Abstract
Glutamic acid decarboxylase (GAD) 65 is one of the major pancreatic antigens targeted by self-reactive T cells in type I diabetes mellitus. T cells specific for GAD65 are among the first to enter inflamed islets and may be important for the initiation of autoimmune diabetes. However, we previously reported that nonobese diabetic (NOD) mice transgenic for a T cell antigen receptor (TCR) specific for one of the immunodominant epitopes of GAD65, peptide 286-300 (G286), are protected from insulitis and diabetes. To examine whether other GAD65-reactive T cells share this phenotype, we have generated TCR transgenic NOD mice for a second immunodominant epitope of GAD65, peptide 206-220 (G206). As in G286 mice, G206 mice do not develop islet inflammation or diabetes. When adoptively transferred along with diabetogenic T cells, activated G206 T cells significantly delayed the onset of diabetes in NOD.scid recipients. Both G206 and G286 T cells produce immunoregulatory cytokines IFN-γ and IL-10 at low levels when activated by cognate antigens. These data suggest that GAD65-specific T cells may play a protective role in diabetes pathogenesis by regulating pathogenic T cell responses. A better understanding of the functions of autoreactive T cells in type I diabetes will be necessary for choosing desirable targets for immunotherapy.
Type I diabetes mellitus in humans is an autoimmune disease that results from the selective destruction of pancreatic β-cells by T cells (1, 2). The nonobese diabetic (NOD) mouse develops spontaneous autoimmune diabetes that shares many characteristics with the human disease (1, 3, 4). The principal islet cell antigens targeted by autoreactive T cells in NOD mice include insulin (5), glutamic acid decarboxylase 65 (GAD65) (6), 65-kDa heat shock protein (7), and islet-specific glucose-6-phosphatase catalytic subunit-related protein (IGRP) (8). Among these, T cell responses to GAD65 and insulin are detected early preceding the onset of clinical disease (3–5 weeks of age in the NOD mouse) (9–11). This result has led to the speculation that GAD65 may be among the first autoantigens targeted by T cells that initiate the diabetes pathogenesis.
The hypothesis for a pathogenic role of GAD65 is supported by a GAD65-specific T cell clone that induces insulitis and diabetes upon adoptive transfer (12) and by the finding that prevention of GAD65 expression in the pancreatic islets by GAD antisense cDNA prevented diabetes (13). In contrast, induction of deletional T cell tolerance by widespread expression of a GAD65 transgene did not alter diabetes pathogenesis (14) but exacerbated the disease (15). In addition, several GAD65-specific T cell clones, lines, and T cell antigen receptor (TCR) transgenic mice were not pathogenic and exhibited a diabetes-delaying capacity (16–18). Thus, it appears that GAD65 may not be a required initiating antigen for diabetes pathogenesis but rather induces a protective response.
To further examine the functions of GAD65-reactive T cells in the pathogenesis of diabetes, we have generated TCR transgenic NOD mice for the two immunodominant CD4+ T cell epitopes of GAD65, p286-300 and p206-220 (19, 20). The phenotype of p206-220-specific TCR transgenic mice (G206) presented here is similar to that seen in p286-300-specific TCR transgenic mice (G286) (18). Neither transgenic line develops diabetes or insulitis (inflammation of islets). Upon adoptive transfer along with diabetogenic T cells, antigen-activated G206 and G286 T cells both delayed diabetes onset. These observations have important implications for the design of antigen-specific immunotherapies and argue against a therapy aimed at deleting GAD65-specific T cells in type I diabetes patients.
Materials and Methods
Generation of G206 NOD Mice Transgenic for a TCR Specific for Peptide 206-220 (p206) of GAD65. A GAD65 p206 (TYEIAPVFVLLEYVT)-specific T cell hybridoma was obtained by fusion of BW5147 T cell hybridoma with CD4+ spleen cells from an unimmunized, unmanipulated 12-week-old NOD female at the time of normal diabetes onset. The p206 TCR (Vα5-Jα45; Vβ8.2-Jβ2.4) was cloned by RT-PCR from this hybridoma by using primers designed at the beginning of the leader segment of V and in the intron ≈200 bp downstream of the J segment for both α and β chains. The TCRα and -β variable region sequences were subcloned from genomic DNA into previously described expression vectors containing TCR regulatory and constant regions (pTαcass, pTβcass) (21).
The linearized ≈20-kb TCRα and ≈22 kb TCRβ constructs were injected into NOD embryos. Potential founders in which >98% of peripheral blood T cells express the transgenic TCRβ chain were identified by flow cytometry using Vβ8.1/8.2-specific antibody. The genomic integration of transgenic TCRα chain was determined by PCR using the following primers for Vα5: CGGGGTGCAGATAGACTCAC (forward) and GTTCTAAGTCAGGCTGAGTG (reverse).
Data presented here were obtained from one (referred to as G206) of the three transgenic lines we established, all of which produced similar results in in vitro and in vivo analyses. Unless indicated, female mice were used in all experiments. All animal studies have been approved by Stanford University's Administrative Panel for Laboratory Animal Care.
Detection of Insulitis and Diabetes. Insulitis was assessed in G206 mice and their nontransgenic littermates by counting inflamed islets in paraffin-embedded pancreas sections that were cut 100 μm apart and stained with hematoxylin and eosin (Histo-Tec, Hayward, CA). At least 10 islets per mouse, three or four mice per group, were scored as no insulitis, periinsulitis (infiltrates surrounding islets), or intrainsulitis (severe infiltration into the islets).
