Abstract
Activation of the ARF–p53 tumor suppressor pathway is one of the cell's major defense mechanisms against cancer induced by oncogenes. The ARF–p53 pathway is dysfunctional in a high proportion of human cancers. The regulation of the ARF–p53 signaling pathway has not yet been well characterized. In this study polyoma virus (Py) is used as a tool to better define the ARF–p53 signaling pathway. Py middle T-antigen (PyMT) induces ARF, which consequently up-regulates p53. We show that Py small T-antigen (PyST) blocks ARF-mediated activation of p53. This inhibition requires the small T-antigen PP2A-interacting domain. Our results reveal a previously unrecognized role of PP2A in the modulation of the ARF–p53 tumor suppressor pathway.
The ARF–p53 tumor suppressor pathway is one of the cell's major defenses against stimulation of uncontrolled cell division induced by activated cellular and viral oncogenes (1–5). ARF and/or p53 are mutated in >70% of human cancers. The inappropriate activation of growth promoting cellular signaling pathways by oncogenes can result in the induction of ARF. Expression of ARF can activate p53 leading to apoptotic cell death or cell-cycle arrest. The mechanisms by which the ARF–p53 pathway is modulated remain to be precisely elucidated.
The small DNA viruses have provided great insight into a number of the cellular signaling pathways that they modulate. During their infectious cycle they overcome cell regulatory control systems and activate signaling pathways required for viral DNA synthesis and virus assembly. Unscheduled activation of these cellular growth signals activates ARF and, consequently, p53, leading to abortive infection. To counteract this outcome, most DNA viruses encode proteins that inhibit p53 function. Most of these viral proteins target p53 directly. For example, the SV40 large T-antigen binds to p53 and inhibits its transcription-factor activity (6–8). Adenovirus E1B 55K and HPV E6 bind to p53 and, in coordination with other proteins, block its function and cause its degradation (9–15).
Polyoma virus (Py) is unusual in that none of its early region proteins, large T-antigen (PyLT), middle T-antigen (PyMT), and small T-antigen (PyST), bind to p53 (16). We have previously shown that the Py oncogene PyMT activates ARF and p53 and therefore will not transform primary mouse cells and REF52 cells that contain an intact ARF–p53 pathway (17). PyMT transforms these cell types when either p53 or ARF is inactivated (17, 18). PyMT will also transform primary mouse and REF52 cells in which PyLT and PyST are also expressed (19, 20). In these transformed cells, wild-type ARF is abundantly expressed, but the level of wild-type p53 is low (17, 18). This finding suggests that PyLT and/or PyST prevent ARF from activating p53. In this paper, we identify and define which Py protein and function is involved in abrogating this critical regulatory step.
Materials and Methods
Cell Culture and Transfection. REF52 and Rat-1 are established lines of rat embryo fibroblasts and are described in refs. 17, 18, and 21. The DNp53REF52 cell line was established after infection of REF52 cells with pBabehygro-p53302–390 (18). The PyREF52 and bc1051 REF52 cells were generated after transfection with pAT153 plasmids containing wild-type Py or the bc1051 mutant (18, 22). All cell lines were routinely cultured in Dulbecco's modified Eagle's medium supplemented with 10% FCS, 100 mg/ml penicillin, and 100 mg/ml streptomycin. All transfections were carried by using 2 μg of DNA per well of a six-well plate using FuGENE 6 (Roche Diagnostics), according to the manufacturer's instructions. Six hours after transfection, the cells from each transfected well were transferred to three 60-cm Petri dishes in which the medium was changed every 3–4 days. After 10–12 days of growth in the case of the transfected Rat-1 and DNp53REF52 cells and after ≈20 days in the case of the transfected REF52 cells, the cells were stained with 0.5% crystal violet in 10% methanol, and the number of transformed foci was assessed.
Generation of J Domain and PP2A Mutants. The generation and properties of the k45Q and Del 42HPDKGG47 J domain mutants and the Ins 107AL PP2A-domain mutant are described in ref. 23. These mutations were incorporated into pAT153 plasmids containing either bc1051 genomic DNA or PyST cDNA by using the QuikChange Mutagenesis Kit (Stratagene), according to the manufacturer's instructions.
