Abstract
The differentiation and maturation of dendritic cells (DCs) is governed by various signals in the microenvironment. Monocytes and DCs circulate in peripheral blood, which contains high levels of natural antibodies (NAbs). NAbs are germ-line-encoded and occur in the absence of deliberate immunization or microbial aggression. To assess the importance of NAbs in the milieu on DC development, we examined the status of DCs in patients with X-linked agammaglobulinemia, a disease characterized by paucity of B cells and circulating antibodies. We demonstrate that the in vitro differentiation of DCs is severely impaired in these patients, at least in part because of low levels of circulating NAbs. We identified NAbs reactive with the CD40 molecule as an important component that participates in the development of DCs. CD40-reactive NAbs restored normal phenotypes of DCs in patients. The maturation process induced by CD40-reactive NAbs was accompanied by an increased IL-10 and decreased IL-12 production. The transcription factor analysis revealed distinct signaling pathways operated by CD40-reactive NAbs compared to those by CD40 ligand. These results suggest that B cells promote bystander DC development through NAbs and the interaction between NAbs and DCs may play a role in steady-state migration of DCs.
Monocytes represent a large pool of circulating precursors that can differentiate into dendritic cells (DCs) or macrophages. Such developmental plasticity is conserved until the late stages of differentiation (1–3). DCs play a critical role in both T cell priming and T cell tolerance (4–7). Signals that determine the differentiation of monocytes into various types of antigen-presenting cells (APCs) and the impact of microenvironmental context on DC differentiation, maturation, and DC-mediated peripheral tolerance are not fully understood. Monocytes and DCs circulate in peripheral blood, which contains high levels of natural antibodies (NAbs). NAbs are the products of germ-line Ig gene expression in B cells that are positively selected during ontogeny (8, 9). They occur in the absence of deliberate immunization or microbial aggression. Most NAbs are autoreactive (10, 11) and participate in the maintenance of immune homeostasis under physiological conditions (12, 13). We thus surmised that NAbs in the milieu are critical elements in the development process of DCs.
To examine this hypothesis, we resorted to X-linked agammaglobulinemia (XLA), a disease consecutive to mutations in the Bruton's tyrosine kinase (btk) gene leading to a paucity of circulating B cells and a marked reduction in circulating IgG antibodies (14, 15). The number and function of T cells are generally normal in patients with XLA (16–18). XLA thus represents a unique model to study the role of NAbs in development of DCs. Our results show that NAbs sustain DC differentiation and maturation, thus suggesting that B cells promote bystander DC development. We further document the role of NAbs reactive with CD40 in this process.
Materials and Methods
Patients and DCs. Heparinized blood samples were obtained from seven patients with XLA at least 21 days after last infusion of intravenous Ig (IVIg). The relevant local ethical committee granted approval, and informed consent was obtained from all the patients. The mean age of the patients was 16.6 ± 9 y (mean ± SD, ranging from 3 to 25 y) (see Table 1, which is published as supporting information on the PNAS web site). At the time of bleeding, the IgG concentration in plasma was 5.3 ± 1.9 mg/ml (ranging from 2 to 8.7 mg/ml). As control, blood samples were obtained also from six adult healthy blood donors. Monocyte-derived DCs (mo-DCs) from patients' blood and control groups were generated as described (19) in the presence of granulocyte/macrophage colony-stimulating factor (GM-CSF), IL-4, and 10% autologous plasma.
Antibodies and Reagents. IVIg (Sandoglobulin), consisting of IgG obtained from plasma pools of healthy donors, was used as a source of NAbs. IVIg was dialyzed extensively and was free from endotoxins. F(ab′)2 fragments were prepared from IVIg by pepsin digestion followed by dialysis and chromatography on protein G-Sepharose to isolate F(ab′)2 fragments from the residual intact IgG molecules and Fc fragments. F(ab′)2 fragments were free of intact IgG and Fc fragments as assessed by SDS/PAGE and ELISA (data not shown).
Mouse fibroblast L cells transfected with the human CD40 and CD40 ligand (CD40L) were gifts from P. Garrone (Dardilly, France) and were maintained as described (20).