Diabetes incidence was followed by biweekly measurement of urine glucose levels by using Chemstrips (Roche Diagnostics). Mice were considered diabetic on two consecutive high glucose readings (>100 μg of glucose per dl) and were killed.
Cyclophosphamide dissolved in sterile PBS (0.2 mg/g of body weight) was injected i.p. to accelerate diabetes onset in 7- to 9-week-old NOD mice (22). A second injection was given 2 weeks later.
Generation of NOD Mice Double-Transgenic for G206 or G286 TCR and Rat Insulin Promoter-Driven Human GAD65 (RIP-huGAD). NOD mice overexpressing human GAD65 from the rat insulin promoter (NOD.RIP-huGAD) (23) were obtained from The Jackson Laboratory. NOD.RIP-huGAD A and Y lines have the RIP-huGAD transgene integrated in chromosome 15 and the Y chromosome, respectively, and both lines overexpress human GAD65 in the pancreas (23).
NOD.RIP-huGAD.A mice were bred with G206 and G286 mice. Peripheral blood leukocytes of all F1 progeny were screened by flow cytometry for G206 or G286 TCR expression. The presence of RIP-huGAD transgene in genomic DNA was determined by PCR as described (23). NOD.RIP-huGAD.Y-line males were crossed with G206 and G286 females. All male F1 progeny (which are RIP-huGAD+) were screened for transgenic TCR expression by flow cytometry.
Flow Cytometry. FITC-, peridinin chlorophyll protein-, and phycoerythrin-conjugated antibodies against CD3 (17A2), CD4 (RM4-5), CD8 (5H-10), and TCR Vβ 8.1/8.2 (RM5-2) (BD Biosciences) were used. Lymphocytes were stained in PBS/1% FCS for 30 min at 4°C following the manufacturer's instructions. Data were collected on unfixed cells immediately after staining and were analyzed by using cellquest software (Becton Dickinson).
T Cell Proliferation and Cytokine Production Assays. Single-cell suspensions were obtained from lymph nodes and spleens, and cells were washed and cultured in RPMI medium 1640 containing 10% FCS, 200 units/ml penicillin, 200 μg/ml streptomycin, 10 mM Hepes, 0.06 μg/ml l-glutamine, and 0.01 μM 2-mercaptoethanol. Red blood cells were removed from splenocyte preparations by incubation in lysis buffer (0.15 M NH4Cl/1 mM KHCO3/0.1 mM Na2EDTA). If necessary, CD4+ T cells were purified by negative selection using magnetic beads coated with anti-CD8 and anti-B220 antibodies or by positive selection using anti-CD4-coated magnetic beads (Dynal, Great Neck, NY).
T cell proliferation was measured by [3H]thymidine incorporation. For pancreatic lymph node cells, 2 × 105 cells were seeded per well in a U-bottomed 96-well plate. For other samples, 106 cells were seeded per well in a flat-bottomed 96-well plate. [3H]Thymidine was added 48 h later at 0.5 μCi (1 Ci = 37 GBq) per well, and cells were further incubated for 12–16 h before scintillation counting. A stimulation index was defined by cpm in the presence of antigen divided by cpm in the absence of antigen.
IL-2, IFN-γ, tumor necrosis factor α (Pharmingen), IL-5, and IL-10 (Endogen, Cambridge, MA) secreted by activated T cells into the culture supernatant were measured by ELISA following the manufacturer's instructions (Pharmingen and Endogen) and as described (18). Detection was by a peroxidase staining system.
Antigens used in in vitro T cell activation include synthetic peptides p206 (TYEIAPVFVLLEYVT), p286 (KKGAAALGIGTDSVI), and pOVA (residues 323–339 of chicken ovalbumin, ISQAVHAAHAEINEAGR) (all three peptides are presented by I-Ag7), as well as recombinant GAD65 produced by baculovirus expression system (19).
Adoptive T Cell Transfer Studies. Spleen cells (2–10 × 106) from newly diabetic NOD mice were injected into the tail vein of 6- to 8-week-old female NOD.scid mice, with or without the addition of G206 splenocytes that were activated or not with p206 (10 μg/ml) for 48 h. Final volume of all injections was 100 μl per mouse in PBS/0.1% BSA.
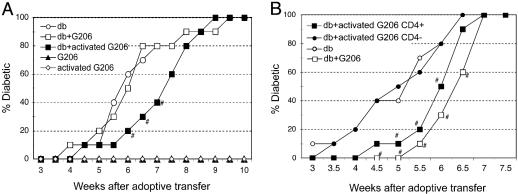
Statistical Analysis. Statistical significance of delay in diabetes onset in adoptive transfer studies (see Fig. 6) was determined by Student's t test.
Fig. 6.
Delay of diabetes onset in NOD.scid recipients after adoptive transfer of activated G206 T cells along with diabetogenic splenocytes. The numbers and types of cells used in A are 107 diabetogenic splenocytes (db); 107 G206 splenocytes, activated or not; and 4 × 106 activated G206 splenocytes (with p206 in vitro). The numbers and types of cells used in B are 107 diabetogenic splenocytes (db); 106 p206-activated G206; 2.5 × 105 CD4+ cells from p206-activated G206 splenocytes; and 8 × 105 CD4– cells from p206-activated G206 splenocytes. n = 10 per group. Diabetes incidence was monitored by measuring urine glucose levels twice a week. Student's t test was performed between “db” and “db+activated G206 (whole or CD4+ splenocytes)” groups. #, Time points showing a significant delay in diabetes (P < 0.05).
