Abstract
Pitcher plants of the genus Nepenthes have highly specialized leaves adapted to attract, capture, retain, and digest arthropod prey. Several mechanisms have been proposed for the capture of insects, ranging from slippery epicuticular wax crystals to downward-pointing lunate cells and alkaloid secretions that anesthetize insects. Here we report that perhaps the most important capture mechanism has thus far remained overlooked. It is based on special surface properties of the pitcher rim (peristome) and insect “aquaplaning.” The peristome is characterized by a regular microstructure with radial ridges of smooth overlapping epidermal cells, which form a series of steps toward the pitcher inside. This surface is completely wettable by nectar secreted at the inner margin of the peristome and by rain water, so that homogenous liquid films cover the surface under humid weather conditions. Only when wet, the peristome surface is slippery for insects, so that most ant visitors become trapped. By measuring friction forces of weaver ants (Oecophylla smaragdina) on the peristome surface of Nepenthes bicalcarata, we demonstrate that the two factors preventing insect attachment to the peristome, i.e., water lubrication and anisotropic surface topography, are effective against different attachment structures of the insect tarsus. Peristome water films disrupt attachment only for the soft adhesive pads but not for the claws, whereas surface topography leads to anisotropic friction only for the claws but not for the adhesive pads. Experiments on Nepenthes alata show that the trapping mechanism of the peristome is also essential in Nepenthes species with waxy inner pitcher walls.
Pitcher plants of the families Cephalotaceae, Nepenthaceae, and Sarraceniaceae are famous for conspicuous leaves that have evolved into organs capable of capturing and digesting arthropods. Nitrogen derived from digested prey helps these plants to survive in nutrient-poor habitats. The functional morphology of pitchers and their trapping mechanism has long attracted the interest of biologists (1–4). It was recognized that pitchers consist of morphologically distinct zones with different functions (2, 5). Insects are attracted by extrafloral nectar, flower fragrance (6), or UV light absorption patterns near the pitcher opening (6, 7). When visiting the pitchers, insects can fall into the traps, from which they are mostly unable to escape, and are digested by enzymes of the pitcher fluid and by the infauna inhabiting it. The numerous studies on the function of Nepenthes pitchers have focused on the mechanism of insect attraction (6, 7), on the trapping of insects by alkaloid anesthesia (8), by slippery epicuticular wax crystals (3, 5, 9, 10) or by downward-pointing lunate cells (3) of the inner pitcher wall, on the properties of the glandular zone (11), and on the nature of the digestive fluid (12–14). Only recently, L. Gaume et al. (15) conducted the first comprehensive study on the trapping mechanism of Nepenthes by comparing the effect of the different pitcher surface zones and by separating the mechanisms of prey capture and retention. Observations of insects placed on Nepenthes alata pitchers suggested that the inner waxy pitcher wall is the most important surface zone for the initial capture of insects (15). These results confirmed earlier observations that insects are trapped when they step on the waxy zone while visiting the nectaries on the inner side of the pitcher rim (peristome) (e.g., refs. 2, 5, 7). The waxy zone of Nepenthes pitchers is characterized by platelet-shaped aldehyde crystals protruding perpendicularly from the surface (9). These platelets not only detach and contaminate the surface of insect adhesive pads but also appear to interact with the insect's adhesive secretion to form an amorphous substance that impedes attachment (10).
However, the consensus about the waxy zone as the principal trapping mechanism in Nepenthes pitchers is impaired by the fact that in several Nepenthes species (e.g., Nepenthes ampullaria, Nepenthes bicalcarata, and Nepenthes ventricosa), the waxy zone is absent. N. ampullaria pitchers were found to accumulate necromass mainly of botanical origin and may thus be considered detritivorous rather than insectivorous (16, 17). However, data on the prey spectrum and capture efficiency of N. bicalcarata clearly show that pitchers of this species are fully functional insect traps despite the absence of a waxy zone (18, 19). Like many other Nepenthes species, N. bicalcarata captures primarily ants (18, 20). What is the capture mechanism in this and other species, where no slippery wax crystal surfaces are present?
