somatic cell variants in vitro. Proc Natl Acad Sci U S A. 1987 Mar;84(6):1634–1637. doi: 10.1073/pnas.84.6.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raizis A. M., Becroft D. M., Shaw R. L., Reeve A. E. A mitotic recombination in Wilms tumor occurs between the parathyroid hormone locus and 11p13. Hum Genet. 1985;70(4):344–346. doi: 10.1007/BF00295375. [DOI] [PubMed] [Google Scholar]
- Rajan T. V., Halay E. D., Potter T. A., Evans G. A., Seidman J. G., Margulies D. H. H-2 hemizygous mutants from a heterozygous cell line: role of mitotic recombination. EMBO J. 1983;2(9):1537–1542. doi: 10.1002/j.1460-2075.1983.tb01620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves R. H., Crowley M. R., O'Hara B. F., Gearhart J. D. Sex, strain, and species differences affect recombination across an evolutionarily conserved segment of mouse chromosome 16. Genomics. 1990 Sep;8(1):141–148. doi: 10.1016/0888-7543(90)90236-n. [DOI] [PubMed] [Google Scholar]
- Rosenberg N., Baltimore D., Scher C. D. In vitro transformation of lymphoid cells by Abelson murine leukemia virus. Proc Natl Acad Sci U S A. 1975 May;72(5):1932–1936. doi: 10.1073/pnas.72.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuler W., Weiler I. J., Schuler A., Phillips R. A., Rosenberg N., Mak T. W., Kearney J. F., Perry R. P., Bosma M. J. Rearrangement of antigen receptor genes is defective in mice with severe combined immune deficiency. Cell. 1986 Sep 26;46(7):963–972. doi: 10.1016/0092-8674(86)90695-1. [DOI] [PubMed] [Google Scholar]
- Shiroishi T., Hanzawa N., Sagai T., Ishiura M., Gojobori T., Steinmetz M., Moriwaki K. Recombinational hotspot specific to female meiosis in the mouse major histocompatibility complex. Immunogenetics. 1990;31(2):79–88. doi: 10.1007/BF00661217. [DOI] [PubMed] [Google Scholar]
- Uematsu Y., Kiefer H., Schulze R., Fischer-Lindahl K., Steinmetz M. Molecular characterization of a meiotic recombinational hotspot enhancing homologous equal crossing-over. EMBO J. 1986 Sep;5(9):2123–2129. doi: 10.1002/j.1460-2075.1986.tb04475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincek V., Kawaguchi H., Mizuno K., Zaleska-Rutczynska Z., Kasahara M., Forejt J., Figueroa F., Klein J. Linkage map of mouse chromosome 17: localization of 27 new DNA markers. Genomics. 1989 Nov;5(4):773–786. doi: 10.1016/0888-7543(89)90119-5. [DOI] [PubMed] [Google Scholar]
- Wahls W. P., Wallace L. J., Moore P. D. Hypervariable minisatellite DNA is a hotspot for homologous recombination in human cells. Cell. 1990 Jan 12;60(1):95–103. doi: 10.1016/0092-8674(90)90719-u. [DOI] [PubMed] [Google Scholar]
- Weiss E. H., Golden L., Fahrner K., Mellor A. L., Devlin J. J., Bullman H., Tiddens H., Bud H., Flavell R. A. Organization and evolution of the class I gene family in the major histocompatibility complex of the C57BL/10 mouse. Nature. 1984 Aug 23;310(5979):650–655. doi: 10.1038/310650a0. [DOI] [PubMed] [Google Scholar]
- Zimmerer E. J., Ogin E., Shreffler D. C., Passmore H. C. Molecular mapping of crossover sites within the I region of the mouse MHC. Analysis of ten recombinant chromosomes. Immunogenetics. 1987;25(4):274–276. doi: 10.1007/BF00404701. [DOI] [PubMed] [Google Scholar]



