Abstract
MdfA is an Escherichia coli multidrug-resistance transporter. Cells expressing MdfA from a multicopy plasmid exhibit multidrug resistance against a diverse group of toxic compounds. In this article, we show that, in addition to its role in multidrug resistance, MdfA confers extreme alkaline pH resistance and allows the growth of transformed cells under conditions that are close to those used normally by alkaliphiles (up to pH 10) by maintaining a physiological internal pH. MdfA-deleted E. coli cells are sensitive even to mild alkaline conditions, and the wild-type phenotype is restored fully by MdfA expressed from a plasmid. This activity of MdfA requires Na+ or K+. Fluorescence studies with inverted membrane vesicles demonstrate that MdfA catalyzes Na+- or K+-dependent proton transport, and experiments with reconstituted proteoliposomes confirm that MdfA is solely responsible for this phenomenon. Studies with multidrug resistance-defective MdfA mutants and competitive transport assays suggest that these activities of MdfA are related. Together, the results demonstrate that a single protein has an unprecedented capacity to turn E. coli from an obligatory neutrophile into an alkalitolerant bacterium, and they suggest a previously uncharacterized physiological role for MdfA in pH homeostasis.
Keywords: MdfA, multidrug transport, sodium proton antiporter, alkaline pH tolerance, Escherichia coli
The simultaneous emergence of resistance in eukaryotic and prokaryotic cells to many chemically unrelated drugs is termed multidrug resistance. Multidrug-resistance transporters (Mdr transporters) that remove the drugs from the cell cytoplasm or cytoplasmic membrane to the external medium cause one major form of multidrug resistance. Secondary Mdr transporters of the major facilitator superfamily group (1) are distributed widely among prokaryotic microorganisms, including pathogenic bacteria (2–7). These transporters are usually driven by the transmembrane proton electrochemical gradient (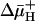 ) (8–11). In addition to their clinical importance (12), the multispecific Mdr transporters pose intriguing questions regarding their true physiological function, their multispecific substrate-recognition properties, and the bioenergetics and mechanism of multidrug transport.
) (8–11). In addition to their clinical importance (12), the multispecific Mdr transporters pose intriguing questions regarding their true physiological function, their multispecific substrate-recognition properties, and the bioenergetics and mechanism of multidrug transport.
MdfA is a major facilitator superfamily-related Escherichia coli Mdr transporter with 12 transmembrane helices (13–15), which has close orthologs only in the following three pathogenic bacteria: Shigella flexneri (99% identity) (16), Salmonella enterica serovar Typhi (90% identity) (17), and Yersinia pestis (73% identity) (18). The substrate-recognition profile of MdfA includes lipophilic cations, zwitterionic drugs, and neutral compounds (10, 11, 19), and Glu-26 in transmembrane helix 1 of the transporter plays an important role in multidrug recognition (14, 20, 21). Transport experiments have shown that MdfA is driven by the proton electrochemical gradient and functions as a drug/proton antiporter (11, 22).
Despite compelling evidence implicating Mdr transporters in multidrug resistance, the ongoing debate concerning the “natural” function of these transport proteins has not abated. The various considerations regarding the physiological role of Mdr transporters have been thoroughly reviewed (4, 23, 24). Recent discoveries support the notion that at least some Mdr transporters have specific physiological substrates and that their multispecificity is a welcomed side effect. One example of such a phenomenon is the transport of spermidine by Blt of Bacillus subtilis (25).
By serendipity, we identified an MdfA-dependent phenotype. E. coli cells expressing MdfA are able to grow in extremely basic media (up to pH 10). Normally, E. coli cells maintain a relatively constant intercellular pH under moderate environmental conditions (pH 6.5–9). The machinery responsible for internal pH stability comprises many cellular factors, and their intricate interplay enables E. coli to maintain an internal proton concentration, which allows growth over a broad range of external pH values. However, at external pH 9, the system begins to collapse and internal alkalization occurs, leading to the arrest of growth and cell death (26–28). Here, we present the surprising findings that the Mdr transporter, MdfA, confers resistance under relatively extreme alkaline pH conditions. Various aspects of this biological activity were analyzed, and the combined results suggest a previously uncharacterized physiological role for MdfA as well as a possible mechanism.
Materials and Methods
Strains and Growth Conditions. E. coli strains UTΔmdfA::kan (R. Edgar and E.B., unpublished data) and EP432 (29) were used for alkaline pH-resistance assays. Cells were transformed with either plasmid pT7–5 or plasmid pT7–5-mdfA-6His (wild type or mutant, as indicated). A swab of colonies from a fresh LB–agar plate was diluted into 2 ml of LB medium (pH 7) and grown for 2–3 h. Cells were then washed five times in a low-salt medium (10 g/liter tryptone/5 g/liter yeast extract) and diluted to OD600 of 0.02 in low-salt medium supplemented with 30 μg/ml kanamycin/200 μg/ml ampicilin/68 mM 1,3-bis[tris(hydroxymethyl)-methylamino]propane (Bistris propane) at the desired pH and the indicated ion concentration. For experiments conducted in a microplate reader (30), 100 μl of mineral oil was placed on top of a 100-μl cell suspension. Cells were grown at 37°C and were vigorously shaken for 20 s every 4 min. OD at 600 nm was measured every 10 min. Growth of cells in flasks was conducted with 10-ml cultures in 50-ml flasks. For growth experiments conducted on solid media, overnight cultures were washed as described and diluted to OD600 of 0.007, and serial 10-fold dilutions were plated on low-salt agar plates with the indicated additions.