Mixed Lymphocyte Reaction (MLR). Responder CD4+ T cells used for allogeneic MLR assays were isolated from peripheral blood mononuclear cells of healthy blood donors by using the MACS cell isolation kit (Miltenyi Biotec, Bergisch Gladbach, Germany). DCs from patients with XLA were differentiated in the presence of GM-CSF and IL-4 alone for 6 days or cultured with CD40-reactive NAbs or CD40 mAb89 (Immunotech, Marseilles, France) or CD40L-transfected fibroblasts (10:1) during the last 48 h of a 6-day culture. Graded doses of DCs then were seeded with 1 × 105 responder allogeneic T cells at DC/T cell ratios of 1:10, 1:20, and 1:50. After 4 days, the cells were pulsed for 16 h with 1 μCi (1 Ci = 37 GBq) of [3H]thymidine. Incorporation of radioactivity was measured, and results were expressed as cpm (mean ± SD of triplicate values) after subtracting values of responder T cell cultures alone.
Transcription Factor Analysis. Six-day-old mo-DCs from healthy donors were stimulated with CD40L-transfected fibroblasts (10:1) or CD40-reactive NAbs (0.6 μM) for 24 h. Nuclear and cytoplasmic extracts were prepared by a TransFactor extraction kit (Clontech). DNA binding by specific transcription factors was detected by ELISA using a Mercury transfactor kit (BD Biosciences/Clontech).
Cytokine Assays. Cytokines were quantified in cell-free culture supernatants by using commercial Quantikine immunoassay kits from Immunotech (IL-10) and R & D Systems (IL-12 p70).
Results
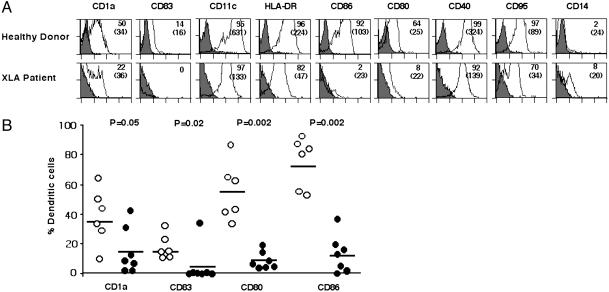
Defective Differentiation of DCs from Patients with XLA. Peripheral blood monocytes of patients with XLA exhibited a markedly impaired differentiation characterized by significantly reduced expression of CD1a and CD83, as compared with DCs of healthy controls (Fig. 1, P ≤ 0.05). The differentiated cells were negative or low-positive for CD14 and CD16, indicating that monocytes were not differentiating toward macrophages (Fig. 1 A and data not shown). The cells also expressed decreased levels of CD80 (10 ± 5%, average ± SD) and CD86 (13 ± 12%) (Fig. 1, P = 0.002). The expression of HLA-DR [mean fluorescence intensity (MFI): 89.9 ± 44.9], CD11c (MFI: 155.9 ± 59.9), and CD40 (MFI: 150.4 ± 47.9) on patients' DCs (n = 7) was also significantly lower than that of DCs from healthy donors (n = 6) (MFI: 261.5 ± 64, 506.7 ± 185.7, and 282.3 ± 47.9 for HLA-DR, CD11c, and CD40, respectively; P < 0.005) (Fig. 1 A). In addition, BDCA-1+ myeloid DCs of patients with XLA also displayed significantly down-regulated expression of HLA-DR (MFI: 646.2 ± 437.6) and CD11c (MFI: 237 ± 170.4), as compared with healthy donors (MFI: 1316.3 ± 168.2 and 657.5 ± 251.7 for HLA-DR and CD11c, respectively; P < 0.05, Mann–Whitney test), whereas CD86 (MFI: 181 ± 130.3 vs. 330.7 ± 217.2 in healthy donors), CD40 (MFI: 471 ± 430.4 vs. 697.3 ± 379.4), and CD54 (MFI: 702.2 ± 484.2 vs. 944 ± 435) showed a tendency to be reduced (Fig. 6, which is published as supporting information on the PNAS web site). However, no defects were observed with BDCA-2+ lymphoid DCs of patients with XLA (data not shown).
Fig. 1.