Materials and Methods
Field Work. Observations and field experiments on N. bicalcarata were conducted at various stands in degraded Shorea albida-dominated peat swamp forests in Brunei, northwest Borneo. We investigated the trapping mechanism of N. bicalcarata by observing the behavior of ants on the pitcher surface. Tests were conducted by using five ant species of different body sizes belonging to the natural prey spectrum of N. bicalcarata at our study site [dry weights: Crematogaster inflata, 0.66 ± 0.12 mg; Camponotus (Colobopsis) sp., 2.4 ± 1.1 mg; Camponotus sp., 2.9 ± 1.8 mg; Polyrhachis hector, 18.4 ± 6.5 mg; and Polyrhachis cf. beccarii, 21.0 ± 8.2 mg; means ± SD of n = 20 workers per species]. To bring large numbers of ants into contact with N. bicalcarata pitchers, we collected partial colonies (≈50–300 workers) and kept them in plastic containers side-coated with slippery Fluon (Whitford, Diez) to prevent ants from escaping. N. bicalcarata pitchers were placed upright on a support inside the plastic container so that the ants had access. We recorded the ants' behavior for 5–10 min using a video camera [Sony (Tokyo) DCR-PC120E]. To correct for varying numbers of foraging ants, we measured trapping efficiency as the number of trapped ants per “peristome visits” (ants with all legs in contact with the peristome passing the peristome center line were assessed as peristome visit). In a first set of experiments, we compared the trapping efficiency of pitchers with natural (dry) and wetted peristomes using the listed ant species. A more extensive experimental protocol was performed with Oecophylla smaragdina. We recorded the ants' behavior on the pitcher (i) with an untreated (dry) peristome, (ii) after wetting the peristome surface using an atomizer, (iii) after drying it with dust-free tissue, and (iv) after rewetting it again.
In a second experiment, we investigated whether ants that have fallen into N. bicalcarata pitchers are able to escape from the traps and which parts of the pitcher are responsible for their retention. Ants were allowed to walk on strips of plastic foil coated at the tip with Fluon. By gently turning the strip when the ant was walking on the slippery part, we made the ants fall into the pitchers from the height of the peristome. Two hundred twenty-eight ants (30–60 ants of each species) were tested and observed for 30 min.
Morphology. Fresh pitcher samples from the greenhouse were frozen in liquid nitrogen and dried overnight in a lyophilizer at –40°C, sputtered with platinum–palladium for 5 min at 25 mA, and observed in a Zeiss DSM 962 scanning electron microscope (working voltage 5–15 kV). To obtain SEM images of ant tarsi in contact with the peristome (Fig. 3A), we anesthetized O. smaragdina ants and glued them with melted wax to N. bicalcarata peristomes. Probes of live ants were observed without sputtering at a working voltage of 3 kV.
Fig. 3.
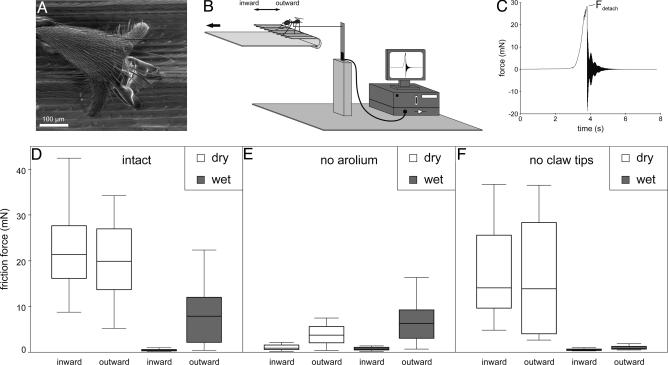
Friction forces of O. smaragdina ants on the peristome of N. bicalcarata pitchers. (A) O. smaragdina tarsus on the peristome of N. bicalcarata; SEM of live ant. (B) Experimental setup. (C) Example of detachment force recording. (D–F) Friction forces measured on dry vs. wet peristomes and for inward vs. outward pulls. (D) Ants with intact hind legs. (E) Ants with arolia removed (but intact claws). (F) Ants with clipped claws (but intact arolia). Horizontal lines denote medians, boxes mark the inner two quartiles, and whiskers mark the maxima and minima.
Friction of O. smaragdina Ants on the Peristome of N. bicalcarata. We investigated the effect of peristome wetting and surface topography on the performance of claws and adhesive pads. Friction forces of O. smaragdina ants were measured on the peristome of N. bicalcarata under variation of peristome wetting and insect pulling direction. We attached ants to a strain gauge force transducer via an ≈20-cm-long thread tied to the thorax with a loose loop between the front and middle legs. The ants were placed on the peristome surface held in horizontal orientation. Due to the small size of the peristome (which made it difficult to bring all six legs into contact) and to the possible variation caused by variable numbers of legs holding onto the surface, we excised the pretarsi of the ants' front and middle legs. As a consequence of this treatment, all ants attained the same body orientation during the experiments (facing toward the thread), with the two hind legs in contact with the peristome (Fig. 3B). We slowly moved the peristome surface away from the force transducer and recorded friction forces until the ant detached. The detachment force peak was used for further analysis (Fig. 3C). For each ant, measurements were performed under four different test conditions. These were “dry” and “wetted” peristome and pulling the ant toward the inside of the pitcher (“inward”) or toward the outside (“outward”). The order of the four consecutive measurements (dry inward, wet inward, dry outward, and wet outward) was randomized. A total of 62 experimental cycles (consisting of four measurements each) were carried out on 13 ants (three to seven cycles per individual). Experiments were performed on three groups of ants. Apart from (i) ants with intact hind legs, we tested (ii) ants with arolia removed (but intact claws) and (iii) ants with clipped claws (but intact arolia). Tethering and ablation operations were conducted on ants anesthetized by CO2, which were allowed to recover for at least 1 hr before the experiments.