The ΔpH Measurements in Whole Cells. Internal pH measurements were performed essentially as described (31) with some modifications. Growing cells were incubated for 5 min with [14C]-methylamine under vigorous agitation. After filtration, the amount of [14C]methylamine accumulated in the cells was counted by liquid scintillation. We used [14C]methylamine accumulation at external alkaline pH (pHout) 7.6 or in the presence of toluene (4%, vol/vol) as controls.
The ΔpH Measurements in Inverted Membrane Vesicles. E. coli strains UTLΔmdfA::kan (R. Edgar and E.B., unpublished data) and EP432 (29) were transformed with plasmid pUC18 or plasmid pUC18-pARA-mdfA-6His (wild type or mutant, as indicated). Cell growth and preparation of inverted membrane vesicles were performed as described (11). Membranes were suspended at a protein concentration of 30–40 mg/ml in 1 mM Tris·HCl, pH 7/10 mM magnesium sulfate/10% glycerol. Sodium-free ATP was prepared by passage through a Dowex column and elution with 1 mM Tris·HCl, pH 7. The final ATP concentration was determined by measuring OD260. For ΔpH dissipation assays, the fluorescent dye 9-amino-6-chloro-2-methoxyacridine (ACMA) was used. We added 0.4 mM Tris·ATP to a 0.6 mg/ml vesicle suspension in 1 mM Tris·HCl/10 mM magnesium sulfate/1 μM ACMA, pH 7. Subsequently, sodium gluconate, potassium gluconate, lithium chloride or choline chloride were added at the indicated concentrations. Carbonyl cyanide m-chlorophenyl hydrazone (CCCP; 2.5 μM) was added for complete dissipation of ΔpH. ACMA fluorescence was monitored at 409 and 474 nm for excitation and emission, respectively. Slit width for both measurements was 3 nm.
Purification and Reconstitution of MdfA. MdfA-6His was purified to homogeneity and reconstituted as described (11), with the following modifications. Detergent removal during the reconstitution into liposomes was achieved by dialysis against 1,000-fold of the detergent-free buffer. The dialysis buffer (150 mM ammonium chloride/5 mM Tris·HCl, pH 7) was supplemented by 0.4 mg/ml Bio-Beads, and dialysis was carried out as described in ref. 32. Protein content of the proteoliposomes was evaluated according to the method of Lowry and by quantitative SDS/PAGE stained with Coomassie brilliant blue.
Fluorescent Measurements in Proteoliposomes. For fluorescence-based assays of transport, the fluorescence dye acridine orange was used. Proteoliposomes were freshly prepared as described (11). Proteoliposomes loaded with 150 mM ammonium chloride/5 mM Tris·HCl (pH 7) were diluted 500-fold into 1 μM acridine orange/150 mM choline chloride/5 mM Tris·HCl, pH 7. Ions were added at the indicated concentrations. CCCP (20 μM) was added for complete dissipation of ΔpH. Acridine orange fluorescence was monitored at 494 and 530 nm for excitation and emission, respectively. Slit width for both measurements was 3 nm.
Transport Assays with Whole Cells. Ethidium bromide (EtdBr) efflux experiments were conducted as described (11, 14). Where indicated, various concentrations of sodium chloride or choline chloride were added to the loading buffer and the reaction mixture.
Results
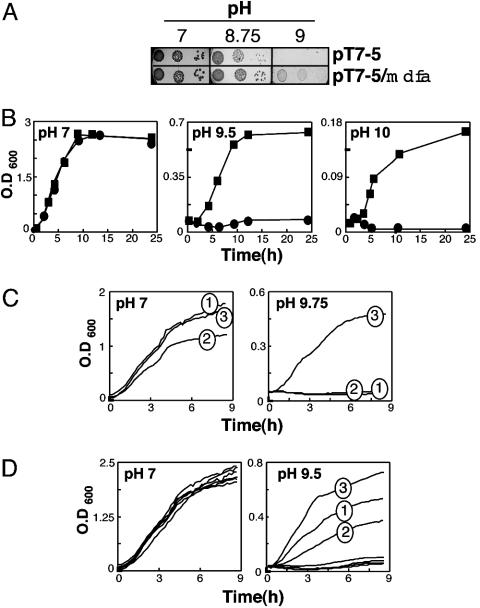
E. coli Expressing MdfA from a Multicopy Plasmid Exhibits Tolerance to Basic pH. We first examined the basic pH tolerance of cells expressing MdfA from a multicopy plasmid by using three different growth regimens. On LB–agar plates, cells expressing MdfA grew better than cells harboring the plain vector already at pH 8.75, and only MdfA-expressing cells grew at pH 9 (Fig. 1A). The advantageous effect of MdfA expression became even more evident in liquid medium. In flasks (Fig. 1B) and 96-well plates (Fig. 1C), control and MdfA-expressing cells grew similarly at pH 7, whereas marked differences were observed under basic pH conditions (Fig. 1 B and C). The observed basic pH tolerance seems to be mediated specifically by MdfA because the (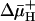 )-driven lactose permease (LacY) (33) did not support cell growth under alkaline pH conditions (Fig. 1C). Interestingly, Fig. 1D shows that partially (Glu26Asp and Glu26Gln) or completely (Glu26Cys) defective MdfA mutants, expressed from plasmids, (21) exhibited a decreased or abolished basic pH-tolerance activity, respectively. Similarly, all of the Mdr transporter-defective mutants harboring substitutions at position 112 (R112C, R112H, and R112K) (S. Molshansky and E.B., unpublished data) failed to support growth at an alkaline pH (Fig. 1D). These results suggest that in E. coli, an extreme alkaline pH tolerance depends on the expression of a functional MdfA (described below).
