Abstract
The c-Jun NH2-terminal kinase (JNK) group of mitogen-activated protein kinases is activated in response to a wide array of cellular stresses and proinflammatory cytokines. Roles for JNK in the developing nervous system and T-cell-mediated immunity have been established by detailed studies of mice with compound mutations in the Jnk genes. However, little is known concerning the roles of JNK in other mammalian tissues. Mice lacking both of the ubiquitously expressed isoforms (JNK1 and -2) die during midgestation with neural tube closure defects and brain abnormalities. Here we show that JNK-deficient mice exhibit delayed epithelial development in the epidermis, intestines, and lungs. In addition, JNK-deficient mice exhibit an eyelid closure defect associated with markedly reduced epidermal growth factor (EGF) receptor function, and loss of expression of the ligand EGF. We further demonstrate that adult mice lacking either JNK1 or -2 display striking differences in epidermal proliferation and differentiation, indicative of distinct roles for these kinases in the skin. We conclude that JNK is necessary for epithelial morphogenesis and is an essential regulator of signal transduction by the EGF receptor in the epidermis.
The c-Jun NH2-terminal kinase (JNK) group of mitogen-activated protein kinases is encoded by three related genes: Jnk1, -2, and -3 (1, 2). JNK1 and -2 are expressed in multiple tissues, whereas JNK3 is primarily expressed in the brain. Mice lacking individual members of the JNK family are viable, but mice lacking both of the ubiquitously expressed JNK isoforms (JNK1 and -2) die during midgestation with neural tube closure defects and brain abnormalities. These defects are associated with regional and developmental stage-specific alterations in apoptosis in the nervous system (1). Although progress toward understanding the role of JNK in brain development has been achieved, little is known about the role of JNK in the development of other mammalian tissues.
Recent studies of the canonical JNK substrate c-Jun indicate a possible connection to mammalian epidermal development. The protooncogene c-Jun is a component of the stress-responsive transcription factor activator protein 1 (AP-1) and is phosphorylated by JNK on sites within the activation domain (1). AP-1 consists of dimers of the Fos (c-Fos, FosB, Fra-1, and Fra-2), Jun (c-Jun, JunB, and JunD), and cAMP-response-element-binding protein/activating transcription factor (CREB/ATF) protein families together with some other bZip proteins. AP-1 in the skin is implicated in keratinocyte differentiation, carcinogenesis, UV response, photoaging, and wound repair (3). Mice lacking c-Jun die during midgestation. This embryonic lethality can be rescued by JunB, but these mice have open eyes at birth (4, 5). The critical role of c-Jun for embryonic eyelid closure was confirmed by an analysis of mice with a conditional disruption of the c-Jun gene in keratinocytes (6, 7). These data suggest that JNK may be important for normal eyelid closure. However, mice with point mutations at the sites of JNK phosphorylation on c-Jun (Ser-63 and -73) have normal eyelid closure at birth (8). Thus, whereas c-Jun is required for eyelid closure, the phosphorylation of c-Jun by JNK is not required. It therefore remains to be established whether mammalian JNK plays a role during embryonic eyelid closure or epidermal development.
Eyelid closure involves the migration of periderm-derived epithelial cells over the surface of the cornea. This epithelial cell migration is regulated by the epidermal growth factor receptor (EGFR) and its ligands, transforming growth factor α (TGF-α), and EGF, together with the α5β1 integrin extracellular matrix receptor. At birth, EGFR-deficient mice are characterized by open eyelids; short, curly whiskers; immature lungs; epidermal defects; and premature death (9). A similar phenotype has been described for waved-2 mice that have a mutated EGFR cytoplasmic domain and consequently attenuated EGFR signaling (9). TGF-α-deficient mice also are born with open eyes, abnormal skin architecture, and wavy hair. Similarly, these defects are observed in waved-1 mice that express reduced levels of TGF-α. Mice lacking three EGFR ligands (EGF, amphiregulin, and TGF-α) also have open eyes at birth and exhibit growth retardation and intestinal defects (9). Furthermore, mice deficient of the protease tumor necrosis factor α converting enzyme have impaired release of several EGF family members and exhibit epidermal defects, including open eyes at birth (9). Together, these studies indicate that EGFR signaling is critical for normal epidermal development.
The purpose of this study was to test whether JNK plays a role in epidermal morphogenesis. We report that JNK-deficient mice exhibit delayed epidermal development and an eyelid closure defect. The mechanism of JNK function is mediated, in part, by markedly reduced EGFR signaling and loss of expression of the ligand EGF. Together, these data indicate that JNK is a critical regulator of EGF signaling in the epidermis.
Methods
Mice. Mice were generated and backcrossed to the C57BL/6 strain (The Jackson Laboratory) for 10 generations (10). These mice were housed in a facility accredited by the American Association of Laboratory Animal Care, and the animal studies were approved by the institutional animal care and use committee of the University of Massachusetts Medical School.