Western Blot Analysis of Protein. Cells were lysed directly from culture in Nonidet P-40 lysis buffer (1% Nonidet P-40/20 mM Tris·HCl, pH 7.5/150 mM NaCl/25 mM NaF/1 mM EDTA/Complete Mini protease inhibitor mixture). Protein concentrations were determined by using the Lowry Protein Assay and DC Protein Assay Reagents (Bio-Rad). All protein samples were loaded for SDS/PAGE onto 4–20% gradient Tris-glycine gels, except the samples to be analyzed for ARF, which were separated by using 16% Tris-glycine gels (Invitrogen). Proteins were immediately transferred to poly(vinylidene difluoride) membrane (Fisher) by using the Bio-Rad Mini Trans-Blot system for 90 min at 90 V. The membrane was blocked by using 5% milk (Bio-Rad) in Tris-buffered saline with Tween 20 (TBST) for an overnight incubation at 4°C with gentle shaking. The membranes were probed by using the following antibodies: PYC for the Py T-antigens, rabbit polyclonal 821B6 for ARF, β-actin (Sigma), p21 mAb (Pharmingen), 2A9, 2A10, and 4B2 for MDM2, and CM5 for p53 (NovoCastra, Newcastle, U.K.). All antibodies were incubated in 5% milk/TBST for 1.5 h with gentle shaking at room temperature. The membranes were then rinsed with TBST and incubated with their appropriate secondary antibody, rat, rabbit, goat, or mouse conjugated to horseradish peroxidase (Jackson ImmunoResearch), which were diluted in 5% milk/TBST for 1 h with gentle shaking at room temperature. Detection was preformed by using ECL reagents (Amersham Biosciences), according to manufacturer's protocol, with Hyperfilm MP (Amersham Biosciences).
Immunofluorescence. Cells were plated in six- or eight-well Lab-Tek slides (Nalge-Nunc) and allowed to grow to desired confluence. They were fixed by using 4% paraformaldehyde (Electron Microscopy Science) for 1 h and subsequently permeabilized by using 1% Triton X-100 in PBS for 10 min. Blocking was preformed by using 5% donkey serum (Jackson ImmunoResearch) in PBS for 1 h. Primary antibodies were incubated for 1 h and diluted in 5% donkey serum. The 762 antibody (kind gift from Steve Dilworth, Imperial College, London) was used to detect the Py T-antigens, and the rabbit polyclonal 821B6 antibody was used to detect ARF. Cells were rinsed, the appropriate secondary antibodies (Molecular Probes) were applied, and the treated cells were incubated for 30 min. Antibodies were conjugated to fluorophores for immunofluorescent detection with a Leica DM RXA fluorescence microscope in conjunction with Zeiss software and lamps. Images were captured by using a Hamamatsu digital camera.
Results
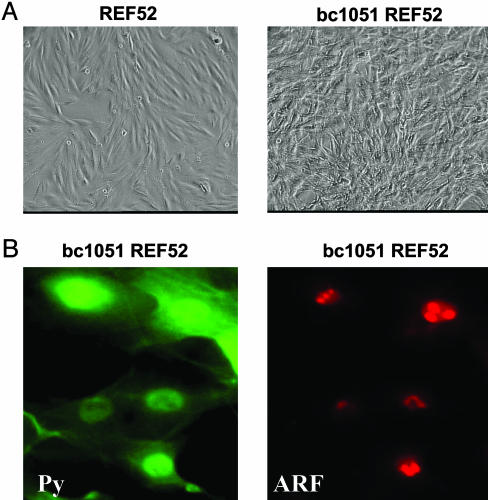
PyST Inhibits ARF Activation of p53. Initial experiments showed that PyLT does not complement PyMT in its ability to transform REF52 cells (data not shown). This finding was in agreement with previous findings that PyLT and PyMT together did not transform primary mouse cells (19, 20). Therefore, we assessed whether PyST could complement PyMT in the transformation of REF52 cells. We initially used the bc1051 Py mutant in which the splice acceptor of PyLT is destroyed resulting in loss of expression of PyLT without impairing the production and function of the PyMT and PyST proteins (22). REF52 cells were easily transformed by bc1051 genomic DNA (Fig. 1A), and these transformed cells, as expected, contained only the PyMT and PyST proteins (Figs. 1B and 2A). The bc1051-transformed REF52 cells (bc1051 REF52) were similar to REF52 cells transformed by the complete Py early region expressing PyLT, PyMT, and PyST (PyREF52 cells) in containing ARF (Figs. 1B and 2B) and low levels of p53 (Fig. 2C). The p53 in both the bc1051- and Py-transformed REF52 cells is functional because it could be stabilized and could activate its p21 and MDM2 downstream genes upon DNA damage with Adriamycin (Fig. 2C). The ARF in the bc1051-transformed REF52 cells was localized within nucleoli (Fig. 1B), as observed in PyREF52 cells (17).