)-driven lactose permease (LacY) (33) did not support cell growth under alkaline pH conditions (Fig. 1C). Interestingly, Fig. 1D shows that partially (Glu26Asp and Glu26Gln) or completely (Glu26Cys) defective MdfA mutants, expressed from plasmids, (21) exhibited a decreased or abolished basic pH-tolerance activity, respectively. Similarly, all of the Mdr transporter-defective mutants harboring substitutions at position 112 (R112C, R112H, and R112K) (S. Molshansky and E.B., unpublished data) failed to support growth at an alkaline pH (Fig. 1D). These results suggest that in E. coli, an extreme alkaline pH tolerance depends on the expression of a functional MdfA (described below).
Fig. 1.
MdfA confers alkaline pH tolerance. (A) Growth on agar–LB plates. Growth of E. coli UTΔmdfA::kan transformed with plasmid pT7-5 or pT7-5-mdfA-6His on solid LB media at pH 7, 8.75, or 9, as indicated. We plated 10-fold dilutions of cells (from left to right). (B) Growth in flasks. E. coli UTΔmdfA::kan transformed with plasmid pT7-5 (•) or pT7-5-mdfA-6His (▪) was grown at pH 7, 9.5, or 10, as indicated. Growth was conducted in LB broth in a 37°C shaker. (C) Growth in a microplate reader. E. coli UTΔmdfA::kan transformed with plasmid pT7-5 (trace 1), pT7-5-LacY (trace 2) or pT7–5-mdfA-6His (trace 3) were grown at pH 7 (Left) or 9.75 (Right). Cells were grown in LB broth at 37°C with intermittent agitation. OD at 600 nm was constantly measured. The results shown are an average of three independent repeats; SEs are <1% of the measured signal and, thus, not shown. (D) Growth in a microplate reader of E. coli UTmdfA::kan expressing various MdfA mutants. At pH 7 (Left), the growth of cells expressing no MdfA, wild-type MdfA (fully active in multidrug resistance), or mutants E26Q, E26D (partially active), E26C, R112C, R112H, or R112K (not active) is indistinguishable. At pH 9.5 (Right), only cells expressing mutants E26Q (trace 1) and E26D (trace 2) exhibit partial growth when compared with those expressing wild-type MdfA (trace 3). All other mutants (E26C, R112C, R112H, and R112K) fail to grow and are indistinguishable from one another.
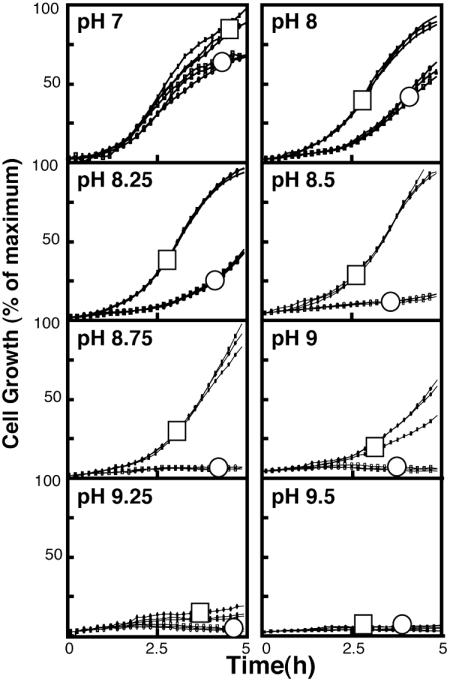
Deletion of the Chromosomal Copy of mdfA Results in an Increased Sensitivity to Basic pH. To examine the physiological relevance of the MdfA-mediated basic-pH tolerance, we characterized a strain that is completely devoid of the chromosomal mdfA gene (E. coli UT5600mdfA::kan) (R. Edgar and E.B., unpublished data). Briefly, the growth of wild-type cells and the deletion mutant were studied under various pH conditions. The OD600 of wild-type cells measured after 5 h at pH 7 was defined as 100% growth. As shown (Fig. 2), wild-type cells grew somewhat better than the mutant even at a neutral pH. As the pH of the medium increased, the negative effect of the chromosomal deletion of mdfA became more evident. For a comparison, under the same conditions, the mdfA-deleted cells were able to grow relatively well even at pH 9.75, provided that they carried mdfA on a multicopy plasmid (Fig. 1). Together, the results suggest that MdfA plays a physiological role in alkaline pH tolerance.