Defective differentiation of mo-DCs in patients with XLA. (A) Flow-cytometric analysis of 6-day-old mo-DCs of healthy donors (Upper) and patients with XLA (Lower). Fluorescence was analyzed with a FACScan flow cytometer, and data were processed by cellquest. The percentage of cells that are positive for the indicated markers is shown, and MFIs are indicated in parentheses (representative of six healthy donors and seven patients). (B) Comparison of the percentage of cells expressing CD1a, CD83, CD80, and CD86 in seven patients with XLA (filled circles) and six healthy donors (open circles) after differentiation of DCs for 6 days. The mean values are indicated with a horizontal bar for each marker. Statistical significance as determined by the Mann–Whitney test is indicated.
Incubation of patient mo-DCs with CD40L-transfected fibroblasts (10:1 ratio), CD40 mAb89 (Fig. 7, which is published as supporting information on the PNAS web site), or lipopolysaccharide (data not shown) induced a normal maturation of the cells, which demonstrates that the DCs of patients with XLA are not intrinsically defective and are able to respond to appropriate maturation signaling. DCs of patients with XLA that were allowed to differentiate in the presence of autologous plasma reconstituted with NAbs (IVIg) to physiological levels expressed increased levels of the maturation markers as compared with cells cultured without in vitro addition of Igs (Fig. 2A). Together, the data suggest that the defective differentiation of DCs in patients with XLA at least in part depends on the low levels of circulating antibodies.
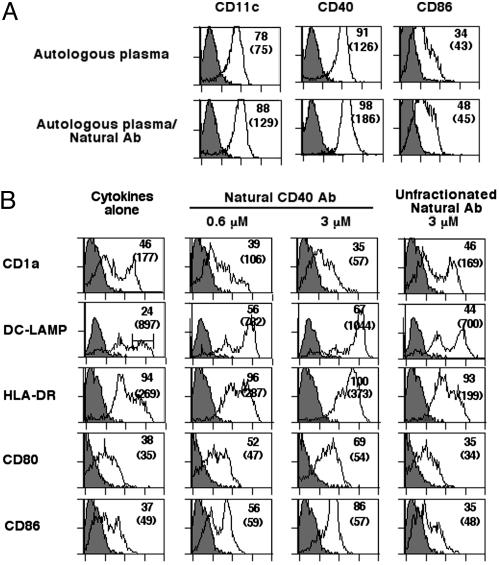
Fig. 2.
NAbs promote DC differentiation and maturation. (A) DCs from patients with XLA were differentiated in the presence of autologous plasma (Upper) or autologous plasma reconstituted with 0.03 mM IVIg as a source of NAbs (Lower) for 6 days. The percentage of cells that are positive for the indicated markers is shown, and MFIs are shown in parentheses. (B) CD40-reactive NAbs induce maturation of DCs of healthy donors. Six-day-old DCs from healthy donors were incubated with CD40-reactive NAbs (Center, 0.6 and 3 μM) or unfractionated NAbs (Right, 3 μM) or cultured in the medium alone (Left) in the presence of GM-CSF and IL-4 for 48 h. The percentage of cells that are positive for the indicated markers is shown, and MFIs are shown in parentheses. The representative of four independent experiments is shown. LAMP, lysosome-associated membrane glycoprotein.
Natural CD40-Reactive Antibodies Induce Maturation of DCs of Healthy Individuals. Having established that NAbs influence the development of DCs, we aimed at identifying the key components within the repertoire of NAbs that may be responsible for differentiation and maturation of DCs. The fact that DCs from patients with XLA undergo normal maturation after CD40 stimulation lead us to hypothesize that CD40-reactive antibodies in the pool of NAbs provide an endogenous stimulation for DC development.
We isolated antibodies reactive with CD40 from a pool of normal Ig (IVIg) by affinity chromatography on a peptide (78HQHKYCDPNLGLRV91) derived from the ligand-binding site of CD40 (21). CD40-reactive antibodies represent up to 1% of the loaded IVIg. To assess whether CD40 peptide absorbed anti-CD40 antibodies, we performed an ELISA by using CD40 peptide and flow-cytometry analysis with CD40 transfected fibroblasts. Both intact IgG (data not shown) and F(ab′)2 fragments of CD40-reactive NAbs recognized immobilized CD40 peptide in a dose-dependent manner as well as native CD40 molecules expressed on transfected mouse fibroblasts (Fig. 8, which is published as supporting information on the PNAS web site).