Fig. 1.
ARF in transformed bc1051 (PyMT + PyST) REF52 cells is located in the nucleolus. (A) On the left is shown a photomicrograph of untransformed REF52 cells that contain a normal morphology and are highly contact inhibited. On the right are shown REF52 cells (bc1051 REF52) transformed by the bc1051 polyoma mutant that only expresses PyMT and PyST (22). The bc1051 REF52 cells have a transformed morphology and are not contact inhibited. (B) The location of the Py T-antigens and ARF in transformed bc1051 REF52 cells were analyzed by immunofluorescence staining. On the left, the membrane-bound PyMT and nuclear and cytoplasmic small T-antigen were detected with 762 anti-polyoma common region antibody (Py) and are stained in green (FITC). On the right, ARF found located in the nucleoli was detected with 821B6 antibody and stained in red (Texas red).
Fig. 2.
ARF, but not p53, is activated in REF52 cells transformed by PyMT and PyST. (A) REF52 cells transformed by wild-type Py DNA (PyREF52) express PyLT, PyMT, and PyST, whereas cells transformed by the bc1051 mutant DNA (bc1051 REF52), which contains a mutation in the LT splice donor site (22), only express PyMT and PyST. Shown is Western analysis of extracts of two different isolates of transformed PyREF52 and three different isolates of bc1051 REF52 cells probed with the PYC antibody that detects common amino acid sequence in PyLT (LT), PyMT (MT), and PyST (ST). (B) REF52 cells transformed by only PyMT and PyST (bc1051 REF52) contain ARF. Western analysis of untransformed REF52 cells, two different isolates of PyREF52 cells transformed with wild-type Py expressing PyLT, PyMT, and PyST, and two different isolates of cells transformed with the bc1051 mutant that only expresses PyMT and PyST (bc1051 REF52) (see A) were probed with the rabbit anti-Rat ARF polyclonal serum 821B6. The differences in the amounts of ARF are most likely due to differences in the levels of the PyMT-induced cellular signaling that activates ARF in the different Py-transformed cell clonal isolates. (C) REF52 cells transformed by either Py wild-type (PyREF52) or the bc1051 mutant (bc1051 REF52) DNA express low levels of wild-type p53 that can be activated by DNA damage. Cells from one line of transformed PyREF52 cells, one isolate of transformed bc1051 REF52 cells, and untransformed REF52 cells were cultured in the presence or absence of the DNA-damaging agent Adriamycin for 7 h before being assessed by Western analysis for the presence of p53, p21, and MDM2.
These data demonstrate that PyST is sufficient to block PyMT-induced ARF from activating p53. PyST contains two known domains. One is the J domain that binds the Hsc70 molecular chaperone (24, 25), and the other is the PP2A domain that allows PyST to mimic the PP2A B subunit and bind to core PP2A dimer composed of the A and C subunits (26, 27). PyMT contains the same J and PP2A domains as PyST (191 of the 195 amino acids of the PyST sequence are also present in the 421-aa PyMT protein), but the cellular locations of the two proteins differ. Whereas PyMT is located in the plasma membrane, PyST is found in both the cytoplasm and the nucleus (28).
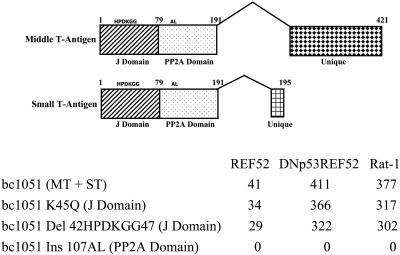
Inactivation of the Py J Domain Does Not Prevent PyMT from Inducing ARF or PyST from Inhibiting ARF Activation of p53. To determine which function of PyST was involved in abrogating the activation of p53 by ARF, mutations in the J domain and PP2A domains of PyST and PyMT were generated in the bc1051 mutant genomic DNA background. Mutations in the J domain that involve a change of the K at amino acid 45 to a Q (K45Q) or the deletion of amino acids 42–47 (HPDKGG) containing the complete J domain binding site do not inhibit transformation of REF52 cells, of DNp53REF52 cells (REF52 cells in which the function of the endogenous rat p53 is inhibited by the presence of a dominant-negative p53), or of Rat-1 cells [lacking endogenous ARF (17) and comprised in its p53 pathway because p21 is not expressed (21)] (Fig. 3).