Fig. 2.
Effect of the chromosomal deletion of the mdfA gene on bacterial growth under various pH conditions. E. coli UT5600 (□) or E. coli UTΔmdfA::kan (○) were grown in 96-well plates at the indicated pH (pH 7–9.5). Three independent growth experiment repeats are shown. Growth of E. coli UT5600 cells at pH 7 was defined as maximal growth (100%). All other growth curves were quantified as a percentage of this maximal curve.
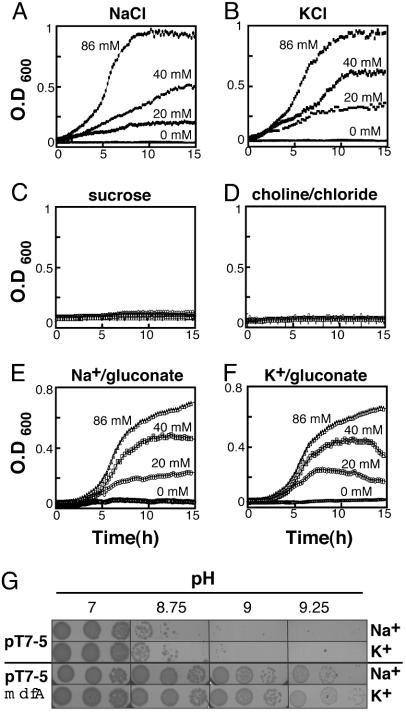
The MdfA-Mediated Alkalitolerance Depends on Na+ or K+. Because pH homeostasis is often related to sodium ions (34), growth experiments were conducted in the absence or presence of various concentrations of sodium chloride. At pH 7, control and MdfA-expressing cells grew equally well, regardless of the sodium chloride concentration (data not shown). At pH 9.5, only MdfA-expressing cells were able to grow, and the growth depended strictly on the concentration of sodium chloride (Fig. 3A). As a control, we performed identical growth experiments with potassium chloride, and to our surprise, identical results were obtained (Fig. 3B), suggesting that the MdfA-mediated alkaline pH tolerance might also be potassium-driven (35). Growth experiments with increasing concentrations of sucrose (Fig. 3C) or cholin chloride (Fig. 3D) ruled out a possible role for osmotic pressure or chloride in alkaline pH tolerance. In contrast, both sodium gluconate and potassium gluconate supported the growth of MdfA-expressing cells at alkaline pH (Fig. 3 E and F, respectively). Collectively, these analyses suggest that the MdfA-mediated alkaline pH tolerance requires either sodium or potassium ions. This conclusion is supported by the results of the growth experiments conducted on solid LB media (Fig. 3G).
Fig. 3.
Growth of E. coli UTΔmdfA::kan transformed with plasmid pT7–5-mdfA-6His under various conditions. (A–F) Growth was monitored continuously at pH 9.5. Cells were grown in the presence of the indicated concentrations of sodium chloride (A); potassium chloride (B); 20, 40, 80, and 100 mM sucrose (C) (growth curves are indistinguishable); 20, 40, and 86 mM choline chloride (D) (growth curves are indistinguishable); sodium gluconate (E); and potassium gluconate (F). (G) Growth of E. coli UTΔmdfA::kan transformed with plasmid pT7–5orpT7–5-mdfA-6His on solid media at pH 7, 8.75, 9, or 9.25 in the presence of 40 mM sodium chloride or potassium chloride, as indicated. We plated 10-fold dilutions of cells (from left to right).
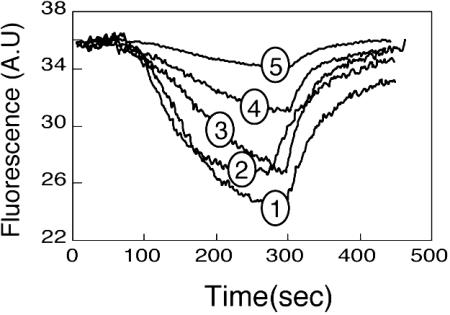
As described above, Mdr transporter-defective mutants of MdfA failed to support growth at alkaline pH (Fig. 1), suggesting a linkage between these two activities of MdfA. To examine this possibility, we monitored the effect of sodium ions on the MdfA-mediated EtdBr efflux activity by using fluorescence spectroscopy. As shown in Fig. 4, increasing concentrations of NaCl drastically inhibited the rate and steady-state level of EtdBr efflux. In contrast, the addition of choline chloride (up to 100 mM) produced no effect (data not shown). These results suggest that the pathways for the transport of sodium and EtdBr by MdfA overlap.