Results
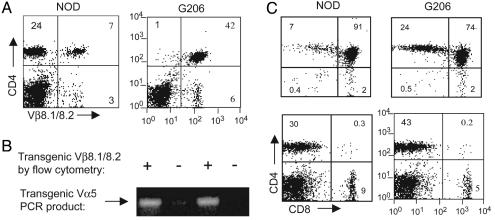
T Cell Development in G206 TCR Transgenic NOD Mice. A TCR specific for p206 of GAD65 was cloned from splenic T cells of an unimmunized NOD mouse at the time of normal diabetes onset (see Materials and Methods). Subsequent studies showed that p206 was one of the immunodominant epitopes of GAD65 in NOD mice (19, 20). We produced NOD mice transgenic for p206-specific TCR (G206) in which >98% of peripheral CD4+ T cells (as well as CD8+ T cells; data not shown) express the transgenic TCRβ chain (Vβ8.2), in comparison with ≈23% in nontransgenic littermates (Fig. 1A). Genomic integration of transgenic TCR Vα5 was confirmed by PCR (Fig. 1B). Because of the lack of specific antibodies and incomplete allelic exclusion (see below), transgenic Vα5 expression at mRNA and protein levels could not be determined. However, the fact that T cells isolated from Vβ8.2+ transgenic mice proliferate and produce cytokines in vitro in a p206-specific manner (see below) indicates that a functional TCR pair is expressed.
Fig. 1.
T cell development in G206 TCR transgenic NOD mice. Single-cell suspensions were obtained from the thymus and spleen of a 6-week-old G206 female (G206 transgenic TCR+) or a nontransgenic littermate (NOD). (A) Splenocytes were costained with anti-TCR Vβ8.1/8.2/FITC and anti-CD4/PerCP antibodies. (B) PCR performed on genomic DNA prepared from NOD and G206 females by using primers that amplify transgenic TCR Vα5 chain (see Materials and Methods). (C) Thymocytes (Upper) and splenocytes (Lower) were costained with anti-CD4/peridinin chlorophyll protein (PerCP) and anti-CD8/phycoerythrin antibodies. Numbers in each quadrant are the percentage of the relevant population after gating on lymphocytes.
In contrast to G286 mice showing no detectable skewing to the CD4+ single-positive phenotype (18), G206 mice exhibited a slight but noticeable skewing to CD4+ T cells in the thymus and periphery when compared with wild-type NOD (Fig. 1C), as is observed in other TCR transgenic systems (24). Histological analysis of thymic architecture did not reveal noticeable differences between G206 mice and NOD mice (E. Ranheim, University of Wisconsin, Madison, personal communication).
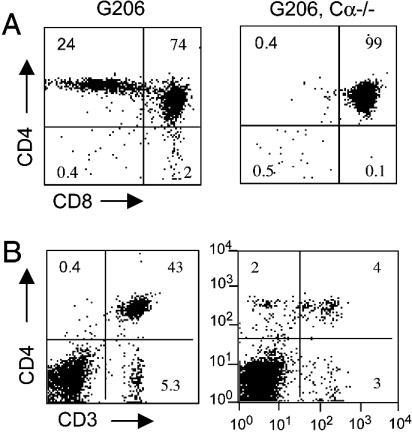
Whereas GAD65-reactive T cells have been isolated from the pancreatic lymph nodes and spleens of diabetic NOD mice (10, 11), it is poorly understood how such T cells escape negative thymic selection and reach the periphery. Skewing to CD4+ T cells observed in G206 mice (Fig. 1B) suggests that p206-specific T cells undergo some positive selection. However, G206 mice crossed onto NOD TCR Cα–/– produced an extremely low number of mature single-positive thymocytes (Fig. 2A) and had a significantly reduced level of peripheral T cells (Fig. 2B). This result indicates that thymic survival of G206 T cells depends on the rearrangement and expression of endogenous TCRα chains. G286 thymic selection is similar, although more G206 T cells are positively selected on the Cα–/– background than G286 T cells (18). Other studies also described rescue of self-reactive TCRs from negative selection by expression of a second TCRα chain (25–27).
Fig. 2.
Negative selection of G206 TCR-transgenic T cells in the absence of endogenous TCR Cα chain rearrangement. Thymocytes (A) and splenocytes (B) of 6-week-old G206 mice and G206.Cα–/– NOD mice were costained with anti-CD3/FITC, anti-CD4/PerCP, and anti-CD8/phycoerythrin antibodies. Numbers in each quadrant are the percentage of the relevant population after gating on lymphocytes.
Despite being an enriched source of G206 TCR transgenic T cells, G206, Cα–/– mice had low numbers of T cells present, limiting the use of those T cells for functional studies. All experiments shown below were performed with T cells isolated from G206 mice.
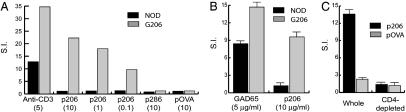
Antigen-Specific Proliferation and Cytokine Production by G206 T Cells. T cells present in the lymph nodes and spleens of G206 mice proliferated specifically in response to peptide p206 and recombinant GAD65 protein upon ex vivo stimulation (Fig. 3). As expected, the observed antigen-specific proliferation is mostly that of CD4+ T cells; depletion of CD4+ T cells from G206 splenocytes almost abrogated p206-specific proliferation (Fig. 3C).
Fig. 3.