Stimulation of 6-day-old DCs from healthy donors with CD40-reactive NAbs for 48 h induced an up-regulation of HLA-DR, DC-lysosome-associated membrane glycoprotein, and costimulatory molecules while down-regulating CD1a (Fig. 2B). The maturation of DCs induced by CD40-reactive NAbs was dose-dependent. Meanwhile, incubation of 6-day-old DCs in the presence of an unfractionated pool of NAbs (IVIg) also induced an early-stage maturation of DCs, as indicated by an up-regulated expression of DC-lysosome-associated membrane glycoprotein (Fig. 2B). To confirm whether natural CD40 antibodies represent the functional activity within IVIg that mediates DC maturation, 6-day-old DCs from healthy donors were incubated with NAbs depleted of CD40 reactivity. DCs cultured in the presence of NAbs depleted of CD40 reactivity failed to undergo maturation, as indicated by the down-regulated expression of CD83 (33% down-regulation), CD95 (35%), and costimulatory markers CD80 (27%) and CD86 (26%). The down-regulation of these markers was not observed in the presence of NAbs that were depleted of antibodies reactive with an irrelevant antigen (Fig. 9, which is published as supporting information on the PNAS web site).
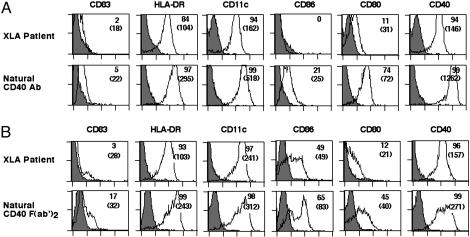
CD40-Reactive NAbs Induce Maturation of DCs from Patients with XLA. The results obtained with DCs of healthy blood donors raise an interesting possibility in that signaling by CD40-reactive NAbs may restore normal phenotypes of DCs from patients with XLA. Stimulation of differentiating DCs from patients with XLA with CD40-reactive NAbs [both IgG or F(ab′)2 fragments] for 48 h resulted in an up-regulation of HLA-DR, CD11c, CD80, CD86, and CD40 (Fig. 3). The up-regulation of CD83, however, was marginal. Thus, stimulation with CD40-reactive antibodies induced a partially matured DC. Because DCs from patients with XLA expressed CD32 (54 ± 35%; MFI: 51 ± 47) and were negative for CD64, we addressed the possibility that CD40-reactive NAbs might couple CD40 signaling with Fc-receptor (FcR) signaling. However, CD40-reactive NAbs induced a similar degree of maturation of DCs from patients with XLA despite FcR blockade (data not shown). The results thus indicate that signaling by CD40-reactive NAbs might not involve signaling through the FcR.
Fig. 3.
Signaling by CD40-reactive NAbs restores normal phenotypes of DCs from patients with XLA. DCs from patients with XLA were differentiated in the presence of GM-CSF, IL-4, and 10% autologous plasma for 6 days. DCs were stimulated with either CD40-reactive natural IgG (3 μM) (A Lower) or F(ab′)2 fragments of CD40-reactive NAbs (1.5 μM) (B Lower) during the last 48 h of culture. The percentage of cells that are positive for the indicated markers is shown, and MFIs are shown in parentheses.
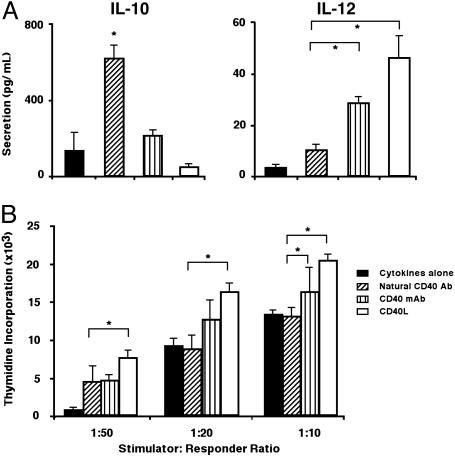
Maturation of DCs by CD40-Reactive NAbs Is Not Associated with IL-12 Production. CD40 ligation on normal DCs triggers the production of high levels of IL-12 (22), which plays an important role in polarizing T cells toward the T helper 1 (Th1) pathway. Surprisingly, CD40-reactive NAbs triggered DCs from patients with XLA to secrete high amounts of IL-10 (P < 0.05) and low levels of bioactive IL-12 (p70) (Fig. 4A). In contrast, stimulation by CD40L-transfected fibroblasts or CD40 mAb resulted in an increased secretion of IL-12 (P < 0.05) and down-regulation of IL-10 (Fig. 4A). We then tested the ability of DCs from patients with XLA stimulated in the presence of CD40-reactive NAbs to induce allogeneic CD4+ T cell proliferation in an MLR. DCs matured in the presence of CD40-reactive NAbs stimulated the proliferation of responder cells but were not particularly more potent as APCs than DCs cultured in the presence of cytokines alone to induce proliferation of allogeneic CD4+ T cells in an MLR (Fig. 4B). These results suggest that although DCs matured in the presence of NAbs secreting IL-10, they do not suppress T cell proliferation. However, the fact that DCs do not secrete IL-12 indicates that their maturation induced by NAbs does not initiate Th1 pathway by default under steady-state conditions.