Fig. 4.
EtdBr efflux from preloaded cells. E. coli UTΔmdfa::kan transformed with plasmid pT7–5orpT7–5mdfA were loaded with 10 μM EtdBr and energized by addition of 0.2% glucose after 90 sec. NaCl was added to both the loading buffer and the reaction mixture. Curves 1–4 show MdfA expressing cells in the presence of 0, 20, 40, and 80 mM NaCl, respectively. Curve 5 shows control cells without NaCl. CCCP was added after 250–300 sec.
MdfA Mediates Proton Transport in the Presence of Na+ or K+. In vivo studies with radiolabelled methylamine were performed to estimate the ΔpH across the membrane of MdfA-expressing cells grown under alkaline pH conditions. The results show that, in contrast to control cells, the internal pH in cells expressing MdfA remains constant even under conditions of high external pH (Fig. 5). These results suggest a mechanism by which MdfA confers alkalitolerance. Because the in vivo experiments described indicated that this process requires Na+ or K+ ions, we wished to study Na+- or K+-dependent MdfA-mediated proton translocation in vitro. To this end, inverted membrane vesicles were prepared from control mdfA-deleted E. coli cells or cells overexpressing either the nonfunctional mutant R112C or wild-type MdfA. A ΔpH (acid inside) was established by the addition of Mg+2 and ATP, and the luminal changes in pH were measured by monitoring ACMA fluorescence (Fig. 6). When unperturbed, the vesicles retained a stable proton gradient after the addition of ATP for at least 30 min (data not shown). The addition of sodium gluconate to vesicles containing no MdfA or the nonfunctional MdfA mutant R112C produced a small but significant increase in fluorescence (Fig. 6A). This MdfA-independent response might reflect the activity of other E. coli transporters (such as Na+/H+ antiporters, described below). Interestingly, however, no response to K+ ions was observed in either control or R112C vesicles, indicating no K+/H+ exchange activity in these membranes. Remarkably, upon the addition of sodium or potassium gluconate, a marked increase in fluorescence was observed in the vesicles containing wild-type MdfA, indicating alkalization of the lumen (Fig. 6A, WT).
Fig. 5.
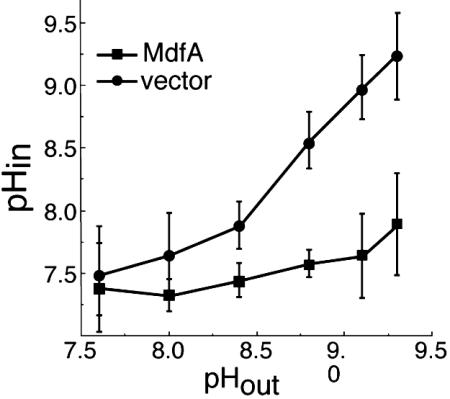
In vivo internal pH measurements. Internal pH (pHin) of cells transformed with plain vector (•) or an MdfA encoding plasmid (▪) was measured under various external alkaline pH (pHout) conditions by the distribution of [14C]methylamine.
Fig. 6.
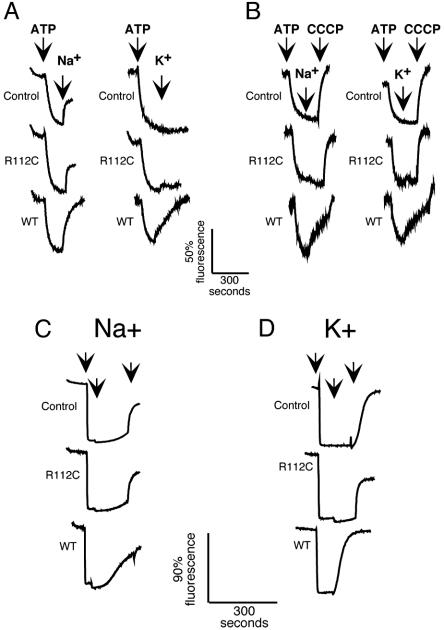
Cation-driven proton translocation by MdfA. (A and B) The ΔpH measurements in inverted membrane vesicles prepared from E. coli strain UTLΔmdfA::kan (A) or E. coli strain EP432 (B). Vesicles were diluted to 0.6 mg/ml membrane proteins in 1 mM Tris·HCl, pH 7/10 mM magnesium sulfate/1 μM ACMA. ATP·Tris (0.4 mM) was added to impose a ΔpH (internal acidic). In A and B Left, the first arrow indicates the time of addition of ATP, and the second arrow indicates addition of 100 mM sodium or potassium gluconate (as indicated). In B Right, a third arrow indicates the time of addition of 2.5 μM CCCP. Traces marked as control refer to vesicles prepared from cells not expressing MdfA. R112C stands for vesicles prepared from cells expressing the nonfunctional mutant R112C. WT refers to vesicles prepared from cells expressing wild-type MdfA. (C and D) ΔpH measurements in proteoliposomes using acridine orange as a fluorescent probe. A ΔpH (inside acidic) was imposed by means of an ammonium chloride chemical gradient across the membranes of liposomes devoid of protein (Control) or containing the nonfunctional mutant R112C, or wild-type MdfA (WT). The left arrow indicates time of addition of liposomes or proteoliposomes, the middle arrow indicates time of addition of 100 mM sodium gluconate (C) or 100 mM potassium gluconate (D), and the right arrow indicates time of addition of 20 μM CCCP.