Morphology and Histology. Embryos from timed matings were fixed in 4% paraformaldehyde for 24 h before processing and embedding in paraffin wax following a standard protocol. Sections were cut at 4 μm and stained with either hematoxylin/ eosin (H&E) or periodic acid/Schiff reagent by using standard methods.
Antibodies. Monoclonal antibodies to JNK1 and -2 from Pharmingen and α-tubulin from Sigma were used in immunoblot assays with chemiluminescence detection. Tissue sections were stained with the following antibodies: phospho-c-Jun, phospho-EGFR, and EGFR from Cell Signaling Technology (Beverly, MA); loricrin and keratin 6 from Covance (Berkeley, CA); p63 from Santa Cruz Biotechnology; keratin 10 from NeoMarkers (Fremont, CA); and proliferating cell nuclear antigen (PCNA) from Zymed. Immunecomplexes were detected by using a biotinylated secondary antibody and streptavidin-conjugated horseradish peroxidase (BioGenex Laboratories, San Ramon, CA) and the substrate 3,3′-diaminobenzidene (Vector Laboratories), followed by brief counterstaining with Mayer's hematoxylin (Sigma).
In Situ Hybridization. Digoxigenin-labeled sense or antisense TGF-α riboprobes were transcribed from linearized plasmids. The probes were hybridized overnight at 52°C in a humid chamber and detected by using biotinylated antidigoxigenin antibody. Digoxigenin-labeled antisense oligoprobes for EGF (5′-GTTCAGTGCTTGGGAGAGAT-3′, 5′-GAAGTCTGTTGTTGGAGGGA-3′,5′-GATGAGTGTGTGCTGGCTAGAT-3′, 5′-GTGTCTCCCTCAGGATTATCCA-3′, 5′-GTGCAATGTAGAGAGGAAGGTG-3′, and 5′-GATGGTACGAATGGTGCAGT-3′), and corresponding sense oligoprobes were hybridized overnight at 37°C in a humid chamber and detected by using tyramide amplification (BioGenex). The TGF-α and EGF mRNA were visualized by using streptavidin-conjugated horseradish peroxidase (BioGenex) and the substrate 3,3′-diaminobenzidine (Vector Laboratories), followed by brief counterstaining with Mayer's hematoxylin (Sigma).
Results
JNK Is Essential for Survival After Birth. To directly assess the role of JNK in development, we investigated the effect of compound Jnk mutations in the C57BL/6 strain background. Mice lacking both of the ubiquitously expressed JNK isoforms (JNK1 and -2) died during midgestation with neural tube closure defects and brain abnormalities (1). We therefore examined the consequence of the expression of a single allele of the ubiquitously expressed JNK isoforms. Mice with a single allele of Jnk1 (Jnk1–/+ Jnk2–/–) survived to adulthood with no obvious phenotype. In contrast, mice with a single allele of Jnk2 (Jnk1–/– Jnk2–/+) were born with the predicted Mendelian frequency, but the majority of these mice died within 48 h after birth (Fig. 1). At birth, these JNK-deficient mice were pale in color and appeared to have difficulty breathing. There was no significant size difference between control and JNK-deficient littermates, and the presence of milk in their stomachs indicated that they were able to feed.
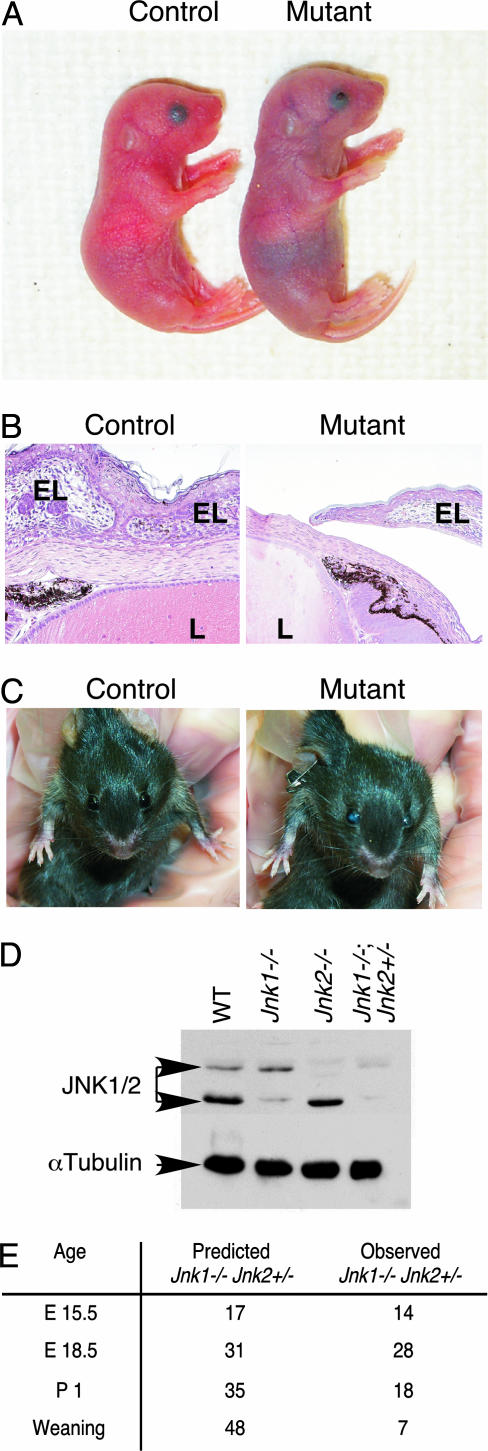
Fig. 1.