Fig. 3.
The J domain is not required for the PyMT induction of ARF or for PyST to block ARF signaling to p53. Mutations in the J and PP2A domains were incorporated into bc1051 genomic DNA and tested for transforming ability on REF52 cells, DNp53REF52 cells (REF52 cells containing a dominant-negative p53), and Rat-1 cells (in which neither ARF nor p21 is expressed, resulting in a compromised p53 response). The number of transformed colonies per microgram is shown for the transfecting bc1051 genomic DNA and bc1051 genomic DNAs containing various J domain and PP2A mutations. The J domain mutations were either a replacement of K with a Q at amino acid 45 in the J domain-binding region (K45Q) or a deletion of amino acids 42–47, which contain the complete J domain-binding region (Del 42HPDKGG47). The PP2A domain was mutated by the insertion of an AL at amino acid 107 (Ins 107AL) that has previously been shown to inactivate the ability of the Py T-antigens to bind PP2A (23). Because genomic DNA was used, the mutations were located in both the middle T-antigen (MT) and small T-antigen (ST) coding regions. At the top, the different splices to generate the middle and small T-antigens from the bc1051 genomic DNA are shown with the location of the mutations in the common J and PP2A domain regions. The numbers indicate the amino acid sequences of the different proteins. The location of the unique middle T-antigen and small T-antigen coding region is also shown.
REF52 cells are similar to primary cells in containing an intact ARF–p53 pathway. After DNA transfection, transformed colonies generated in REF52 cells are fewer and take longer to appear than in cells in which the p53 pathway is compromised (Fig. 3). It is most likely that this lower transformation rate is due to the inability of the REF52 cells to fully recover from the consequences of p53 activation due to a DNA damage response as a result of introducing large amounts of damaged DNA into the cells as a result of transfection.
In cells transformed by the bc1051 genomic DNA containing mutations in the J domain, ARF was abundant and p53 was minimal, similar to cells transformed by the bc1051 genomic DNA in which the J domain is not mutated (Figs. 1 B and C and 2). These data show that mutations that inactivate the J domain of PyMT do not inhibit the ability of PyMT to induce ARF, and mutations that inactivate the PyST J domain do not affect the ability of PyST to block ARF signaling to p53.
PP2A Plays a Role in ARF Signaling to p53. Mutations in the genomic sequence specifying the bc1051 PP2A domain affect both PyMT and PyST and are transformation-deficient in REF52 cells, as well as in REF52 cells containing a dominant-negative p53 and Rat-1 cells lacking functional ARF and p21 genes (Fig. 3). This is because the binding of PP2A subunits A and C to PyMT is required for the stable binding of the SRC family kinases to PyMT (29). Once bound, these cellular kinases can phosphorylate PyMT tyrosines 250, 315, and 322, allowing the binding of cellular proteins to these phosphorylated tyrosines and resulting in the unscheduled activation of the cellular PI3 kinase, PLC-γ, and the RAS-activated RAF → MEK → ERG signaling pathways leading to cell transformation (28). Thus, to evaluate the effect of mutations that only affect the PyST PP2A domain, we assessed the transformation of REF52 cells after cotransfection of either wild-type or mutant PyST cDNAs together with wild-type PyMT cDNA. Fig. 4 shows the average number of transformed colonies per microgram of transfecting DNA derived from three separate transfections. Both wild-type PyST cDNA and PyST cDNA mutated in the J domain were able to complement wild-type PyMT for transformation of REF52 cells, whereas PyST mutated in the PP2A domain was unable to generate transformed REF52 cells in conjunction with wild-type PyMT cDNA (Fig. 4). The lack of transformation is not due to toxicity of the PP2A PyST mutant, because we can easily isolate REF52 cells containing this PyST mutant when linked to an antibiotic resistance marker (data not shown). These results identify the PP2A domain of PyST as playing a role in the ARF-dependent mediated activation of p53.