Because both control and R112C vesicles produced a substantial response to sodium ions ascribed to Na+/H+ antiporters (Fig. 6A), we performed similar studies with vesicles incapable of Na+/H+ antiport activity prepared from E. coli strain EP432 (29, 36). Indeed, no sodium- or potassium-mediated change in fluorescence was observed in control or R112C vesicles prepared from E. coli EP432 (Fig. 6B). In contrast, when sodium or potassium gluconate was added to wild-type MdfA containing EP432 vesicles, a rapid dissipation of the fluorescent signal was observed (Fig. 6B, WT). In all experiments, the addition of either lithium or choline ions did not cause dissipation of ΔpH (data not shown), whereas the addition of CCCP completely dissipated the ΔpH (Fig. 6B, right arrow). Dose–response experiments with increasing sodium and potassium concentrations suggest an apparent kMs for sodium and potassium ions in the range of 50–100 mM (data not shown).
To test the possibility that MdfA alone mediates Na+- or K+-dependent H+ transport, wild type and R112C mutant of MdfA were purified and functionally reconstituted in liposomes (11). A ΔpH (acid inside) was imposed, by creating an ammonium chloride chemical gradient, and internal pH changes were monitored by using acridine orange. Only upon dilution of the ammonium chloride-loaded liposomes in ammonium chloride-free buffer was an instantaneous quench of fluorescence observed (Fig. 6 C and D, left arrows and data not shown), representing the formation of a ΔpH (acid inside). The addition of CCCP reversed the quench in all cases, indicating that a ΔpH had been formed (Fig. 6 C and D, right arrows). When sodium gluconate (Fig. 6C) or potassium gluconate (Fig. 6D) was added to liposomes devoid of MdfA, or to the R112C-proteoliposomes, no change in the fluorescent signal was recorded. In contrast, when sodium (Fig. 6C) or potassium (Fig. 6D) gluconate was added to wild-type MdfA-proteliposomes, the fluorescent quench was abolished, indicating that ΔpH had been dissipated, reflecting the Na+/H+ or K+/H+ antiport activity of MdfA.
Discussion
Recently, we demonstrated that at alkaline pH, MdfA is unable to confer resistance to positively charged drugs because of the electroneutral nature of their exchange reaction (11). Surprisingly, in control experiments, we observed that cells expressing MdfA grew better than cells harboring plain vector under alkaline pH conditions (pH >9). This observation was investigated further, and as shown here, MdfA indeed enables the growth of E. coli cells, even at pH 10 (Fig. 1B). This phenotype is MdfA-specific because a direct correlation was found between growth at alkaline pH and the Mdr transport function of MdfA mutants. Most important, an alkaline pH sensitivity was revealed in cells harboring a chromosomal deletion of the mdfA gene. The growth of the mdfA-deleted strain was already greatly inhibited at pH 8, and it almost ceased at pH 8.5. The magnitude of the effect of the chromosomal deletion clearly suggests a physiological role for MdfA in pH homeostasis. Because the multidrug resistance phenotype of the mdfA knockout strain is almost identical to that of the parental strain (14), and only expression from a multicopy plasmid results in a measurable multidrug resistance, we think that other Mdr transporters (such as AcrB) (37) mask the mdfA-deletion phenotype because they are natively expressed at higher levels than MdfA. However, these Mdr transporters are unable to complement the alkaline pH-resistance function of MdfA in mdfA-deleted cells.
In line with the in vivo studies, in which MdfA-mediated alkaline pH tolerance was found to depend on sodium or potassium ions, the in vitro assays also suggest a mechanistic role for the monovalent cations in MdfA activity. The experiments with MdfA-containing vesicles or proteoliposomes showed that the addition of either sodium gluconate or potassium gluconate resulted in dissipation of a preestablished proton gradient. Therefore, we propose that MdfA couples proton movement to antiport of Na or K ions. The required concentrations of these ions for growth at alkaline pH (Fig. 3) or for ΔpH dissipation in proteoliposomes (data not shown) indicate that the affinity of MdfA to Na and K ions is very low (in the range of 50 mM). Because of this low affinity, we were unable to measure directly the uptake of [22Na] in proteoliposomes. Proton influx should prevent internal alkalization, thus enabling cell growth at alkaline pH. In the absence of both ions, no ΔpH dissipation occurred in vitro and no alkaline pH tolerance occurred in vivo. Interestingly, in this regard, H+/K+(Na+) antiport activities have been described for the tetracycline-specific transporters from B. subtilis (e.g., TetL) (38), which play a primary role in pH homeostasis of this bacterium. As shown here with MdfA, (i) a TetL-deleted strain exhibited substantial pH sensitivity, and (ii) complementing growth at alkaline pH (8.3) by a plasmid harboring the tetL gene also required monovalent cations (39). These results suggest that MdfA in E. coli and TetL in B. subtilis might perform similar pH homeostasis-related functions, although the magnitude of the MdfA-mediated alkaline pH tolerance is extraordinary. Interestingly, in both cases the drug resistance and the alkaline pH tolerance functions are linked (this study and ref. 40).