Effect of Peristome in Nepenthes Species with Waxy Inner Pitcher Walls. To identify the surfaces responsible for insect capture in Nepenthes species with waxy inner pitcher walls, we conducted experiments on N. alata pitchers and Messor barbarus ants. We placed ants individually onto the outer wall of a fresh pitcher directly below the peristome and observed them for 5 min. To obtain detailed information on the position of the ants' legs before falling, we used two high-speed video cameras [Redlake (San Diego) PCI 1000 B/W], which recorded the peristome and upper part of the waxy inner wall of the entire pitcher at 50 frames per second. In most cases, the ants were trapped between the peristome and the waxy zone with their legs standing on both surfaces. We determined the number of legs in contact with the peristome and the waxy zone immediately before falling. Capture events were compared between dry and wetted peristomes on two pitchers with 30 ants for each pitcher and experimental condition.
To evaluate the contribution of the peristome to the total trapping efficiency in N. alata, we used a pitcher where the left half of the peristome was dry and the right half was wetted with water. To prevent water from spreading onto the dry section of the peristome, we cut a thin vertical gap into the peristome. The pitcher was brought into contact with a colony compartment containing ≈50 ants and filmed for 160 sec (n = 7 repetitions). We determined (i) the absolute number of ants trapped on both sides of the pitcher and (ii) the number of trapped ants per peristome visit (defined as all legs being in contact with the peristome).
Results
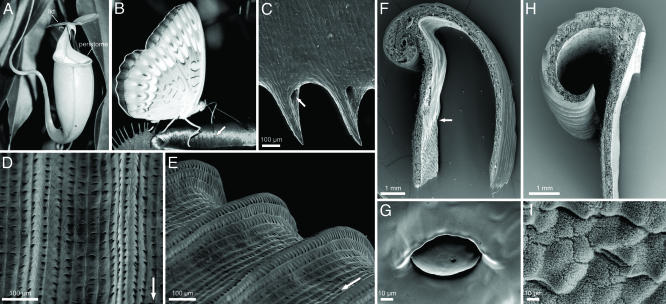
Morphology. Because the general morphology of Nepenthes pitchers has been covered extensively by others (2, 5, 21), only aspects relevant for this study will be presented here. The edge of the pitcher mouth (peristome) is a broad collar-shaped structure (Fig. 1A). In transverse section, it is T-shaped, with both horizontal arms of the T sloping downward (Fig. 1 F and H). In N. bicalcarata, the outer arm of the T is short and reflexed, whereas the inner arm is strongly elongated (≈10–20 mm; Fig. 1F), so that the peristome has a long, almost vertical slope toward the inside of the pitcher (the inner arm is shorter, ≈1–5 mm in N. alata; Fig. 1H). The peristome surface has a very regular microstructure consisting of first- and second-order radial ridges formed by straight rows of epidermal cells (Fig. 1 D and E). Each epidermal cell overlaps the cell adjacent to the pitcher inside, so that the surface contains a series of steps toward the pitcher inside and is anisotropic. The zone adjoining the peristome toward the pitcher inside is covered with wax crystals in N. alata (Fig. 1I) but is smooth in N. bicalcarata (Fig. 1G). The glands with hoods characteristic of the digestive zone (Fig. 1 F and G) indicate that in N. bicalcarata, this zone extends upward to the level of the peristome, whereas the digestive zone in N. alata is restricted to the lower part of the pitcher beneath the waxy zone.
Fig. 1.
Nepenthes pitcher and peristome morphology. (A–G) N. bicalcarata. (A) Pitcher. (B) Butterfly (probably Tanaecia pelea pelea) harvesting nectar from the peristome surface. Note the visible line of peristome channels filled with nectar secreted from pores at the inner margin of the peristome (arrow). (C) Underside of inner margin of peristome with tooth-like projections and nectar pores (arrow). (D and E) Peristome surface with first- and second-order radial ridges. Arrows indicate direction toward the inside of the pitcher (F) Transverse section of peristome. Note the transition from the digestive zone to the smooth surface under the peristome (arrow). (G) Inner pitcher wall with digestive gland at the height of the inner peristome margin (H and I) N. alata.(H) Transverse section of peristome. (I) Waxy inner pitcher wall at the height of the inner peristome margin.