Fig. 4.
Cytokine production and T cell stimulation by DC upon stimulation with CD40-reactive NAbs. (A) Maturation of DCs by CD40-reactive NAbs leads to production of high amounts of IL-10 (Left) but not IL-12 (Right). Cytokine production by 6-day-old DCs of patients with XLA cultured in the presence of GM-CSF and IL-4 alone (filled bars) or after stimulation with CD40-reactive NAbs (3 μM; diagonally hatched bars) or CD40 mAb89 (3 μg/ml; vertical hatched bars) or CD40L-transfected fibroblasts (10:1; open bars) during the last 48 h of a 6-day culture. *, P < 0.05, unpaired t test. (B) Allogeneic stimulation of CD4+ T cells by DCs in an MLR. DCs from patients with XLA were differentiated in the presence of GM-CSF and IL-4 alone for 6 days (filled bars) or cultured with CD40-reactive NAbs (3 μM; diagonally hatched bars), CD40 mAb89 (3 μg/ml; vertical hatched bars), or CD40L-transfected fibroblasts (10:1; open bars) during the last 48 h of a 6-day culture. Graded doses of DCs were then used to stimulate allogeneic CD4+ T cells as described. The level of [3H]thymidine uptake by T cells in medium alone was in the range of 1,010 ± 40 cpm. *, P < 0.05, unpaired t test.
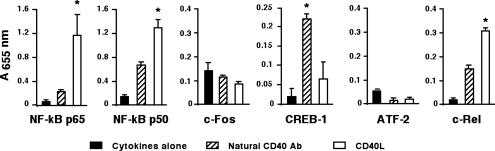
Natural CD40-Reactive Antibodies Operate Through Distinct Signaling Pathways. To explore the mechanistic insight into the pathways used by CD40-reactive NAbs, we analyzed downstream signaling through various transcription factors. Six-day-old DCs from healthy donors stimulated with CD40L-transfected fibroblasts (10:1) exhibited significantly increased levels of NF-κB p65, NF-κB p50, and c-Rel (Fig. 5), whereas stimulation with CD40-reactive NAbs induced enhanced levels of cAMP-response element-binding protein 1 (CREB-1) (Fig. 5). These results suggest that CD40-reactive NAb-induced maturation involves distinct signaling pathways.
Fig. 5.
Stimulation of DCs by CD40-reactive NAbs and CD40L operates through distinct signaling pathways. Six-day-old mo-DCs from healthy donors were cultured in the presence of GM-CSF and IL-4 alone (filled bars) or cultured with CD40-reactive NAbs (diagonally hatched bars) or CD40L-transfected fibroblasts (open bars) for 24 h. Nuclear extracts were prepared, and transcription factor profiles in nuclear extracts were detected by ELISA. Data are expressed as the mean ± SD of two experiments. *, P < 0.05, ANOVA.