Antigen-specific ex vivo proliferation of G206 T cells. Pooled lymph node cells (A) and pancreatic lymph node cells (B) from an 8-week-old G206 female and its transgene-negative littermate (NOD) were used in [3H]thymidine incorporation assays. Numbers in parentheses are antigen concentrations in μg/ml. (C) G206 splenocytes before and after depletion of CD4+ cells with anti-CD4 magnetic beads were stimulated with 10 μg/ml indicated peptides. Results from representative triplicate experiments are shown as mean stimulation index (S.I.) ± SD.
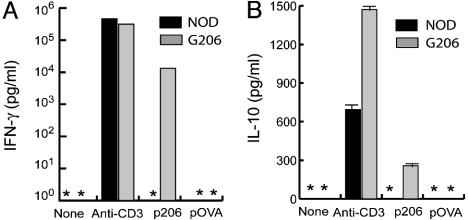
When activated with p206, G206 T cells produced low levels of IFN-γ and IL-10 that were undetectable in nontransgenic T cell cultures (Fig. 4). Whereas anti-CD3 stimulation induced IL-5 and tumor necrosis factor α production by G206 T cells, these cytokines were not detectable when the same cells were stimulated with p206 (data not shown). The amount of IFN-γ produced by G206 T cells was typically ≈10 ng/ml and far lower than that produced by conventional T helper (Th)1 cells (>300 ng/ml) (28, 29). The amount of IL-10 produced by G206 T cells was ≈200 pg/ml, much lower than that produced by Th2 cells (28, 29). The concomitant production of low-level IFN-γ and IL-10 was also observed with G286 T cells (18) and in other systems, including the GAD65 epitope p521-535-specific T cell response in NOD mice (30–32). These studies have not yet resolved whether IFN-γ and IL-10 are produced by the same T cells or by different subsets of T cells of the same antigen specificity.
Fig. 4.
Low-level production of IFN-γ and IL-10 by activated G206 T cells. Splenocytes were activated for 48 h with anti-CD3 (5 μg/ml) and indicated peptides (10 μg/ml). Cytokines in culture supernatant were measured by ELISA. *, A value below the lower limit of detection (15 pg/ml).
T cells capable of responding to their antigen in vitro can be tolerant to the same antigen in vivo (33–35). To address this possibility, G206 mice were immunized with p206 in incomplete Freund's adjuvant (IFA), and ex vivo T cell proliferation assays were performed 5 days later with draining lymph node cells. In a representative experiment, T cells from IFA-injected and IFA/p206-immunized G206 mice showed stimulation indexes of 5 and 20, respectively, suggesting G206 T cells can be activated in vivo. This result suggests that the lack of disease in G206 mice is not due to the tolerant state of otherwise pathogenic G206 T cells.
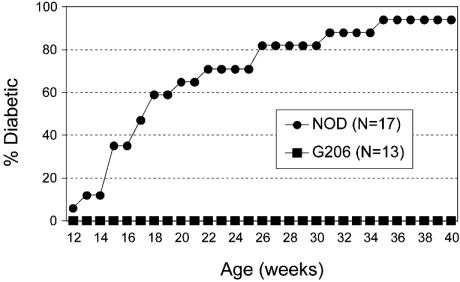
Lack of Insulitis and Diabetes in G206 Mice. None of the G206 females developed diabetes, whereas ≈90% of the nontransgenic littermates were diabetic by 40 weeks of age (Fig. 5). G206 mice displayed no signs of insulitis at 12 weeks of age (50 islets scored from five mice), whereas age-matched, nontransgenic littermates showed 30% periinsulitis and 70% intrainsulitis (40 islets scored from four mice). At 25 weeks of age, wild-type NOD exhibited severe intrainsulitis in the few remaining islets (10 islets scored from five mice), but G206 mice still lacked any signs of islet inflammation (70 islets scored from seven mice).
Fig. 5.
Lack of diabetes in G206 TCR-transgenic NOD mice. Diabetes incidence was determined in G206 females and their nontransgenic female littermates (NOD) by measuring urine glucose levels.
Cyclophosphamide treatment, known to accelerate diabetes onset in NOD mice (22), failed to induce diabetes in all eight G206 mice tested (7–9 weeks of age) after two cyclophosphamide injections, whereas seven of nine age-matched, nontransgenic littermates became diabetic.
Delay of Diabetes Onset by Activated G206 T Cells. To examine the role of G206 T cells in diabetes pathogenesis, we used an adoptive transfer model in which splenocytes from overtly diabetic NOD females are transferred to NOD.scid recipients that do not normally develop autoimmune diabetes caused by the lack of T and B cells. More than 80% of the NOD.scid mice develop diabetes within 6 weeks of receiving diabetogenic splenocytes (Fig. 6). Interestingly, a significant delay in diabetes onset was observed when p206-activated G206 splenocytes were transferred along with diabetogenic splenocytes (Fig. 6A). CD4+ T cells positively sorted from p206-activated G206 splenocytes alone delayed the onset of diabetes induced by diabetogenic splenocytes, whereas the CD4-depleted fraction did not (Fig. 6B). Adoptive transfer of G206 T cells alone, regardless of in vitro activation with cognate peptide before transfer, did not cause disease in the NOD.scid recipients for the duration of the 10-week study (Fig. 6A).
A similar diabetes-delaying capacity was observed with G286 T cells (18). The protection by activated G206 and G286 T cells was not complete, and all mice that received diabetogenic splenocytes eventually developed diabetes (Fig. 6) (18). The lack of complete protection may be because pathogenic cells have a greater proliferative potential than in vitro-activated G206 and G286 T cells, and over time cell populations with pathogenic potential outnumber and overwhelm the disease-delaying capacity of activated G206 or G286 cells.