Trapping Function of the Pitcher Peristome in N. bicalcarata. We discovered the trapping effect of the peristome in the field after a heavy rainfall. Our initial observations on N. bicalcarata had seemingly confirmed earlier reports that the trapping of insects by a Nepenthes pitcher was a rare event witnessed only sporadically (18). However, 1 hr after a heavy afternoon rainfall, we observed a stand of N. bicalcarata visited by numerous Crematogaster ants. In striking contrast to our previous observations, most of the ants that stepped on the peristome helplessly slipped on its surface and fell into the pitchers. The large number of ants still moving inside the digestive fluid or on its surface gave evidence that large numbers of ants had been captured within a very short period.
The slippery peristome was completely wetted by rain water; its surface appeared glossy, and no individual droplets could be seen. We verified the exceptional wettability of the peristome surface by placing small water droplets on dry N. bicalcarata peristomes. The droplets spread out rapidly across the peristome with a speed of ≈20 mm/sec. Spreading was not uniform in all directions but clearly followed the radial ridges (inward and outward). Direction-dependent wetting explains the presence of visible “nectar lines” transported from nectaries at the inner margin of the peristome onto its surface (Fig. 1B).
We tested the effect of peristome wetting in the field by bringing large numbers of ants into contact with N. bicalcarata pitchers (Table 1). On untreated dry peristome surfaces, the ants had no problem walking, and only few workers were trapped by the pitchers. In all four ant species tested, the wetted peristome was significantly more effective at capturing ants than the dry peristome. The wet peristome was so slippery that most (or all) of the visiting ants fell into the pitchers (Table 1). Only in C. inflata, 11 ants did not fall into the pitcher directly but slid to the inner margin of the peristome and were unable to climb out again. Ultimately, these ants may either fall prey to the pitcher or may escape once the peristome has dried and is less slippery again. The results of a more extensive field experiment conducted with O. smaragdina, which included drying and rewetting the peristome, show the same effect (Fig. 2A). After the peristome was wiped dry, its surface was no longer slippery. Subsequent rewetting of the peristome completely restored its slipperiness (Fig. 2 A).
Table 1. Effect of peristome wetting on the trapping efficiency in N. bicalcarata.
| Peristome condition
|
|||
|---|---|---|---|
| Ant species | Natural (dry), visits/falls | Wetted, visits/falls | P |
| C. inflata | 89/4 | 53/37 | <0.001 |
| Camponotus (Colobopsis) sp. | 37/0 | 14/14 | <0.001 |
| O. smaragdina | 18/0 | 21/21 | <0.001 |
| Polyrhachis cf. beccarii | 26/0 | 12/10 | <0.001 |
P values give Fisher's exact test statistics. Ants with all legs in peristome contact that passed the peristome center line were counted as peristome visits.
Fig. 2.
Initial capture and prey retention in N. bicalcarata. (A) Effect of peristome wetting, drying, and rewetting on the capture efficiency in N. bicalcarata pitchers; O. smaragdina ants. (B) Retention of prey ants in N. bicalcarata pitchers. Data show the condition of ants 30 min after being dropped into the pitcher. Ci, C. inflata; Cc, Camponotus (Colobopsis) sp.; Cs, Camponotus sp.; Ph, P. hector; Pb, Polyrhachis cf. beccarii.
Retention of Insect Prey. Fig. 2B shows that ant species differed strongly in their capacity to escape from N. bicalcarata pitchers (escaped vs. not escaped: χ2 = 89.0, df = 4, P < 0.001; drowned vs. not drowned; χ2 = 38.3, df = 4, P < 0.001). Retention was based on different mechanisms, depending on the ant species. When falling into the pitcher fluid, most of the small C. inflata (and some Camponotus spp.) remained stuck to the fluid meniscus. Due to surface tension forces, they mostly did not manage to get a grip on the pitcher wall. In contrast, most of the larger ants passed the surface film upon falling. Once submerged in the fluid, most ants failed to escape and stopped moving after ≈60 sec. The only conspicuous exception among the tested ant species was Polyrhachis cf. beccarii, which showed coordinated underwater swimming movements. They mostly managed to hold onto the pitcher surface, walk out of the digestive fluid, and climb up the inner pitcher wall. However, they did have great difficulty leaving the pitcher across the peristome. Seventy-three percent of the ants needed more than one attempt (median 2.5 attempts) to overcome the peristome. In one particularly dramatic case, a P. beccarii worker fell back into the fluid 48 times before it finally managed to escape from the pitcher. The difficulty of passing the peristome was apparently enhanced by the fact that these ants carried digestive fluid adhering to their body, which visibly wetted the peristome once they tried to step on it and made it more slippery.