Discussion
XLA is associated with mutations in the btk gene that lead to abortive B cell differentiation (23–25) and is characterized by low levels of circulating antibodies of all isotypes. Mutations in btk seem to have no effect on myeloid cell function, and the number and function of T cells are generally normal (16, 17). We demonstrate that XLA is associated with dramatically impaired differentiation of DCs characterized by a significantly reduced expression of markers, as compared to that of healthy donors. Stimulation by CD40L, CD40 mAb, or lipopolysaccharide led to normal differentiation and maturation of DCs from patients with XLA, confirming that altered DC differentiation is not a result of the btk mutations. Because the mean serum concentration of IgG in healthy individuals is 12–13 mg/ml serum (26), patient DCs were allowed to differentiate in the presence of autologous plasma reconstituted up to 12–13 mg/ml with IVIg, a privileged source of NAbs. DCs differentiated in the presence of IVIg at a physiological IgG level showed a tendency to up-regulate some of the phenotypic markers. Interestingly, we previously observed an inhibitory effect on the maturation process of DCs by IVIg at a high concentration corresponding to that used in the therapy of autoimmune and inflammatory conditions (19, 27). These results thus suggest that IVIg at a concentration corresponding to that of a physiological level of IgG is not inhibitory and point toward a dual role of IVIg on DCs depending on concentration. We suggest that the prolonged exposure of bone marrow progenitors or monocytes to an agammaglobulinemic microenvironment might lead to a down-regulated differentiation process of DCs of patients with XLA. Together, these results point toward a role for NAbs in the development of DCs under physiological conditions.
The interaction between CD40 and CD40L is involved in a wide range of immunological cross-talks among cells of the immune system, including those between DCs and T cells (22, 28–30). There is evidence that triggering of CD40 by CD40L promotes differentiation and maturation of DCs (18, 31). Because NAbs were able to enhance differentiation of DCs from patients with XLA, we investigated the presence of CD40-reactive antibodies within the repertoire of NAbs and determined the functional relevance of the CD40-reactive antibodies isolated from the pool of NAbs on the development of DCs of patients with XLA. The following lines of evidence support that the concentration of IVIg and CD40-reactive NAbs used in the experiments are representative of physiological levels of circulating antibodies. Because the mean serum concentration of IgG in healthy individuals is 12–13 mg/ml serum and CD40-reactive NAbs represent up to 1% of the pool of NAbs, the fraction of CD40-reactive NAbs would correspond to ≈120–130 μg (0.72–0.78 μM) of total circulating IgG. At 0.6 μM concentration, CD40-reactive NAbs induced maturation of DCs from healthy donors as well as patients with XLA. However, all individuals may not have similar levels of circulating CD40-reactive NAbs. The contribution from a large number of donors may account for the consistent level of individual antibody activities within IVIg (13).
We investigated the induction of transcription factors to further delineate the signaling pathways operated by CD40-reactive NAbs from those by CD40L. Although CD40 mAbs induced the maturation of DCs from patients with XLA, we favored CD40L as a control for CD40-reactive NAbs, because both CD40L (in the context of cross-talk between T cells and DCs and being an important stimulus for DC activation) and CD40-reactive NAbs represent a physiological state, whereas CD40 mAbs are induced by hyperimmunization in other species and thus are not equivalent for NAbs in a steady state. The transcription factor NF-κB is widely recognized as a critical mediator of immune and inflammatory responses. In the nucleus, NF-κB regulates genes encoding cytokines, cytokine receptors, cell adhesion molecules, proteins involved in coagulation, and genes involved in cell-growth control and the inflammatory process (32, 33). The transcription factors of the Rel/NF-κB family are expressed by DCs and play an important role in their activation and are a critical signal for cross-priming APCs (34, 35). The maturation of DCs results in an increase in nuclear RelB, p50, p52, and c-Rel (36, 37). Because NF-κB activation is required for functional maturation of DCs, signals that enhance the APC function of DCs most effectively would be expected to have potent effects on NF-κB. Our study clearly demonstrates the differences in the patterns of signaling through CD40-reactive NAbs and CD40L. Thus, signaling by CD40L implies activation of NF-κB components: NF-κB p65, NF-κB p50, and c-Rel. In contrast, CD40-reactive NAb-mediated signaling of DCs involves activation of cAMP-response element-binding protein 1, the transcription factor involved in cAMP-mediated signaling and low levels of Rel/NF-κB proteins compared to CD40L signaling. cAMP-responsive elements are involved in the transcriptional activation of the human IL-10 gene in monocytic cells (38) and is consistent with the observed increased production of IL-10 by DCs after stimulation with CD40-reactive NAbs. It is not understood at this stage whether the observed effect is caused by variations in the CD40 epitope recognition by CD40-reactive NAbs vs. CD40L (39), differences in the affinity between CD40-reactive NAbs and CD40L, or cross-reactivity of CD40-reactive NAbs with other signaling molecules caused by the inherent polyreactive property of NAbs (40).