Mutant Jnk1–/– Jnk2–/+ pups exhibit open eyes at birth. (A) Mutant (Jnk1–/– Jnk2–/+) pups are pale in color and have open eyes at birth, compared with control (Jnk1+/+ Jnk2–/+) littermates. Throughout this study, Jnk1+/+ Jnk2–/+ embryos were used as littermate controls, and in all cases, these results were consistent with those observed in wild-type C57BL/6 embryos of the same age. Ocular anomalies have been reported in a small percentage of C57BL/6 mice; however, the ocular phenotype reported here is genotype-specific and not similar to wild-type mice. (B) H&E-stained sections of E18.5 eyes indicate that mutant (Jnk1–/– Jnk2–/+) embryos are born with open eyes. L, lens; EL, eyelid. (C) Adult mutant (Jnk1–/– Jnk2–/+) mice have opaque eyes. (D) The expression of JNK1 and -2 was examined by immunoblot analysis of whole-head lysates from newborn pups. Control immunoblots were performed by using an antibody to α-tubulin. (E) JNK-deficient mice die shortly after birth. JNK-deficient mice were mated, and the number of viable progeny with the genotype Jnk1–/– Jnk2–/+ was examined at E15.5, E18.5, P1, and weaning. The expected number of Jnk1–/– Jnk2–/+ mice based on Mendelian inheritance is indicated. Most Jnk1–/– Jnk2–/+ pups found alive at birth died within 48 h.
JNK Is Required for Eyelid Closure. Jnk1–/– Jnk2–/+ mutant mice were born with open eyes (Fig. 1). During the development of wild-type mice, the eyelids migrate across the surface of the eye and fuse at embryonic day (E) 16.5 (11). The eyelids remain closed during birth and do not open until approximately postnatal day (P) 14, a process accelerated by EGF (12). The eyes of wild-type embryos were fully open at E15.5 but completely closed at E16.5. In contrast, all Jnk1–/– Jnk2–/+ embryos had open eyelids at E15.5, which remained open until birth (Fig. 1). Histological analysis demonstrated that the eyelids of control embryos displayed fusion of the opposing epithelia, whereas the eyelids of JNK-deficient littermates exhibited variable extension across the cornea but no contact of the opposing epithelia (Fig. 1). At E18.5, these mutant embryos exhibited a number of developmental defects in the eye, including severe lens abnormality and retinal coloboma (10). Protein extracts prepared from the head of day-1 pups indicated the presence of a small amount of JNK2 but not JNK1 in Jnk1–/– Jnk2–/+ mutants (Fig. 1). A small number of the Jnk1–/– Jnk2–/+ mutant mice survived to adulthood but were sterile and had opaque eyes (Fig. 1). No defect in eyelid closure was observed in mice lacking individual Jnk genes (Jnk1–/–, -2–/–, or -3–/–) or in mice with compound mutations in either Jnk1 and -3 (Jnk1–/– Jnk3–/–) or -2 and -3 (Jnk2–/– Jnk3–/–).
We investigated the defect in mechanism that might account for the failure of eyelid closure in JNK-deficient embryos. JNK phosphorylates c-Jun on sites within the activation domain (1). We therefore used immunohistochemical staining of phosphorylated c-Jun as an in situ indicator of the biological activity of JNK in vivo. Phosphorylated c-Jun was present at the leading edge of the eyelid epithelium during eyelid closure in control embryos, whereas very little phosphorylated c-Jun was detectable in JNK-deficient littermates (Fig. 2). These data demonstrate that JNK-dependent phosphorylation of c-Jun occurs during normal eyelid closure, and that the JNK signaling pathway is severely attenuated in JNK-deficient (Jnk1–/– Jnk2–/+) mice.
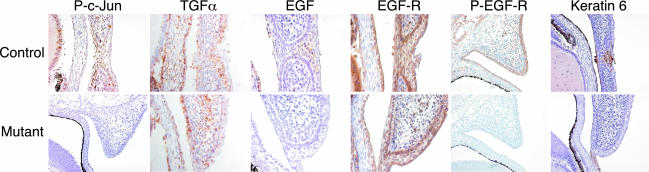
Fig. 2.