Fig. 4.
The PP2A domain is required for PyST to block ARF signaling to p53. The number of transformed colonies generated after REF52 cells were co-transfected with wild-type middle T-antigen cDNA (MT) together with wild-type or mutant small T-antigen cDNAs (ST) or transfected with bc1051 genomic DNA alone is presented. The average number of transformed colonies per microgram of middle-T antigen DNA from three experiments is shown. The small T-antigen mutants used were the Del 42HPDKGG47 J domain deletion and the Ins 107AL PP2A insertion mutant (see Fig. 3). At the top is shown the organization of the middle and small T-antigen domains in the cDNAs. The numbers indicate the amino acid sequence of the two proteins, and the location of the mutations in the small T-antigen cDNA is shown.
Discussion
PP2A is a major cellular serine–threonine phosphatase with many cellular protein targets (30–34). PyST is known to replace the PP2A B regulatory subunit in the PP2A holoenzyme and to form a new complex with the PP2A A scaffold and C catalytic subunits (26, 27, 35). There are four distinct families of regulatory B subunits comprising at least 22 different isoforms that are encoded by different genes and different splice variants of the same B gene (31, 33, 34). It is not known whether PyST replaces a specific family of B subunits or whether it can replace multiple B subunits. It is suspected that when PyST enters into the PP2A complex, it inhibits PP2A activity, but it has not been ruled out that the new PP2A complex containing PyST has a novel PP2A specificity. At this time, we can only speculate at the role of PP2A in the ARF–p53 signaling pathway. ARF itself may be phosphorylated, because we have detected multiple ARF forms on 2D gels (M.G.M. and M.F., unpublished results). ARF may be complexed with other proteins whose phosphorylation state may affect ARF's ability to activate p53.
ARF is thought to activate p53 by inhibiting the ability of MDM2 to degrade p53 (36–39). Although ARF has been shown to bind to MDM2 in vitro, there is mounting evidence that in vivo ARF does not need to form a stable interaction with MDM2 or sequester MDM2 to the nucleoli to activate p53 (17, 40, 41). Thus, ARF may affect the ability of MDM2 to degrade p53 by indirect as well as direct means that may involve a PP2A-dependent step. Both p53 and its regulator MDM2 are known to be phosphorylated at multiple sites, and the binding of MDM2 to p53 and the resultant inhibition of p53 activity have been reported to depend on phosphorylation (42–44). It is also possible that PyST affects the phosphorylation state of other unidentified proteins involved in the ARF–p53 pathway.
SV40 and Py have a similar genome size and related gene organizations, but each virus blocks p53 activation by a different mechanism. The small T-antigens from both viruses are of similar size and share similar J and PP2A domains. The PP2A domains of the two small T-antigens may have distinct biological functions. It recently has been demonstrated that the perturbation of PP2A by the SV40 small T-antigen is required for the immortalization of human cells transformed by the Ras oncogene (45). PyST has not yet been reported to function in a similar manner. It is not clear that the SV40 small T-antigen PP2A domain can or needs to interfere with ARF signaling to p53 in a similar fashion as has been determined here for the PyST PP2A domain. In the case of SV40, p53 is directly targeted and functionally inactivated by the binding of SV40 large T-antigen. In contrast, in the case of Py, PyLT does not bind to p53, and it is the PyST PP2A domain that is involved in inhibiting p53 up-regulation by blocking ARF signaling. The SV40 small T-antigen can also replace B regulatory subunits in the PP2A holoenzyme (26, 46). A recent report has identified at least two different B isoforms that SV40 small T-antigen can replace in the PP2A complex (47). At present, it is not known whether PyST replaces these or other B subunits in the PP2A complex. Differences have been noted between the binding of the SV40 and Py small T-antigens to the PP2A A subunits (48) that may reflect a difference in their effects on PP2A holoenzyme activities.
Our study demonstrates that Py utilizes a unique strategy to abrogate activation of p53. In contrast to the other small DNA viruses, which encode proteins that target and inactivate p53 directly, Py targets and inhibits critical ARF-mediated signals for p53 activation through the PyST PP2A binding domain. This study reveals a previously unrecognized role for PP2A in modulating the ARF–p53 tumor suppressor pathway and highlights the utility of viral proteins to identify important regulatory mechanisms involved in cellular signaling pathways.