Friction of O. smaragdina Ants on the Peristome of N. bicalcarata. We investigated the effects of peristome wetting and surface anisotropy on the performance of insect claws and adhesive pads by measuring friction forces of O. smaragdina ants on the N. bicalcarata peristome (Fig. 3). In ants with intact tarsi, friction mainly depended on peristome wetting. Pads slid and generated only minute friction forces on the wetted peristome (Fig. 3D and Table 2). When the peristome was dry, friction forces were generally large and did not significantly depend on pulling direction (Fig. 3D and Table 3). On the wetted peristome, however, the overall smaller forces were significantly lower when ants were pulled toward the inside of the pitcher (Fig. 3D and Table 3).
Table 2. Statistics of the effects of peristome wetting on friction force in O. smaragdina (Wilcoxon's signed rank tests; Bonferroni correction applied for multiple testing; Pcorrected = 4 × P).
| Tarsus | Pulling direction | n | z | Pcorrected |
|---|---|---|---|---|
| Intact | Inward | 22 | 4.107 | <0.001 |
| Intact | Outward | 22 | 4.074 | <0.001 |
| No arolium | Inward | 27 | 1.586 | >0.1 |
| No arolium | Outward | 27 | 2.354 | 0.074 |
| No claws | Inward | 19 | 3.823 | <0.001 |
| No claws | Outward | 19 | 3.724 | <0.001 |
Table 3. Statistics of the effects of pulling direction on friction force in O. smaragdina (Wilcoxon's signed rank tests; Bonferroni correction applied for multiple testing; Pcorrected = 4 × P).
| Tarsus | Peristome condition | n | z | Pcorrected |
|---|---|---|---|---|
| Intact | Dry | 22 | 0.243 | >0.1 |
| Intact | Wet | 22 | 3.912 | <0.001 |
| No arolium | Dry | 27 | 3.820 | <0.001 |
| No arolium | Wet | 27 | 4.541 | <0.001 |
| No claws | Dry | 19 | 0.563 | >0.1 |
| No claws | Wet | 19 | 2.817 | 0.019 |
Ablation experiments on the tarsi of O. smaragdina demonstrate that the two experimental factors, i.e., peristome wetting and pulling direction, have very different effects on the attachment structures of the tarsus (claws and arolium). The strong effect of peristome wetting was equally present in ants with clipped claws (Fig. 3F) but almost absent (and not significant) in ants where the arolia had been removed (Fig. 3E and Table 2). This shows that peristome wetting primarily impedes attachment of the arolium. In contrast, pulling direction had a significant effect when forces due to the arolium were small (i.e., in tarsi with no arolia or in intact tarsi on the wet peristome; Fig. 3 D and E and Table 3). The larger forces in the direction toward the outside of the pitcher are probably due to interlocking of the claws with the surface profile. The weaker significance of pulling direction in the claw-amputated tarsi on the wet peristome (Fig. 3F and Table 3) indicates that also other parts of the tarsus (and possibly the arolium itself) may slightly contribute to anisotropic friction.
Effect of Peristome in Nepenthes Species with Waxy Inner Pitcher Walls. We investigated which surfaces are responsible for insect capture in a Nepenthes species with waxy inner pitcher walls, N. alata. Table 4 compares the results for N. alata with dry vs. wetted peristome. Due to the relatively long observation times, most of the M. barbarus ants investigated were trapped in this experiment (capture efficiency was greater for the wet peristome, but the difference was marginally nonsignificant; Fisher's exact test: P = 0.066). However, the trapping mechanism differed between the dry and wet conditions of the peristome. The number of legs in contact with the waxy surface before capture was significantly smaller when the peristome was wet (Mann–Whitney test: U = 75.5, P < 0.001). When the peristome was dry, most of the ants fell into the pitchers when they were holding onto the peristome with only one leg (median = one leg on the peristome). On the wetted peristome, 50% of the ants slipped into the pitcher without even touching the wax (median = 5.5 legs on the peristome).
Table 4. Surfaces responsible for the capture of ants in N. alata with a dry or wetted peristome (5-min observations of M. barbarus ants placed individually on the pitchers).
| Fallen from
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Peristome/wax (no. legs on peristome)
|
|||||||||||
| Peristome condition | n | Lid | Peristome only | 5 | 4 | 3 | 2 | 1 | Wax only | Not fallen | No peristome visit |
| Dry | 60 | - | - | - | - | 1 | 3 | 38 | 4 | 13 | 1 |
| Wet | 60 | 1 | 22 | 3 | 7 | 8 | 1 | 3 | - | 4 | 11 |
To evaluate the contribution of the peristome to the general trapping efficiency in N. alata, ants were released on a pitcher where 50% of the peristome was dry and 50% wet. On the wetted side of the peristome, 60 of 73 visitors (82%) were trapped, whereas on the dry peristome half, only 24 of 99 visiting ants (24%) fell into the pitcher. The absolute number of ants trapped on the dry and wet side of the pitcher differed significantly from a random distribution (χ2 = 15.4, df = 1, P < 0.001). This difference was clearly not due to a lower number of ants visiting the dry side of the pitcher. When considering not the absolute numbers but the proportion of peristome visitors that were trapped, the increase of pitcher capture rate for the wet peristome was also highly significant (χ2 = 56.5, df = 1, P < 0.001).