JNK regulates EGF expression during eyelid closure. E16.5 eyelids of control (Jnk1+/+ Jnk2–/+) and mutant (Jnk1–/– Jnk2–/+) littermates were examined by immunohistochemical analysis (EGFR, Ser-63-phosphorylated c-Jun, and keratin 6) and by in situ hybridization (TGF-α and EGF mRNA). Immunohistochemical staining of E15.5 eyelids was performed by using an antibody to the Tyr-1068-phosphorylated EGFR.
Decreased Signaling by the EGFR in the Epidermis of JNK-Deficient Mice. It is established that the EGFR is required for embryonic eyelid closure (9). Because JNK-deficient mice have open eyes at birth, we tested whether these mice exhibited defects in EGFR activation in the skin. It has been reported that c-Jun-deficient mice express low levels of the EGFR in the skin (5). In contrast, no difference in EGFR expression between control and JNK-deficient eyelids was detected at E16.5 (Fig. 2). However, a marked decrease in tyrosine-phosphorylated EGFR was detected in JNK-deficient embryos, compared with control littermates, at both E16.5 (data not shown) and E15.5 (Fig. 2). These observations indicate that JNK-deficient embryos exhibit normal levels of EGFR expression but decreased EGFR signaling during development. This decrease in EGFR signaling may account for the observation that JNK-deficient mice are born with open eyes.
JNK Deficiency Causes Decreased Expression of EGF in the Epidermis.
To test whether the decreased EGFR signaling observed in the epidermis of JNK-deficient mice was caused by defects in the expression of a ligand for the EGFR, we examined the expression of TGF-α, a ligand that has been implicated in the regulation of eyelid closure (9). Consistent with previous reports, we observed TGF-α expression in the advancing eyelid tip epithelium during eyelid closure in wild-type embryos. However, no difference in TGF-α expression was detected when JNK-deficient embryos were compared with control littermates (Fig. 2).
We investigated EGF mRNA expression by in situ hybridization to examine whether altered expression of this ligand may be responsible for the markedly reduced EGFR activation observed in the JNK-deficient epidermis. EGF was expressed in the eyelid tip epithelium at E16.5 in control embryos but was not detected in the eyelids of JNK-deficient embryos (Fig. 2). This observation implies that JNK is required for EGF expression in the epidermis.
Periderm cells participating in temporary epithelial fusions, such as eyelid closure, express keratins 6 and 17 (13). At E16.5, keratin 6 was expressed in the eyelids of control embryos, but only a small amount of keratin 6 was detected at the leading edges of the eyelid epithelium in JNK-deficient embryos (Fig. 2). Interestingly, EGF can induce the expression of keratin 6 via the AP-1 transcription factor site in its promoter (14). This observation is consistent with the finding that neither EGF nor keratin 6 are expressed in the eyelids of E16.5 JNK-deficient mice.
JNK-Deficient Mice Exhibit Epidermal Immaturity. The formation and maintenance of the interfollicular epidermis relies on a tightly balanced process of proliferation and differentiation. The initial stratification of the single-layered ectoderm during embryonic development gives rise to an outer periderm layer and an inner basal layer. The embryonic basal layer acts as a precursor to all skin epithelia in the adult and undergoes a high level of proliferation to increase its surface area as the organism grows in size. All newborn Jnk1–/– Jnk2–/+ pups had pale skin with fewer hair follicles than did control littermates, reflecting immature development (Fig. 3). Interfollicular epidermal proliferation, which was monitored by immunohistochemistry for PCNA, was reduced in E15.5 JNK-deficient skin, compared with control littermates (Fig. 3). This reduction in cell proliferation was primarily restricted to the epidermis, because no reduction was observed in tissues such as the liver or skeletal muscle. The p63 transcription factor is a p53 homolog that is essential for regenerative proliferation in epithelial development. p63 is a marker of keratinocyte stem cells because it is expressed only in keratinocyte stem cells and not in their transient amplifying progeny (15). Consistent with decreased epidermal proliferation, p63 was expressed in the epidermis of control embryos, but the expression of p63 was markedly reduced in JNK-deficient littermates at E15.5 (Fig. 3).
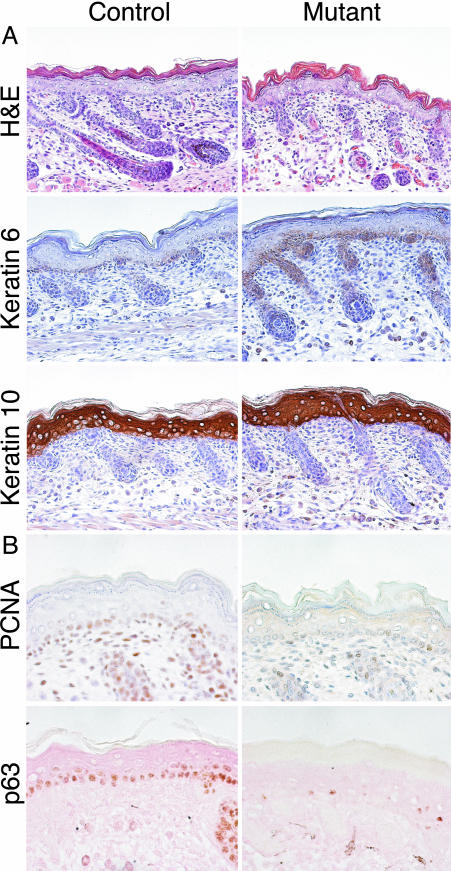
Fig. 3.