Patients with XLA are biased to predominant Th1 responses and are associated with an increased incidence of several Th1-oriented pathological conditions (14, 41, 42). Patient DCs displayed defective differentiation yet produced IL-12, a Th1-priming cytokine, after engagement of the surface CD40 by CD40L. Thus, the absence of induction of IL-10, consecutive to the lack of stimulation by NAbs caused by the primary Ig deficiency, may, in the context of efficient CD40L signaling, further participate in the development of pathological Th1 responses in patients with XLA.
DCs originate from either myeloid or lymphoid precursors in the bone marrow (1, 2). Under the influence of pathogens and inflammation, tissue-resident DCs undergo activation and maturation followed by migration to the draining lymph nodes, where they present antigens for T cell response (1). The spontaneous migration to lymph nodes of tissue-resident DCs has also been described under physiological conditions (43–45). The signals that induce maturation of steady-state migratory DCs is not totally understood. Our data demonstrate that NAbs are able to induce a maturation of mo-DCs from healthy individuals and from patients with XLA and suggest that they may play a similar role in the development of mo-DCs and in migration of DCs under physiological conditions. Indeed, MLR results indicate no difference between DCs matured in the presence of CD40-reactive NAbs and DCs cultured in the presence of cytokines alone in their capacity to act as APCs. The fact that signaling by NAbs does not initiate IL-12 production makes teleological sense, because it ensures that maturation of DCs does not lead to Th1 differentiation by default. Thus, induction of maturation of DCs without enhancing their immunostimulatory capacity seems to be a function of NAbs in steady state. Whether NAbs in the peripheral tissues exert similar effects on the tissue-resident DC lineage remains to be determined.
Supplementary Material
Acknowledgments
We thank Drs. A. Galy, A. Hosmalin, and A. Nicoletti for critically reading the manuscript, and F. Prost and A. Lengagne for technical assistance. This work was supported by grants from Institut National de la Santé et de la Recherche Médicale, Centre National de la Recherche Scientifique, and ZLB Bioplasma AG (Bern, Switzerland). J.B., C.C., and N.M. are recipients of fellowships from the Fondation de la Recherche Médicale, Ministère de l'Education Nationale, and Agence Nationale de Recherches sur le SIDA (France), respectively.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: DC, dendritic cell; APC, antigen-presenting cell; NAb, natural Ab; XLA, X-linked agammaglobulinemia; btk, Bruton's tyrosine kinase; IVIg, intravenous Ig; mo-DC, monocyte-derived DC; GM-CSF, granulocyte/macrophage colony-stimulating factor; CD40L, CD40 ligand; MLR, mixed lymphocyte reaction; MFI, mean fluorescence intensity; Th1, T helper 1.
References
- 1.Banchereau, J. & Steinman, R. M. (1998) Nature 392, 245–252. [DOI] [PubMed] [Google Scholar]
- 2.Randolph, G. J., Beaulieu, S., Lebecque, S., Steinman, R. M. & Muller, W. A. (1998) Science 282, 480–483. [DOI] [PubMed] [Google Scholar]
- 3.Liu, Y. J. (2001) Cell 106, 259–262. [DOI] [PubMed] [Google Scholar]