Acknowledgments
We thank Drs. Gerard Evan, Nancy Hogg, Clodagh O'Shea, and David Stokoe for their helpful advice and comments during the preparation of the manuscript and the course of the research. This research was supported in part by National Institutes of Health Grants CA92454 and CA101967.
Abbreviations: Py, polyoma virus; PyLT, Py large T-antigen; PyMT, Py middle T-antigen; PyST, Py small T-antigen.
References
- 1.Sharpless, N. E. & DePinho, R. A. (1999) Curr. Opin. Genet. Dev. 9, 22–30. [DOI] [PubMed] [Google Scholar]
- 2.Sherr, C. J. & Weber, J. D. (2000) Curr. Opin. Genet. Dev. 10, 94–99. [DOI] [PubMed] [Google Scholar]
- 3.Sherr, C. J. (2001) Nat. Rev. Mol. Cell Biol. 2, 731–737. [DOI] [PubMed] [Google Scholar]
- 4.Sherr, C. J. & McCormick, F. (2002) Cancer Cell 2, 103–112. [DOI] [PubMed] [Google Scholar]
- 5.Sherr, C. J. (2004) Cell 116, 235–246. [DOI] [PubMed] [Google Scholar]
- 6.Mietz, J. A., Unger, T., Huibregtse, J. M. & Howley, P. M. (1992) EMBO J. 11, 5013–5020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiang, D., Srinivasan, A., Lozano, G. & Robbins, P. D. (1993) Oncogene 8, 2805–2812. [PubMed] [Google Scholar]
- 8.Segawa, K., Minowa, A., Sugasawa, K., Takano, T. & Hanaoka, F. (1993) Oncogene 8, 543–548. [PubMed] [Google Scholar]
- 9.Sarnow, P., Ho, Y. S., Williams, J. & Levine, A. J. (1982) Cell 28, 387–394. [DOI] [PubMed] [Google Scholar]
- 10.Scheffner, M., Werness, B. A., Huibregtse, J. M., Levine, A. J. & Howley, P. M. (1990) Cell 63, 1129–1136. [DOI] [PubMed] [Google Scholar]
- 11.Huibregtse, J. M., Scheffner, M. & Howley, P. M. (1991) EMBO J. 10, 4129–4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scheffner, M., Munger, K., Huibregtse, J. M. & Howley, P. M. (1992) EMBO J. 11, 2425–2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yew, P. R. & Berk, A. J. (1992) Nature 357, 82–85. [DOI] [PubMed] [Google Scholar]
- 14.Querido, E., Marcellus, R. C., Lai, A., Charbonneau, R., Teodoro, J. G., Ketner, G. & Branton, P. E. (1997) J. Virol. 71, 3788–3798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steegenga, W. T., Riteco, N., Jochemsen, A. G., Fallaux, F. J. & Bos, J. L. (1998) Oncogene 16, 349–357. [DOI] [PubMed] [Google Scholar]
- 16.Dilworth, S. M. (1990) Semin. Cancer Biol. 1, 407–414. [PubMed] [Google Scholar]
- 17.Lomax, M. & Fried, M. (2001) Oncogene 20, 4951–4960. [DOI] [PubMed] [Google Scholar]
- 18.Mor, O., Read, M. & Fried, M. (1997) Oncogene 15, 3113–3119. [DOI] [PubMed] [Google Scholar]
- 19.Rassoulzadegan, M., Cowie, A., Carr, A., Glaichenhaus, N., Kamen, R. & Cuzin, F. (1982) Nature 300, 713–718. [DOI] [PubMed] [Google Scholar]
- 20.Cuzin, F. (1984) Biochim. Biophys. Acta 781, 193–204. [DOI] [PubMed] [Google Scholar]
- 21.Allan, L. A., Duhig, T., Read, M. & Fried, M. (2000) Mol. Cell. Biol. 20, 1291–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nilsson, S. V. & Magnusson, G. (1983) EMBO J. 2, 2095–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Campbell, K. S., Auger, K. R., Hemmings, B. A., Roberts, T. M. & Pallas, D. C. (1995) J. Virol. 69, 3721–3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walter, G., Carbone, A. & Welch, W. J. (1987) J. Virol. 61, 405–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pallas, D. C., Morgan, W. & Roberts, T. M. (1989) J. Virol. 63, 4533–4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pallas, D. C., Shahrik, L. K., Martin, B. L., Jaspers, S., Miller, T. B., Brautigan, D. L. & Roberts, T. M. (1990) Cell 60, 167–176. [DOI] [PubMed] [Google Scholar]