JNK-deficient mice display immature epithelial development. (A) H&E staining of E18.5 skin illustrates disorganized and immature development of the hair follicles and skin structure in mutant (Jnk1–/– Jnk2–/+) embryos, compared with control littermates. Immunohistochemical staining illustrates that keratin 6 is expressed more strongly in mutant P1 pups than in control littermates, whereas the expression of keratin 10 is comparable. (B) Immunohistochemical analysis of E15.5 mutant embryos demonstrates decreased expression of PCNA and p63 in the epidermis, compared with control littermates.
Skin differentiation begins at E13.5 and is completed by E16.5. Differentiation-specific gene expression is tightly regulated by a wide range of growth factors including EGF, keratinocyte growth factor (KGF), TGF-α, and TGF-β (16). To determine whether differentiation also was perturbed in JNK-deficient embryos, we examined several markers of differentiation, including keratin 10, which is expressed in the suprabasal layer of the epidermis, and keratin 6, which is rapidly up-regulated during epidermal wound healing and can be an indication of damaged tissue (16). There were no major differences in keratin 10 staining, but JNK mutant (Jnk1–/– Jnk2–/+) mice expressed keratin 6 in the skin and ectopically in the region just above the hair follicle (Fig. 3). This finding is consistent with the keratin 6 expression observed in EGFR-deficient mice (9) and is indicative of skin defects.
JNK- and EGFR-Deficient Mice Have Similar Defects in Epidermal Development. The EGFR and its ligands are expressed in the intestinal mucosa where they regulate of the proliferation of enterocytes. EGFR-deficient mice exhibit reduced enterocyte proliferation, haemorrhagic intestines, and destruction of villi, and mice lacking tumor necrosis factor α converting enzyme display delayed intestinal maturation (9). To investigate whether JNK-deficient mice exhibited decreased expression of EGF in the intestines, we performed in situ hybridization. Consistent with previous reports (9), EGF was expressed in the intestinal mucosa of control embryos, with strong expression in villous epithelium. This expression was severely reduced in JNK-deficient littermates, suggesting that JNK is necessary for EGF expression in the intestines (Fig. 4).
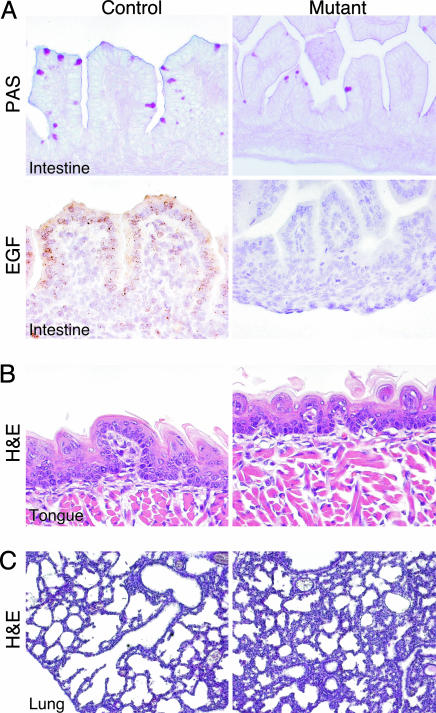
Fig. 4.
JNK-deficient mice exhibit developmental defects in the intestines, lungs, and tongue. (A) The number of periodic acid/Schiff reagent-staining mucous cells was reduced in the intestines of mutant E15.5 embryos, compared with control littermates. The villi also were smaller and developmentally immature. EGF mRNA expression was detected by in situ hybridization in the intestines of control E15.5 embryos but not in the intestinal epithelium of mutant (Jnk1–/– Jnk2–/+) littermates. (B) The epithelium of the tongue of mutant embryos was immature at E18.5 with no fungiform taste papillae (H&E). (C) JNK-deficient P1 mice have immature lungs with thicker alveolar septae than littermate controls (H&E).
JNK-deficient pups could feed and had milk in their stomachs, but their intestines were immature, compared with control littermates. The intestines of JNK-deficient pups had all of the differentiated cell types but exhibited irregular loops and shorter villi, compared with control littermates (Fig. 4). The delayed maturation of the villi was apparent at E14.5, soon after the villi start to form. The number of periodic acid/Schiff reagent-staining surface mucous cells was reduced in JNK-deficient embryos, compared with controls. The small intestinal crypt–villus architecture was preserved, and absorptive epithelial and goblet cell lineages were present. Defects in the JNK-deficient intestines were more severe in embryos than after birth, indicative of delayed development. Milk, especially colostrum, contains high levels of EGF and TGF-α that stimulate proliferation and maturation of the gastrointestinal tract of newborn infants (17). The presence of EGF in milk may account for the more normal intestinal morphology of JNK-deficient mice after birth.