Lack of Insulitis and Diabetes in G206 and G286 Mice Overexpressing Human GAD65 in the Pancreas. The dose of antigen applied to stimulate naive T cells is one factor that influences differentiation of CD4+ T lymphocytes into Th1 or Th2 subsets (36, 37). Exposure to a high antigen dose tends to drive differentiation of naive CD4+ T cells into Th1-type cells producing IFN-γ, whereas exposure to a low dose of the same antigen induces the T cells to differentiate into Th2-type cells producing IL-4 (38–41). We speculated that ≈10-fold lower expression levels of GAD65 in the pancreas of mice than those in rats and humans (42) might be preventing G206 and G286 T cells from developing into pathogenic effectors in the periphery. To examine this possibility, we crossed G206 and G286 mice with NOD mice overexpressing human GAD65 under the rat insulin promoter (NOD. RIP-huGAD) (23). Two different NOD.RIP-huGAD lines (23) were used: the NOD.RIP-huGAD65.A line carrying the transgene as a hemizygous autosomal trait and the NOD.RIP-huGAD65.Y line carrying the transgene in the Y chromosome.
Diabetes incidence in NOD.RIP-huGAD65.A mice maintained in our colony was significantly lower than in wild-type NOD (Fig. 7) or the same strain originally described in ref. 23. NOD.RIP-huGAD65.A in our colony developed insulitis at a much slower rate than wild-type NOD; at 10 weeks of age, NOD.RIP-huGAD.A mice lacked any insulitis (30 islets scored from three mice), whereas age-matched wild-type NOD exhibited 50% no insulitis, 15% periinsulitis, and 35% intrainsulitis (40 islets scored from 40 mice). At 16 weeks of age, the levels of severity of insulitis in two groups were similar (≈20% no insulitis, ≈30% intrainsulitis, and ≈50% periinsultis). Repeated backcrosses to the NOD background since the original description of NOD.RIP-huGAD.A mice may be contributing factors to the observed decrease in disease progression.
Fig. 7.
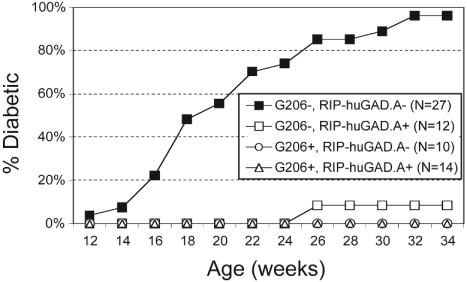
Lack of diabetes in G206+, RIP-huGAD+ NOD mice. Diabetes incidence was assessed in (G206 × RIP.huGAD.A)F1 progeny by measuring urine glucose levels.
NOD mice double-transgenic for RIP-huGAD.A and G206 TCR (Fig. 7) or G286 TCR (data not shown) did not develop diabetes and had no insulitis at all ages examined (data not shown). Likewise, none of the double-transgenic mice from the crosses between G206 (or G286) females and NOD.RIP-huGAD.Y males developed insulitis or diabetes (data not shown). Thus, G206 and G286 T cells do not acquire a pathogenic phenotype in the NOD mouse regardless of GAD65 expression levels in the pancreas.
The presence of the RIP-huGAD transgene did not alter CD4/CD8 ratios in G206 and G286 mice (data not shown). Splenocytes from the G206+, RIP-huGAD.A+ and G286+, RIP-huGAD.A+ double-transgenic mice showed an in vitro proliferative response and cytokine production profiles that were indistinguishable from G206 and G286 mice (data not shown).
Discussion
GAD65 is one of the potential target autoantigens in immunotherapies for treatment and prevention of type I diabetes. Therefore, the role of GAD65-specific immune responses in the pathogenesis of diabetes should be better understood. To that end, we generated NOD mice transgenic for TCRs specific for I-Ag7-restricted CD4+ T cell epitopes p286 (G286 mice) (18) and p206 (G206 mice; present study). Neither transgenic line developed diabetes or insulitis. Upon adoptive transfer along with diabetogenic T cells, activated G206 and G286 T cells significantly delayed the onset of diabetes in recipient NOD.scid mice.
This report adds to the growing evidence that the GAD65-specific immune response does not per se induce disease. In fact, with one exception (12), most T cell clones, T cell lines, and TCR-transgenic T cells specific for GAD65 either failed to induce diabetes or in some cases protected against diabetes progression (17, 43). T cells shown to be capable of causing insulitis and/or diabetes are specific either for insulin (44) or for unknown pancreatic islet antigens (45, 46). Regarding immunotherapies, we emphasize that the GAD65-specific response is not associated with disease induction but may play a protective role by down-modulating pathogenic responses.
The mechanisms by which G206 and G286 T cells delay the onset of diabetes are unclear. We speculated that the lack of a proinflammatory, pathogenic phenotype in G206 and G286 mice might be due to the low-level expression of GAD in the pancreas of mice compared with those of rats and humans. This possibility has been excluded because increased GAD65 expression in the pancreas of G206 and G286 mice did not induce insulitis or diabetes (Fig. 7 and accompanying text).