- 27.Walter, G., Ruediger, R., Slaughter, C. & Mumby, M. (1990) Proc. Natl. Acad. Sci. USA 87, 2521–2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ichaso, N. & Dilworth, S. M. (2001) Oncogene 20, 7908–7916. [DOI] [PubMed] [Google Scholar]
- 29.Glover, H. R., Brewster, C. E. & Dilworth, S. M. (1999) Oncogene 18, 4364–4370. [DOI] [PubMed] [Google Scholar]
- 30.Millward, T. A., Zolnierowicz, S. & Hemmings, B. A. (1999) Trends Biochem. Sci. 24, 186–191. [DOI] [PubMed] [Google Scholar]
- 31.Janssens, V. & Goris, J. (2001) Biochem. J. 353, 417–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sontag, E. (2001) Cell Signalling 13, 7–16. [DOI] [PubMed] [Google Scholar]
- 33.Zolnierowicz, S. (2000) Biochem. Pharmacol. 60, 1225–1235. [DOI] [PubMed] [Google Scholar]
- 34.Lechward, K., Awotunde, O. S., Swiatek, W. & Muszynska, G. (2001) Acta Biochim. Pol. 48, 921–933. [PubMed] [Google Scholar]
- 35.Mumby, M. (1995) Semin. Cancer Biol. 6, 229–237. [DOI] [PubMed] [Google Scholar]
- 36.Pomerantz, J., Schreiber-Agus, N., Liegeois, N. J., Silverman, A., Alland, L., Chin, L., Potes, J., Chen, K., Orlow, I., Lee, H. W., et al. (1998) Cell 92, 713–723. [DOI] [PubMed] [Google Scholar]
- 37.Zhang, Y., Xiong, Y. & Yarbrough, W. G. (1998) Cell 92, 725–734. [DOI] [PubMed] [Google Scholar]
- 38.Honda, R. & Yasuda, H. (1999) EMBO J. 18, 22–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kamijo, T., Weber, J. D., Zambetti, G., Zindy, F., Roussel, M. F. & Sherr, C. J. (1998) Proc. Natl. Acad. Sci. USA 95, 8292–8297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Llanos, S., Clark, P. A., Rowe, J. & Peters, G. (2001) Nat. Cell Biol. 3, 445–452. [DOI] [PubMed] [Google Scholar]
- 41.Korgaonkar, C., Zhao, L., Modestou, M. & Quelle, D. E. (2002) Mol. Cell. Biol. 22, 196–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Woods, D. B. & Vousden, K. H. (2001) Exp. Cell Res. 264, 56–66. [DOI] [PubMed] [Google Scholar]
- 43.Shimizu, H. & Hupp, T. R. (2003) Trends Biochem. Sci. 28, 346–349. [DOI] [PubMed] [Google Scholar]
- 44.Meek, D. W. & Knippschild, U. (2003) Mol. Cancer Res. 1, 1017–1026. [PubMed] [Google Scholar]
- 45.Hahn, W. C., Dessain, S. K., Brooks, M. W., King, J. E., Elenbaas, B., Sabatini, D. M., DeCaprio, J. A. & Weinberg, R. A. (2002) Mol. Cell. Biol. 22, 2111–2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang, S. I., Lickteig, R. L., Estes, R., Rundell, K., Walter, G. & Mumby, M. C. (1991) Mol. Cell. Biol. 11, 1988–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen, W., Possemato, R., Campbell, K. T., Plattner, C. A., Pallas, D. C. & Hahn, W. C. (2004) Cancer Cell 5, 127–136. [DOI] [PubMed] [Google Scholar]
- 48.Zhou, J., Pham, H. T., Ruediger, R. & Walter, G. (2003) Biochem. J. 369, 387–398. [DOI] [PMC free article] [PubMed] [Google Scholar]