Discussion
Our study shows that N. bicalcarata pitchers capture insects with the help of their slippery peristome. We discovered that when wet, its surface is extremely slippery, so that most insects stepping on the peristome fall prey, whereas virtually no insects are captured when the peristome is dry.
Despite the century-long interest in Nepenthes pitcher plants and their trapping mechanism, the effective trapping function of the peristome reported in this study has thus far remained unnoticed. Based on morphological structure and supposed function, Hooker (22) divided the inside of the pitcher into an “attractive” zone, which included the lid and the peristome, a “conductive” (waxy part of inner wall) and a “digestive” zone (lower part of the pitcher). Later authors also did not consider the peristome to be directly involved in the trapping mechanism. Lloyd (2) noticed that the peristome surface is “... not slippery (...), for as a matter of observation, small insects (ants, etc.) can walk freely on it, using their footpads.” Juniper and Burras (23) remarked that “... ants seem to be able to walk about freely on it,” and that “the peristome by itself does not seem likely to prove a serious obstacle to the escape of all types of insects.” Moran et al. (7) noted that “... the peristome appears to offer a secure foothold for most visiting invertebrates.” Only Juniper and Burras (23) hypothesized that the peristome may be a “precarious foothold” for some insects due to its smoothness. However, the smoothness of a surface by itself is no problem for insects equipped with tarsal adhesive pads. Many insects are able to cope with detachment forces of >100 times their own body weight on perfectly smooth substrates (24, 25).
The mechanism of peristome slipperiness is based on the presence of lubricating water or nectar films and on the microstructured surface of the peristome. The peristome surface contains microscopic cavities between overlapping epidermal cells that may be in the appropriate size range to provide anchorage for insect claws (26) but only in the direction toward the outside of the pitcher. Apart from claws, many insects possess adhesive pads that are used on smooth surfaces where the claws fail. The analysis of friction forces of O. smaragdina ants on the peristome surprisingly revealed that surface anisotropy has hardly any effect on friction forces of the adhesive pads. However, when only claws were present, friction forces were smaller toward the inside of the pitcher but larger toward the outside, as expected from surface topography. In contrast, the presence of water films on the peristome had no effect on forces generated by the claws but did strongly disrupt attachment for the adhesive pads. We assume that slipperiness of the wet peristome is caused by aquaplaning, i.e., by the lubricating effect of a water or nectar film between adhesive pads and the peristome surface. On a smooth surface, insect adhesive pads can generate large friction forces if their soft cuticle either comes into very close contact or directly interacts with the substrate (27). If the surface is covered with fluid, adhesive pads can make contact only by squeezing out the liquid, which may be a slow process depending on the fluid's viscosity. The stability of a very thin liquid film intercalated between a soft adherend and a surface depends on the film's tendency to dewet and to establish dry adhesive contacts (28). Dewetting is determined by the sign of the spreading coefficient S = γSA – (γSL + γLA), where γSA, γSL, and γLA are the solid/adherend, solid/liquid, and liquid/adherend interfacial tensions, respectively. Lubrication is enhanced if S is positive (liquid film stable) or negative and small [slow dewetting (29)], both of which are facilitated if the substrate is well wettable (small γSL).
Wettability is also the fundamental prerequisite for the formation of homogenous water or nectar films on the peristome. Complete wettability is uncommon among plant leaf surfaces (30). Epicuticular surface lipids are hydrophobic and typically give rise to water contact angles >90°. When hydrophobicity is combined with surface roughness (as is the case in epicuticular wax crystal surfaces), plant surfaces can be superhydrophobic with contact angles as large as 170° (e.g., ref. 31). If a material is hydrophilic with Young contact angles <90°, however, surface roughness has an inverse effect and increases wettability (32). We assume that the corrugations of the peristome surface are necessary for its complete wettability, which in turn enhances the peristome trapping function.