The epithelium of the tongue appeared to be immature at E18.5, with no fungiform taste papillae (Fig. 4). At E15.5, there was a reduction in proliferation, as determined by PCNA immunohistochemistry, in the tongue epidermis of JNK-deficient embryos (data not shown). Keratin 6 was expressed in the oral epithelium of E15.5 control embryos, but this expression was markedly reduced in JNK-deficient littermates (data not shown). Together, these data indicate that JNK-deficient mice, like EGFR-deficient mice, exhibit delayed development of the tongue epithelium.
The newborn JNK-deficient mice were pale in color and appeared to have difficulty breathing. Consistent with these breathing difficulties, the lungs of the JNK-deficient mice were abnormally developed. In contrast to the thin alveolar epithelium of control mice, the alveolar septae of JNK-deficient mice were thicker and more cellular (Fig. 4). This immaturity of lung development resembles neonatal distress syndrome. However, there was no reduction in the expression of the surfactant proteins A and C in the lungs of JNK-deficient mice, compared with control littermates (data not shown). The defective lung morphology of JNK-deficient mice was similar to that observed in EGFR-deficient mice (9).
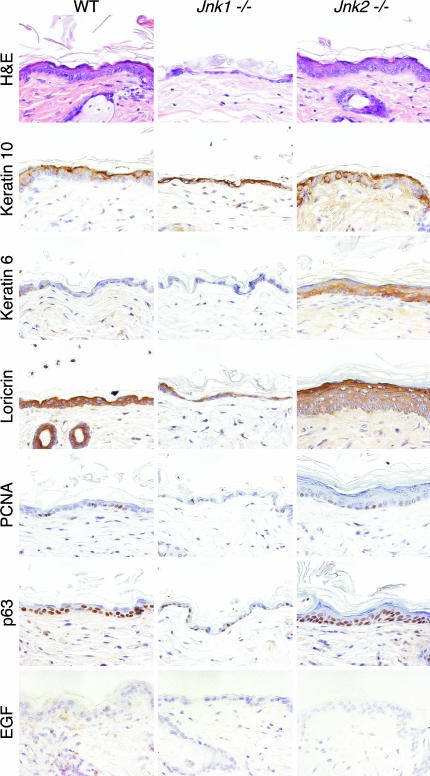
JNK-Deficient Adult Mice Exhibit Skin Defects. The majority of JNK-deficient (Jnk1–/– Jnk2–/+) mice died within 48 h after birth; it was therefore not possible to study the adult skin. We therefore examined the skin of adult mice lacking only either Jnk1 or -2. H&E staining of skin sections from age-matched mice revealed that the epidermis of Jnk1–/– mice was thin and consisted of fewer cell layers, compared with skin from wild-type controls (Fig. 5). Keratohyalin granules, which are characteristic markers of epidermal differentiation, were missing in Jnk1–/– mice, and the number of proliferating cells in the stratum basale was markedly reduced (Fig. 5). This finding was confirmed by the strongly reduced expression of the late differentiation marker loricrin (Fig. 5). Keratin 10 was present in the epidermis of Jnk1–/– mice, but there was no expression of keratin 6 (Fig. 5). These defects were associated with markedly reduced expression of EGF mRNA (Fig. 5). Interestingly, the organization of the skin in Jnk1–/– mice resembles that observed in a reconstituted skin model composed of human keratinocytes and c-Jun–/– fibroblasts (18).
Fig. 5.
The epidermis of adult JNK-deficient mice is abnormal, with aberrant expression of markers of differentiation. H&E staining of skin sections from 16-week-old mice demonstrates that Jnk1–/– mice have a thin epidermis, whereas Jnk2–/– mice have a thick epidermis, compared with wild-type controls. Immunohistochemical staining for keratins 6 and 10, loricrin, PCNA, and p63 indicates abnormal expression in JNK-deficient animals. Markedly reduced expression of EGF mRNA in the skin of Jnk1–/– and -2–/– animals, compared with wild-type controls, was detected by in situ hybridization.
Mice lacking JNK2 exhibited a very different phenotype in the skin, compared with mice lacking JNK1. Tissue sections from the skin of Jnk2–/– mice revealed keratinocyte hyperplasia, resulting in an increased number of epithelial cell layers (Fig. 5). The zone of keratin 10 expression in the skin was enlarged, and more keratohyalin granules were observed in the stratum granulosum, compared with wild-type mice (Fig. 5). The localization of loricrin-expressing cells was markedly increased (Fig. 5), and keratin 6 was up-regulated, indicative of skin defects. In addition to enhanced cell proliferation, there was an increase in p63-positive cells (Fig. 5), indicating a greater number of keratinocyte stem cells in Jnk2–/– skin, compared with wild-type skin (19). These data indicate that JNK1 and -2 have both redundant and nonredundant functions in epidermal development.