Another plausible mechanism is the regulation of pathogenic autoimmune responses by G206 and G286 T cells by means of the production of low-level IFN-γ and IL-10. In contrast to the proinflammatory effects of IFN-γ at relatively high concentrations, low-dose IFN-γ appears to exert global suppressive effects on T cell trafficking and may have an antiinflammatory effect (47). In some autoimmune disease models, such as experimental autoimmune encephalomyelitis and collagen-induced arthritis (48), disease incidence and severity are frequently increased in IFN-γ-deficient animals. A possible mechanism for this may be that IFN-γ can induce indoleamine 2,3-dioxygenase expression and subsequent tryptophan catabolism in antigen-presenting cells (49), which in turn down-regulate T cells interacting with affected antigen presenting cells. Low-level production of IFN-γ may be one mechanism for avoiding autoimmunity and may at least partially account for the lack of inflammatory disease in G206 and G286 mice. In addition, G206 and G286 T cells produce an immunosuppressive cytokine, IL-10 (50, 51), which may further contribute to their diabetes-delaying capacity. The importance of IFN-γ and IL-10 production by G206 and G286 T cells is being investigated by use of blocking antibodies in transgenic mice and by crossing onto IL-10-null NOD mice.
Low-level expression of the TCR may also be responsible for the protective phenotype of G206 and G286 T cells against diabetes. In addition to expressing a second α-chain (Fig. 2 and ref. 18), G206 and G286 T cells may markedly down-regulate the transgenic TCR to escape thymic negative selection. In G286, for which functional MHC tetramers are available, only 10–20% of splenic CD4+ T cells express the transgenic TCR (18). This down-regulation of TCR causes G206 and G286 TCRs to exhibit low avidity (not necessarily low affinity) for MHC/peptide ligands as was observed in G286 splenic T cells (E. Ranheim, personal communication). The low-avidity TCR-MHC/peptide interaction may account for the low-level IFN-γ production and protection against diabetes. In line with this possibility, some TCR-transgenic models of β-islet-specific autoimmunity have revealed that low levels of transgenic TCR expression tend to exert a diabetes-protective effect (52–54). Low-level expression of self-specific TCRs may be a general mechanism for protecting against autoimmune diseases.
Finally, negative thymic selection of G206 and G286 T cells and their diabetes-protective phenotype in the periphery may be due to the expression of GAD65 in the thymus. Many “tissue-specific” molecules (55), especially of endocrine organ-related proteins including GAD and insulin (56–58), are now known to be expressed at low levels in small numbers of thymic medullary epithelial cells under the control of the AIRE gene (59). In AIRE-null mutants, T cells arising spontaneously in the thymus and specific for various self peptides escape thymic negative selection and induce organ-specific autoimmune disease in the periphery (60). G206 and G286 TCR transgenic lines may be crossed to NOD.AIRE–/– mice to examine whether the AIRE-null mutation results in decreased thymic negative selection and increased transgenic TCR expression in G206 and G286 T cells. The absence of AIRE may allow positive selection of high-affinity G206 and G286 T cells and their development into pathogenic effector cells.
It is important to further examine the role of GAD65-specific T cells in the pathogenesis of diabetes. Available data suggest that responses to GAD65, at least those targeted to p206 and p286, may down-modulate pathogenic responses targeted to, for example, insulin or other unknown islet antigens. Two contradicting types of T cell responses may ensue in response to different autoantigens and have different contributions to disease progression. The balance between the two opposing responses may in part be responsible for the long lag period seen between the onset of autoimmunity (detection of T cell and antibody responses) and clinical diabetes in NOD mice (61), as well as in humans (62). When designing GAD65-specific immunotherapy for treatment and prevention of type I diabetes, consideration must taken to activate responses to “protective” epitopes while suppressing responses to “pathogenic” epitopes.
Acknowledgments
This research was supported by National Institutes of Health Grant DK51667 and Juvenile Diabetes Research Foundation Grant 1-2000-162.
Abbreviations: GAD, glutamic acid decarboxylase; NOD, nonobese diabetic; p206 and p286, peptides 206-220 and 286-300 of GAD65; TCR, T cell antigen receptor; G206 and G286, NOD mice expressing a transgenic TCR specific for p206 and p286, respectively; RIP-huGAD, rat-insulin promoter-driven human GAD; Th, T helper.