There are several possible mechanisms of how Nepenthes peristomes become wetted under natural conditions: (i) rain, (ii) condensation, and (iii) nectar secretion. Even though the pitcher lid may prevent pitchers from being flooded in some Nepenthes species, it usually does not completely shield the peristome from rain. N. bicalcarata peristomes were indeed wet and slippery for insects after rainfalls. Due to its strong wettability, the peristome surface may also be an area where dew can condensate at air humidities very close to 100%. Last, nectar secreted from the large peristome nectaries can form fluid films (Fig. 1B). The location of the pores of these nectaries between the tooth-like projections at the inner margin of the peristome (Fig. 1C) is ideal for spreading the nectar onto the peristome surface. However, because the volume of nectar and the time course of nectar production have not been quantified, the contribution of nectar to the slipperiness of the peristome is still unclear. The presence of nectar (instead of pure water) on the peristome surface, apart from being attractive to insects, may have implications for peristome slipperiness. First, sugar remaining on the surface after nectar evaporation may be hygroscopic and could facilitate condensation. Second, nectar is more viscous than pure water due to its sugar content (33), which may render the peristome even more slippery.
The mechanism of peristome slipperiness in Nepenthes pitchers has fascinating ecological implications. Independent of the detailed mechanism of peristome wetting, it can be predicted that there is strong temporal variation of trapping efficiency. In most Nepenthes species, including N. bicalcarata, ants dominate the prey spectrum (7, 20). It has been hypothesized that when pitcher plants capture ants, generally low capture rates are beneficial, because surviving scouts can recruit more nestmates to the pitcher's nectar resources (20, 34). Our findings show that the capture efficiency of Nepenthes pitchers is temporarily high due to the presence of water films on the peristome but low when it is dry. As a consequence, ant recruitment to pitcher nectar will operate effectively while the peristome is dry, but large groups of recruited ants may become trapped when the peristome is slippery. Slipperiness of the peristome not only varies temporarily but may also be unpredictable for the arriving ants. Temporal separation of ant recruitment and prey capture could represent a strategy for capturing ants superior to a simple generally low trapping rate. We predict that pitcher-trapping efficiency will vary according to daytime and weather conditions. Nectar secretion and temperature-induced condensation may exhibit daytime-dependent maxima. Peristome wetting by rain may result in higher capture rates during rainy seasons, which may profitably coincide with periods of enhanced growth and greater demand of nutrients.
Our findings demonstrate that the trapping mechanism of the peristome is not restricted to N. bicalcarata but is also important in other Nepenthes species with waxy inner pitcher walls such as N. alata. Only when the peristome was dry was the waxy surface of N. alata the most important trapping zone, consistent with previous observations (presumably made on dry peristomes; refs. 2, 5, and 15). When the peristome was wet, however, most ants slipped into the pitcher from the peristome, often without even touching the waxy surface. Most importantly, wetting of the peristome resulted in a >3-fold increase of the capture rate. Thus, even though the peristome is wetted only temporarily, its contribution to the overall prey capture rate might be considerable. The functional significance of the waxy zone could lie more in the retention of insects, which appears to be less effective in the wax-free N. bicalcarata pitchers (see Fig. 2B and ref. 15). Further work is needed to investigate the relative importance of different trapping mechanisms (waxy walls vs. peristome) under field conditions.
N. bicalcarata is an exceptional member of the genus Nepenthes because of its myrmecophytic association with the ant Camponotus schmitzi Stärke (35). These specialized ants nest in the swollen hollow pitcher tendrils of their host plant. Apart from harvesting extrafloral nectar, C. schmitzi feed on large prey items captured by their host plant, which they transport out of the pitcher fluid (19). In striking contrast to other ants, C. schmitzi ants are capable not only of swimming and diving through the digestive pitcher fluid but also of running across wet slippery peristomes; they never become trapped in the pitchers. We are currently investigating the proximate mechanisms of these fascinating adaptations.
Acknowledgments
We acknowledge David Marshall (Universiti Brunei Darussalam), Marlis and Dennis Merbach, Ulrich Maschwitz (J. W. Goethe University, Frankfurt am Main), and Reinhard Jetter (University of British Columbia, Vancouver) for help in the field and fruitful discussions. We are indebted to the staff of the Botanical Garden in Würzburg and the Palmengarten in Frankfurt am Main. The Universiti Brunei Darussalam, the Brunei Museum, and the Department of Forestry of Brunei are gratefully acknowledged for permission to conduct field work. This study was financially supported by research grants from the Deutsche Forschungsgemeinschaft (SFB 567/C6 and Emmy–Noether fellowship FE 547/1–2 to W.F.).
References
- 1.Darwin, C. (1875) Insectivorous Plants (Appleton, London).
- 2.Lloyd, F. E. (1942) The Carnivorous Plants (Ronald Press, New York).