Discussion
JNK deficiency causes defective epidermal development in multiple tissues, including the skin, intestines, and lungs (Figs. 3 and 4). Thus, the skin of JNK-deficient mice is altered with changes in both proliferation and cellular differentiation (Fig. 5). In contrast to these major changes in epidermal development in JNK-deficient mice, the development of the skin in c-Jun-deficient mice was normal (6, 7). These data indicate that whereas JNK is essential for normal epidermal development, c-Jun is not.
Although the skin phenotype of c-Jun- and JNK-deficient mice is very different, there is one similar defect in the epidermis of these mice. Both c-Jun- (6, 7) and JNK-deficient mice (Fig. 1) have open eyes at birth because of a failure of embryonic eyelid closure at E15.5. The eyelid closure defect in the c-Jun-deficient mice was caused by reduced expression of both the EGFR and heparin-binding EGF-like growth factor (HB-EGF) in eyelids (6, 7). In contrast, no decrease in EGFR expression was detected in JNK-deficient eyelids (Fig. 2). However, decreased EGFR signaling in JNK-deficient eyelids (detected by staining with an antibody to the tyrosine-phosphorylated EGFR) was observed (Fig. 2). The decreased EGFR signaling in JNK-deficient mice correlated with a marked reduction in the expression of EGF in the skin and intestine (Figs. 2, 4, and 5). Interestingly, the decreased expression of EGF was found in multiple epidermal tissues and was not restricted to the eyelids. This loss of EGF expression most likely accounts for the effect of JNK deficiency to cause widespread defects in epidermal development. In contrast, the epidermal defects in the development of c-Jun-deficient mice were restricted to the eyelids.
One implication of the conclusion that different epidermal phenotypes are caused by JNK/c-Jun deficiency is that c-Jun is not an essential mediator of JNK function during epidermal development. This implication is consistent with the finding that mice with point mutations at the sites of JNK phosphorylation on c-Jun (Ser-63 and -73, replaced with Ala) do not have an eyelid closure defect or obvious epidermal developmental defects (8). Similarly, epidermal developmental defects were not reported for mice in which c-Jun was replaced with JunB, although these mice were born with open eyes, consistent with a role for c-Jun during eyelid closure (4). Together, these data indicate that the major effects of JNK on epidermal development are independent of c-Jun.
EGF Is a Target of the JNK Signaling Pathway. There are striking similarities in the phenotypes of JNK-deficient mice (Figs. 1, 4, and 5) and mice with defective EGFR signaling (9). Like EGFR-deficient mice, we observed epithelial immaturity in the skin, intestines, lungs, and the tongue of JNK-deficient mice (Figs. 3, 4, 5). Both EGFR- and JNK-deficient mice exhibit a major delay in the development of these tissues. This phenotypic similarity can be accounted for by the observation that JNK-deficient mice display reduced EGFR signaling, monitored by examination of EGFR tyrosine phosphorylation, and markedly reduced expression of the ligand EGF. The reduced expression of EGF may contribute to the decrease in EGFR signaling. Detailed analysis of the EGF promoter indicates the presence of multiple regulatory elements, including two AP-1 sites (20). Because it is established that JNK can control gene expression by regulating AP-1 transcription activity (21), it is likely that the loss of EGF expression caused by JNK deficiency is caused, at least in part, by decreased AP-1 transcription activity.
JNK1 and -2 Play Different Roles in Interfollicular Epidermal Development. The specialized nonredundant roles of JNK1 and -2 during epidermal development appear to be different (Fig. 5). Major differences in epidermal structure, keratinocyte proliferation, and the expression of markers of differentiation were observed. The epidermis of Jnk1–/– mice was thinner than that of wild-type mice, with reduced proliferation, reduced numbers of p63-positive keratinocyte stem cells, and decreased expression of differentiation markers. This finding is consistent with a role for JNK in epidermal proliferation (22). In contrast, the epidermis of Jnk2–/– mice was thicker than that of wild-type mice, with hyperproliferation, increased numbers of p63-positive keratinocyte stem cells, and increased expression of differentiation markers. The difference between the skin of Jnk1–/– and -2–/– mice may reflect the different biochemical properties of the JNK1 and -2 protein kinases (23). It is therefore interesting that Jnk1-deficient mice have enhanced skin tumor formation in response to a chemical carcinogen (24), whereas Jnk2-deficient mice exhibit suppression of skin tumorigenesis (25). The differences we describe in epidermal structure between Jnk1–/– and -2–/– mice might therefore contribute to their opposing susceptibility to skin tumorigenesis.
Acknowledgments
We thank D. Lee (University of North Carolina, Chapel Hill) for the TGF-α probe, J. Reilly and L. Lesco for expert technical assistance, and K. Gemme for administrative assistance. These studies were supported by a grant from the National Cancer Institute. R.A.F. and R.J.D. are Investigators of the Howard Hughes Medical Institute.