As has been mentioned, one of the most important and noteworthy features of many alkaliphiles is their ability to regulate their intracellular pH, which always remains lower than that of the external medium. Thus, alkaliphily is maintained by these organisms through bioenergetic and transport mechanisms and does not necessarily rely on alkali-resistant intracellular enzymes (41). However, all living cells also have extracellularly exposed protein domains, usually those of integral membrane proteins. In E. coli, as in other Gram-negative bacteria, there are also periplasmic and outer-membrane proteins that should function under various external conditions. Therefore, our results suggest that the exposure of E. coli extracytoplasmic proteins to an extreme alkaline pH (up to pH 10) does not represent a major growth constraint in rich medium. In a marked contrast, pH homeostasis is sufficient and essential for growth at extreme alkaline pH.
The E. coli sodium proton antiporter NhaA confers high sodium resistance under mild alkaline pH conditions (pH <9) (34). To investigate possible parallelism and/or complementarity in the function of NhaA and MdfA, we tested the ability of MdfA to rescue E. coli strain EP432, whose NhaA and NhaB sodium proton antiporters had been deleted. Although not shown, MdfA was able to support the growth of this mutant strain only in the presence of rather low sodium concentrations (40–80 mM). At these sodium concentrations, growth was observed also at pH 9.75, whereas at high sodium concentrations (>100 mM), MdfA does not support the growth of this strain even under mild alkaline pH conditions. Therefore, these studies support the notion that MdfA deals with the pH problem per se and not with regard to sodium toxicity at alkaline pH. Experiments with NhaA expressed from a plasmid showed that it does not allow growth at a high alkaline pH (pH >9), even in the presence of a physiological concentration of sodium (data not shown). Therefore, we suggest that MdfA provides alkaline pH protection also under conditions in which NhaA does not function, such as (i) at pH > and (ii) in the presence of potassium as the main monovalent cation. Together, the results of this study demonstrate that a single transport protein has an unprecedented capacity to turn E. coli from an obligatory neutrophile into an alkalitolerant bacterium, and they suggest a previously uncharacterized physiological role for MdfA in pH homeostasis.
Acknowledgments
We thank Terry Krulwich for helpful discussions during the work and comments on the manuscript. This work was supported by a grant from the Y. Leon Benoziyo Institute for Molecular Medicine at the Weizmann Institute of Science (to E.B.) and the Israel Science Foundation (E.P.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: Mdr, multidrug resistance; EtdBr, ethidium bromide; ACMA, 9-amino-6-chloro-2-methoxyacridine; CCCP, carbonyl cyanide m-chlorophenyl hydrazone.
References
- 1.Saier, M. H., Jr., & Paulsen, I. T. (2001) Semin. Cell. Dev. Biol. 12, 205–213. [DOI] [PubMed] [Google Scholar]
- 2.Levy, S. B. (1992) Antimicrob. Agents Chemother. 36, 695–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paulsen, I. T. & Skurray, R. A. (1993) Gene 124, 1–11. [DOI] [PubMed] [Google Scholar]
- 4.Lewis, K. (1994) Trends Biochem. Sci. 19, 119–123. [DOI] [PubMed] [Google Scholar]
- 5.Nikaido, H. (1994) Science 264, 382–388. [DOI] [PubMed] [Google Scholar]
- 6.Markham, P. N. & Neyfakh, A. A. (2001) Curr. Opin. Microbiol. 4, 509–514. [DOI] [PubMed] [Google Scholar]
- 7.Poole, K. (2001) Curr. Opin. Microbiol. 4, 500–508. [DOI] [PubMed] [Google Scholar]
- 8.Bolhuis, H., van Veen, H. W., Molenaar, D., Poolman, B., Driessen, A. J. & Konings, W. N. (1996) EMBO J. 15, 4239–4245. [PMC free article] [PubMed] [Google Scholar]