References
- 1.Tisch, R. & McDevitt, H. O. (1996) Cell 85, 291–297. [DOI] [PubMed] [Google Scholar]
- 2.Castano, L. & Eisenbarth, G. S. (1990) Annu. Rev. Immunol. 8, 647–680. [DOI] [PubMed] [Google Scholar]
- 3.Leiter, E. H. & Serreze, D. V. (1992) Reg. Immunol. 4, 263–273. [PubMed] [Google Scholar]
- 4.Makino, S., Kunimoto, K., Muraoka, Y., Mizushima, Y., Katagiri, K. & Tochino, Y. (1980) Jikken Dobutsu 29, 1–13. [DOI] [PubMed] [Google Scholar]
- 5.Palmer, J. P., Asplin, C. M., Clemons, P., Lyen, K., Tatpati, O., Raghu, P. K. & Paquette, T. L. (1983) Science 222, 1337–1339. [DOI] [PubMed] [Google Scholar]
- 6.Baekkeskov, S., Anstoot, H. J., Christgau, S., Reetz, A., Solimena, M., Cascalho, M., Folli, F., Richter-Olesen, H., De Camilli, P. & Camilli, P. D. (1990) Nature 347, 151–156. [DOI] [PubMed] [Google Scholar]
- 7.Elias, D., Markovits, D., Reshef, T., van der Zee, R. & Cohen, I. R. (1990) Proc. Natl. Acad. Sci. USA 87, 1576–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lieberman, S. M., Evans, A. M., Han, B., Takaki, T., Vinnitskaya, Y., Caldwell, J. A., Serreze, D. V., Shabanowitz, J., Hunt, D. F., Nathenson, S. G., et al. (2003) Proc. Natl. Acad. Sci. USA 100, 8384–8388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wegmann, D. R., Gill, R. G., Norbury-Glaser, M., Schloot, N. & Daniel, D. (1994) J. Autoimmun. 7, 833–843. [DOI] [PubMed] [Google Scholar]
- 10.Tisch, R., Yang, X.-D., Singer, S. M., Liblau, R. S., Fugger, L. & McDevitt, H. O. (1993) Nature 366, 72–75. [DOI] [PubMed] [Google Scholar]
- 11.Kaufman, D. L., Clare-Salzier, M., Tian, J., Forsthuber, T., Ting, G. S. P., Robinson, P., Atkinson, M. A., Sercarz, E. E., Tobin, A. J. & Lehmann, P. V. (1993) Nature 366, 69–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zekzer, D., Wong, F. S., Ayalon, O., Millet, I., Altieri, M., Shintani, S., Solimena, M. & Sherwin, R. S. (1998) J. Clin. Invest. 101, 68–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoon, J. W., Yoon, C. S., Lim, H. W., Huang, Q. Q., Kang, Y., Pyun, K. H., Hirasawa, K., Sherwin, R. S. & Jun, H. S. (1999) Science 284, 1183–1187. [DOI] [PubMed] [Google Scholar]
- 14.Jaeckel, E., Klein, L., Martin-Orozco, N. & von Boehmer, H. (2003) J. Exp. Med. 197, 1635–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geng, L., Solimena, M., Flavell, R. A., Sherwin, R. S. & Hayday, A. C. (1998) Proc. Natl. Acad. Sci. USA 95, 10055–10060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schloot, N. C., Daniel, D., Norbury-Glaser, M. & Wegmann, D. R. (1996) J. Autoimmun. 9, 357–363. [DOI] [PubMed] [Google Scholar]
- 17.Tisch, R., Wang, B., Atkinson, M. A., Serreze, D. V. & Friedline, R. (2001) J. Immunol. 166, 6925–6936. [DOI] [PubMed] [Google Scholar]
- 18.Tarbell, K. T., Lee, M., Ranheim, E., Chao, C. C., Sanna, M., Kim, S. K., Dickie, P., Teyton, L., Davis, M. & McDevitt, H. (2002) J. Exp. Med. 196, 481–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chao, C. C. & McDevitt, H. O. (1997) Immunogenetics 46, 29–34. [DOI] [PubMed] [Google Scholar]
- 20.Chao, C. C., Sytwu, H.-K., Chen, E. L., Toma, J. & McDevitt, H. O. (1999) Proc. Natl. Acad. Sci. USA 96, 9299–9304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kouskoff, V., Signorelli, K., Benoist, C. & Mathis, D. (1995) J. Immunol. Methods 180, 273–280. [DOI] [PubMed] [Google Scholar]
- 22.Yasunami, R. & Bach, J. F. (1988) Eur. J. Immunol. 18, 481–484. [DOI] [PubMed] [Google Scholar]
- 23.Bridgett, M., Cetkovic-Cvrlje, M., O'Rourke, R., Shi, Y., Narayanswami, S., Lambert, J., Ramiya, V., Baekkeskov, S. & Leiter, E. H. (1998) Diabetes 47, 1848–1856. [DOI] [PubMed] [Google Scholar]
- 24.Pearson, C. I., van Ewijk, W. & McDevitt, H. O. (1997) J. Exp. Med. 185, 583–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heath, W. R. & Miller, J. F. (1993) J. Exp. Med. 178, 1807–1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zal, T., Weiss, S., Mellor, A. & Stockinger, B. (1996) Proc. Natl. Acad. Sci. USA 93, 9102–9107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sarukhan, A., Garcia, C., Lanoue, A. & von Boehmer, H. (1998) Immunity 8, 563–570. [DOI] [PubMed] [Google Scholar]
- 28.Tau, G. Z., von der Weid, T., Lu, B., Cowan, S., Kvatyuk, M., Pernis, A., Cattoretti, G., Braunstein, N. S., Coffman, R. L. & Rothman, P. B. (2000) J. Exp. Med. 192, 977–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harris, D. P., Haynes, L., Sayles, P. C., Duso, D. K., Eaton, E. M., Lepak, N. M., Johnson, L. L., Swain, S. L. & Lund, F. E. (2000) Nat. Immunol. 1, 475–482. [DOI] [PubMed] [Google Scholar]