Abbreviations: AP-1, activator protein 1; EGF, epidermal growth factor; EGFR, EGF receptor; En, embryonic day n; H&E, hematoxylin/eosin; JNK, c-Jun NH2-terminal kinase; PCNA, proliferating cell nuclear antigen; Pn, postnatal day n; TGF, transforming growth factor.
References
- 1.Davis, R. J. (2000) Cell 103, 239–252. [DOI] [PubMed] [Google Scholar]
- 2.Weston, C. R. & Davis, R. J. (2002) Curr. Opin. Genet. Dev. 12, 14–21. [DOI] [PubMed] [Google Scholar]
- 3.Angel, P., Szabowski, A. & Schorpp-Kistner, M. (2001) Oncogene 20, 2413–2423. [DOI] [PubMed] [Google Scholar]
- 4.Passegue, E., Jochum, W., Behrens, A., Ricci, R. & Wagner, E. F. (2002) Nat. Genet. 30, 158–166. [DOI] [PubMed] [Google Scholar]
- 5.Grose, R. (2003) Curr. Biol. 13, R678–R680. [DOI] [PubMed] [Google Scholar]
- 6.Li, G., Gustafson-Brown, C., Hanks, S. K., Nason, K., Arbeit, J. M., Pogliano, K., Wisdom, R. M. & Johnson, R. S. (2003) Dev. Cell 4, 865–877. [DOI] [PubMed] [Google Scholar]
- 7.Zenz, R., Scheuch, H., Martin, P., Frank, C., Eferl, R., Kenner, L., Sibilia, M. & Wagner, E. F. (2003) Dev. Cell 4, 879–889. [DOI] [PubMed] [Google Scholar]
- 8.Behrens, A., Sibilia, M. & Wagner, E. F. (1999) Nat. Genet. 21, 326–329. [DOI] [PubMed] [Google Scholar]
- 9.Wong, R. W. (2003) Cell Mol. Life Sci. 60, 113–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weston, C. R., Wong, A., Hall, J. P., Goad, M. E., Flavell, R. A. & Davis, R. J. (2003) Genes Dev. 17, 1271–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xia, Y. & Karin, M. (2004) Trends Cell Biol. 14, 94–101. [DOI] [PubMed] [Google Scholar]
- 12.Cohen, S. (1962) J. Biol. Chem. 237, 1555–1562. [PubMed] [Google Scholar]
- 13.Mazzalupo, S. & Coulombe, P. A. (2001) Mech. Dev. 100, 65–69. [DOI] [PubMed] [Google Scholar]
- 14.Bernerd, F., Magnaldo, T., Freedberg, I. M. & Blumenberg, M. (1993) Gene Expression 3, 187–199. [PMC free article] [PubMed] [Google Scholar]
- 15.McKeon, F. (2004) Genes Dev. 18, 465–469. [DOI] [PubMed] [Google Scholar]
- 16.Fuchs, E. & Raghavan, S. (2002) Nat. Rev. Genet. 3, 199–209. [DOI] [PubMed] [Google Scholar]
- 17.Okada, M., Ohmura, E., Kamiya, Y., Murakami, H., Onoda, N., Iwashita, M., Wakai, K., Tsushima, T. & Shizume, K. (1991) Life Sci. 48, 1151–1156. [DOI] [PubMed] [Google Scholar]
- 18.Szabowski, A., Maas-Szabowski, N., Andrecht, S., Kolbus, A., Schorpp-Kistner, M., Fusenig, N. E. & Angel, P. (2000) Cell 103, 745–755. [DOI] [PubMed] [Google Scholar]
- 19.Pellegrini, G., Dellambra, E., Golisano, O., Martinelli, E., Fantozzi, I., Bondanza, S., Ponzin, D., McKeon, F. & De Luca, M. (2001) Proc. Natl. Acad. Sci. USA 98, 3156–3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fenton, S. E., Groce, N. S. & Lee, D. C. (1996) J. Biol. Chem. 271, 30870–30878. [DOI] [PubMed] [Google Scholar]
- 21.Ventura, J. J., Kennedy, N. J., Lamb, J. A., Flavell, R. A. & Davis, R. J. (2003) Mol. Cell. Biol. 23, 2871–2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang, J. Y., Green, C. L., Tao, S. & Khavari, P. A. (2004) Genes Dev. 18, 17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gupta, S., Barrett, T., Whitmarsh, A. J., Cavanagh, J., Sluss, H. K., Derijard, B. & Davis, R. J. (1996) EMBO J. 15, 2760–2770. [PMC free article] [PubMed] [Google Scholar]
- 24.She, Q. B., Chen, N., Bode, A. M., Flavell, R. A. & Dong, Z. (2002) Cancer Res. 62, 1343–1348. [PubMed] [Google Scholar]
- 25.Chen, N., Nomura, M., She, Q. B., Ma, W. Y., Bode, A. M., Wang, L., Flavell, R. A. & Dong, Z. (2001) Cancer Res. 61, 3908–3912. [PubMed] [Google Scholar]