- 9.Paulsen, I. T., Brown, M. H. & Skurray, R. A. (1996) Microbiol. Rev. 60, 575–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edgar, R. & Bibi, E. (1997) J. Bacteriol. 179, 2274–2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lewinson, O., Adler, J., Poelarends, G. J., Mazurkiewicz, P., Driessen, A. J. & Bibi, E. (2003) Proc. Natl. Acad. Sci. USA 100, 1667–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nikaido, H. (1998) Curr. Opin. Microbiol. 1, 516–523. [DOI] [PubMed] [Google Scholar]
- 13.Bibi, E., Adler, J., Lewinson, O. & Edgar, R. (2001) J. Mol. Microbiol. Biotechnol. 3, 171–177. [PubMed] [Google Scholar]
- 14.Edgar, R. & Bibi, E. (1999) EMBO J. 18, 822–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adler, J. & Bibi, E. (2002) J. Bacteriol. 184, 3313–3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jin, Q., Yuan, Z., Xu, J., Wang, Y., Shen, Y., Lu, W., Wang, J., Liu, H., Yang, J., Yang, F., et al. (2002) Nucleic Acids Res. 30, 4432–4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parkhill, J., Dougan, G., James, K.D., Thomson, N.R., Pickard, D., Wain, J., Churcher, C., Mungall, K. L., Bentley, S. D., Holden, M. T., et al. (2001) Nature 413, 848–852. [DOI] [PubMed] [Google Scholar]
- 18.Parkhill, J., Wren, B.W., Thomson, N. R., Titball, R.W., Holden, M. T., Prentice, M. B., Sebaihia, M., James, K. D., Churcher, C., Mungall, K. L., et al. (2001) Nature 413, 523–527. [DOI] [PubMed] [Google Scholar]
- 19.Bohn, C. & Bouloc, P. (1998) J. Bacteriol. 180, 6072–6075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adler J., Lewinson, O. & Bibi, E. (2004) Biochemistry 43, 518–525. [DOI] [PubMed] [Google Scholar]
- 21.Adler J. & Bibi, E. (2004) J. Biol. Chem. 279, 8957–8965. [DOI] [PubMed] [Google Scholar]
- 22.Mine, T., Morita, Y., Kataoka, A., Mizushima, T. & Tsuchiya, T. (1998) J. Biochem. (Tokyo) 124, 187–193. [DOI] [PubMed] [Google Scholar]
- 23.Neyfakh, A. A. (1997) Trends Microbiol. 5, 309–313. [DOI] [PubMed] [Google Scholar]
- 24.Neyfakh, A. A. (2002) Mol. Microbiol. 44, 1123–1130. [DOI] [PubMed] [Google Scholar]
- 25.Woolridge, D. P., Vazquez-Laslop, N., Markham, P. N., Chevalier, M. S., Gerner, E. W. & Neyfakh, A. A. (1997) J. Biol. Chem. 272, 8864–8866. [DOI] [PubMed] [Google Scholar]
- 26.Booth, I. R. (1985) Microbiol. Rev. 49, 359–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Padan, E. & Schuldiner, S. (1986) Methods Enzymol. 125, 337–352. [DOI] [PubMed] [Google Scholar]
- 28.Padan, E., Venturi, M., Gerchman, Y. & Dover, N. (2001) Biochim. Biophys. Acta 1505, 144–157. [DOI] [PubMed] [Google Scholar]
- 29.Pinner, E., Kotler, Y., Padan, E. & Schuldiner, S. (1993) J. Biol. Chem. 268, 1729–1734. [PubMed] [Google Scholar]
- 30.Kalir, S., McClure, J., Pabbaraju, K., Southward, C., Ronen, M., Leibler, S., Surette, M. G. & Alon, U. (2001) Science 292, 2080–2083. [DOI] [PubMed] [Google Scholar]
- 31.Zilberstein, D., Agmon, V., Schuldiner, S. & Padan, E. (1982) J. Biol. Chem. 257, 3687–3691. [PubMed] [Google Scholar]
- 32.Rigaud, J. L., Pitard, B. & Levy, D. (1995) Biochim. Biophys. Acta 1231, 223–246. [DOI] [PubMed] [Google Scholar]
- 33.Kaback, H. R., Sahin-Toth, M. & Weinglass, A. B. (2001) Nat. Rev. Mol. Cell. Biol. 2, 610–620. [DOI] [PubMed] [Google Scholar]
- 34.Padan, E. & Schuldiner, S. (1994) J. Exp. Biol. 196, 443–456. [DOI] [PubMed] [Google Scholar]
- 35.Brey, R. N., Rosen, B. P. & Sorensen, E. N. (1980) J. Biol. Chem. 255, 39–44. [PubMed] [Google Scholar]
- 36.Padan, E., Maisler, N., Taglicht, D., Karpel, R. & Schuldiner, S. (1989) J. Biol. Chem. 264, 20297–20302. [PubMed] [Google Scholar]
- 37.Nikaido, H. & Zgurskaya, H. I. (2001) J. Mol. Microbiol. Biotechnol. 3, 215–218. [PubMed] [Google Scholar]
- 38.Krulwich, T. A., Jin, J., Guffanti, A. A. & Bechhofer, H. (2001) J. Mol. Microbiol. Biotechnol. 3, 237–246. [PubMed] [Google Scholar]
- 39.Cheng, J., Guffanti, A. A., Wang, W., Krulwich, T. A. & Bechhofer, D. H. (1996) J. Bacteriol. 178, 2853–2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jin, J., Guffanti, A. A., Bechhofer, D. H. & Krulwich, T. A. (2002) J. Bacteriol. 184, 4722–4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krulwich, T. A. & Guffanti, A. A. (1983) Adv. Microb. Physiol. 24, 173–214. [DOI] [PubMed] [Google Scholar]