- 30.Boyton, R. J., Lohmann, T., Londei, M., Kalbacher, H., Halder, T., Frater, A. J., Douek, D. C., Leslie, D. G., Flavell, R. A. & Altmann, D. M. (1998) Int. Immunol. 10, 1765–1776. [DOI] [PubMed] [Google Scholar]
- 31.Katsikis, P. D., Cohen, S. B., Londei, M. & Feldmann, M. (1995) Int. Immunol. 7, 1287–1294. [DOI] [PubMed] [Google Scholar]
- 32.Windhagen, A., Anderson, D. E., Carrizosa, A., Williams, R. E. & Hafler, D. A. (1996) J. Immunol. 157, 1127–1131. [PubMed] [Google Scholar]
- 33.Hoffman, M. W., Heath, W. R., Ruschmeyer, D. & Miller, J. F. (1995) Proc. Natl. Acad. Sci. USA 92, 9851–9855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hammerling, G. J., Schonrich, G., Ferber, I. & Arnold, B. (1993) Immunol. Rev. 133, 93–104. [DOI] [PubMed] [Google Scholar]
- 35.Hawiger, D., Masilamani, R. F., Bettelli, E., Kuchroo, V. K. & Nussenzweig, M. C. (2004) Immunity 20, 695–705. [DOI] [PubMed] [Google Scholar]
- 36.Constant, S. L. & Bottomly, K. (1997) Annu. Rev. Immunol. 15, 297–322. [DOI] [PubMed] [Google Scholar]
- 37.Leitenberg, D. & Bottomly, K. (1999) Semin. Immunol. 11, 283–292. [DOI] [PubMed] [Google Scholar]
- 38.Constant, S., Pfeiffer, C., Woodard, A., Pasqualini, T. & Bottomly, K. (1995) J. Exp. Med. 182, 1591–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fitch, F. W., McKisic, M. D., Lancki, D. W. & Gajewski, T. F. (1993) Annu. Rev. Immunol. 11, 29–48. [DOI] [PubMed] [Google Scholar]
- 40.Hosken, N. A., Shibuya, K., Heath, A. W., Murphy, K. M. & O'Garra, A. (1995) J. Exp. Med. 182, 1579–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ruedl, C., Bachmann, M. F. & Kopf, M. (2000) Eur. J. Immunol. 30, 2056–2064. [DOI] [PubMed] [Google Scholar]
- 42.Kim, J., Richter, W., Aanstoot, H. J., Shi, Y., Fu, Q., Rajotte, R., Warnock, G. & Baekkeskov, S. (1993) Diabetes 42, 1799–1808. [DOI] [PubMed] [Google Scholar]
- 43.Wegmann, D. R., Shehadeh, N., Lafferty, K. J., Norbury-Glaser, M., Gill, R. G. & Daniel, D. (1993) J. Autoimmun. 6, 517–527. [DOI] [PubMed] [Google Scholar]
- 44.Haskins, K. & Wegmann, D. (1996) Diabetes 45, 1299–1305. [DOI] [PubMed] [Google Scholar]
- 45.Katz, J. D., Wang, B. & Haskins, K., Benoist, C. & Mathis, D. (1993) Cell 74, 1089–1100. [DOI] [PubMed] [Google Scholar]
- 46.Schmidt, D., Verdaguer, J., Averill, N. & Santamaria, P. (1997) J. Exp. Med. 186, 1059–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Flaishon, L., Topilski, I., Shoseyov, D., Hershkoviz, R., Fireman, E., Levo, Y., Marmor, S. & Shachar, I. (2002) J. Immunol. 168, 3707–3711. [DOI] [PubMed] [Google Scholar]
- 48.Rosloniec, E. F., Latham, K. & Guedez, Y. B. (2002) Arthritis Res. 4, 333–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Munn, D. H., Shafizadeh, E., Attwood, J. T., Bondarev, I., Pashine, A. & Mellor, A. L. (1999) J. Exp. Med. 189, 1363–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moore, K. W., de Waal Malefyt, R., Coffman, R. L. & O'Garra, A. (2001) Annu. Rev. Immunol. 19, 683–765. [DOI] [PubMed] [Google Scholar]
- 51.Billiau, A. (1996) Cytokine Growth Factor Rev. 7, 25–34. [DOI] [PubMed] [Google Scholar]
- 52.Gebe, J. A., Falk, B. A., Rock, K. A., Kochik, S. A., Heninger, A. K., Reijonen, H., Kwok, W. W. & Nepom, G. T. (2003) Eur. J. Immunol. 33, 1409–1417. [DOI] [PubMed] [Google Scholar]
- 53.Fossati, G., Cooke, A., Papafio, R. Q., Haskins, K. & Stockinger, B. (1999) J. Exp. Med. 190, 577–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kanagawa, O., Militech, A. & Vaupel, B. A. (2002) J. Immunol. 168, 6159–6164. [DOI] [PubMed] [Google Scholar]
- 55.Derbinski, J., Schulte, A., Kyewski, B. & Klein, L. (2001) Nat. Immunol. 2, 1032–1039. [DOI] [PubMed] [Google Scholar]
- 56.Sospedra, M., Ferrer-Francesch, X., Dominguez, O., Juan, M., Foz-Sala, M. & Pujol-Borrell, R. (1998) J. Immunol. 161, 5918–5929. [PubMed] [Google Scholar]
- 57.Pleau, J. M., Esling, A., Geutkens, S., Dardenne, M. & Homo-Delarche, F. (2001) Biochem. Biophys. Res. Commun. 283, 843–848. [DOI] [PubMed] [Google Scholar]
- 58.Pugliese, A., Brown, D., Garza, D., Murchison, D., Zeller, M., Redondo, M., Diez, J., Eisenbarth, G. S., Patel, D. D. & Ricordi, C. (2001) J. Clin. Invest. 107, 555–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Anderson, M. S., Venanzi, E. S., Klein, L., Chen, Z., Berzins, S. P., Turley, S. J., von Boehmer, H., Bronson, R., Dierich, A., Benoist, C. & Mathis, D. (2002) Science 298, 1395–1401. [DOI] [PubMed] [Google Scholar]
- 60.Liston, A., Lesage, S., Wilson, J., Peltonen, L. & Goodnow, C. C. (2003) Nature Immunol. 4, 350–354. [DOI] [PubMed] [Google Scholar]
- 61.Andre, I., Gonzalez, A., Wang, B., Katz, J., Benoist, C. & Mathis, D. (1996) Proc. Natl. Acad. Sci. USA 93, 2260–2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gottlieb, P. A. & Eisenbarth, G. S. (1996) J. Autoimmun. 9, 277–281. [DOI] [PubMed] [Google Scholar]