- 3.Knoll, F. (1914) Jahrb. Wiss. Bot. 54, 448–497. [Google Scholar]
- 4.Adams, R. M. & Smith, G. W. (1977) Am. J. Bot. 64, 265–272. [Google Scholar]
- 5.Juniper, B. E., Robins, R. J. & Joel, D. M. (1989) The Carnivorous Plants (Academic, London).
- 6.Moran, J. A. (1996) J. Ecol. 84, 515–525. [Google Scholar]
- 7.Moran, J. A., Booth, W. E. & Charles, J. K. (1999) Ann. Bot. 83, 521–528. [Google Scholar]
- 8.Ratsirarson, J. & Silander, J. A. (1996) Biotropica 28, 218–227. [Google Scholar]
- 9.Riedel, M., Eichner, A. & Jetter, R. (2003) Planta 218, 87–97. [DOI] [PubMed] [Google Scholar]
- 10.Gaume, L., Perret, P., Gorb, E., Gorb, S., Labat, J.-J. & Rowe, N. (2004) Arthropod. Struct. Dev. 33, 103–111. [DOI] [PubMed] [Google Scholar]
- 11.Gorb, E. V. (2004) Comp. Biochem. Physiol. A 137, S82–S83. [Google Scholar]
- 12.Hepburn, J. S. (1918) Proc. Am. Philos. Soc. 57, 112–129. [Google Scholar]
- 13.Amagase, S. (1972) J. Biochem. 72, 765–767. [DOI] [PubMed] [Google Scholar]
- 14.Tokés, Z. A. (1974) Planta 119, 39–46. [DOI] [PubMed] [Google Scholar]
- 15.Gaume, L., Gorb, S. & Rowe, N. (2002) New Phytol. 156, 479–489. [DOI] [PubMed] [Google Scholar]
- 16.Cresswell, J. E. (1998) Oecologia 113, 383–390. [DOI] [PubMed] [Google Scholar]
- 17.Moran, J. A., Clarke, C. M. & Hawkins, B. J. (2003) Int. J. Plant Sci. 164, 635–639. [Google Scholar]
- 18.Merbach, M. A., Zizka, G., Fiala, B., Maschwitz, U. & Booth, W. E. (2001) Flora 196, 153–160. [Google Scholar]
- 19.Clarke, C. M. & Kitching, R. L. (1995) J. Trop. Ecol. 11, 589–602. [Google Scholar]
- 20.Clarke, C. & Wong, K. M. (1997) Nepenthes of Borneo (Natural History Publications, Kota Kinabalu, Malaysia).
- 21.Owen, T. P. & Lennon, K. A. (1999) Am. J. Bot. 86, 1382–1390. [PubMed] [Google Scholar]
- 22.Hooker, J. D. (1859) Trans. Linn. Soc. 22, 415–424. [Google Scholar]
- 23.Juniper, B. E. & Burras, J. K. (1962) New Sci. 13, 75–77. [Google Scholar]
- 24.Eisner, T. & Aneshansley, D. J. (2000) Proc. Natl. Acad. Sci. USA 97, 6568–6573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Federle, W., Rohrseitz, K. & Hölldobler, B. (2000) J. Exp. Biol. 203, 505–512. [DOI] [PubMed] [Google Scholar]
- 26.Dai, Z., Gorb, S. N. & Schwarz, U. (2002) J. Exp. Biol. 205, 2479–2488. [DOI] [PubMed] [Google Scholar]
- 27.Federle, W., Baumgartner, W. & Hölldobler, B. (2004) J. Exp. Biol. 207, 67–74. [DOI] [PubMed] [Google Scholar]
- 28.Martin, A., Buguin, A. & Brochard-Wyart, F. (2001) Langmuir 17, 6553–6559. [Google Scholar]
- 29.Martin, P. & Brochard-Wyart, F. (1998) Phys. Rev. Lett. 80, 3296–3299. [Google Scholar]
- 30.Juniper, B. E. & Jeffree, C. E. (1983) Plant Surfaces (Edward Arnold, London).
- 31.Holloway, P. J. (1969) J. Sci. Food Agric. 20, 124–128. [Google Scholar]
- 32.Bico, J., Thiele, U. & Quéré, D. (2002) Colloids Surfaces A 206, 41–46. [Google Scholar]
- 33.Wolf, A. V., Brown, M. G. & Prentiss, P. G. (1984) in CRC Handbook of Chemistry and Physics, ed. Weast, R. C. (CRC, Boca Raton, FL), pp. D223–D272.
- 34.Joel, D. M. (1988) Biol. J. Linn. Soc. 35, 185–197. [Google Scholar]
- 35.Schuitemaker, J. P. & Staercke, A. (1933) Overdr. Natuurhist. Maandbl. 22, 29–31. [Google Scholar]