- 4.Banchereau, J., Briere, F., Caux, C., Davoust, J., Lebecque, S., Liu, Y. J., Pulendran, B. & Palucka, K. (2000) Annu. Rev. Immunol. 18, 767–811. [DOI] [PubMed] [Google Scholar]
- 5.Heath, W. R. & Carbone, F. R. (2001) Annu. Rev. Immunol. 19, 47–64. [DOI] [PubMed] [Google Scholar]
- 6.Guermonprez, P., Valladeau, J., Zitvogel, L., Thery, C. & Amigorena, S. (2002) Annu. Rev. Immunol. 20, 621–667. [DOI] [PubMed] [Google Scholar]
- 7.Steinman, R. M., Hawiger, D. & Nussenzweig, M. C. (2003) Annu. Rev. Immunol. 21, 685–711. [DOI] [PubMed] [Google Scholar]
- 8.Hayakawa, K., Asano, M., Shinton, S. A., Gui, M., Allman, D., Stewart, C. L., Silver, J. & Hardy, R. R. (1999) Science 285, 113–116. [DOI] [PubMed] [Google Scholar]
- 9.Marchalonis, J. J., Adelman, M. K., Zeitler, B. J., Sarazin, P. M., Jaqua, P. M. & Schluter, S. F. (2001) Adv. Exp. Med. Biol. 484, 13–30. [DOI] [PubMed] [Google Scholar]
- 10.Avrameas, S. (1991) Immunol. Today 12, 154–159. [DOI] [PubMed] [Google Scholar]
- 11.Notkins, A. L. & Prabhakar, B. S. (1986) Ann. N.Y. Acad. Sci. 475, 123–134. [DOI] [PubMed] [Google Scholar]
- 12.Coutinho, A. (2003) Trends Immunol. 24, 53–54. [DOI] [PubMed] [Google Scholar]
- 13.Kazatchkine, M. D. & Kaveri, S. V. (2001) N. Engl. J. Med. 345, 747–755. [DOI] [PubMed] [Google Scholar]
- 14.Rosen, F. S., Cooper, M. D. & Wedgwood, R. J. (1995) N. Engl. J. Med. 333, 431–440. [DOI] [PubMed] [Google Scholar]
- 15.Conley, M. E. & Cooper, M. D. (1998) Curr. Opin. Immunol. 10, 399–406. [DOI] [PubMed] [Google Scholar]
- 16.Paroli, M., Accapezzato, D., Francavilla, V., Insalaco, A., Plebani, A., Balsano, F. & Barnaba, V. (2002) Blood 99, 2131–2137. [DOI] [PubMed] [Google Scholar]
- 17.Futatani, T., Miyawaki, T., Tsukada, S., Hashimoto, S., Kunikata, T., Arai, S., Kurimoto, M., Niida, Y., Matsuoka, H., Sakiyama, Y., et al. (1998) Blood 91, 595–602. [PubMed] [Google Scholar]
- 18.Shreedhar, V., Moodycliffe, A. M., Ullrich, S. E., Bucana, C., Kripke, M. L. & Flores-Romo, L. (1999) Immunity 11, 625–636. [DOI] [PubMed] [Google Scholar]
- 19.Bayry, J., Lacroix-Desmazes, S., Carbonneil, C., Misra, N., Donkova, V., Pashov, A., Chevailler, A., Mouthon, L., Weill, B., Bruneval, P., et al. (2003) Blood 101, 758–765. [DOI] [PubMed] [Google Scholar]
- 20.Garrone, P., Neidhardt, E. M., Garcia, E., Galibert, L., van Kooten, C. & Banchereau, J. (1995) J. Exp. Med. 182, 1265–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bajorath, J., Chalupny, N. J., Marken, J. S., Siadak, A. W., Skonier, J., Gordon, M., Hollenbaugh, D., Noelle, R. J., Ochs, H. D. & Aruffo, A. (1995) Biochemistry 34, 1833–1844. [DOI] [PubMed] [Google Scholar]
- 22.Cella, M., Scheidegger, D., Palmer-Lehmann, K., Lane, P., Lanzavecchia, A. & Alber, G. (1996) J. Exp. Med. 184, 747–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Conley, M. E., Parolini, O., Rohrer, J. & Campana, D. (1994) Immunol. Rev. 138, 5–21. [DOI] [PubMed] [Google Scholar]
- 24.Tsukada, S., Rawlings, D. J. & Witte, O. N. (1994) Curr. Opin. Immunol. 6, 623–630. [DOI] [PubMed] [Google Scholar]
- 25.Desiderio, S. (1997) Curr. Opin. Immunol. 9, 534–540. [DOI] [PubMed] [Google Scholar]
- 26.Abbas, A. K., Lichtman, A. H. & Pober, J. S. (1991) in Cellular and Molecular Immunology (Saunders, Philadelphia), p. 44.
- 27.Bayry, J., Lacroix-Desmazes, S., Delignat, S., Mouthon, L., Weill, B., Kazatchkine, M. D. & Kaveri, S. V. (2003) Arthritis Rheum. 48, 3497–3502. [DOI] [PubMed] [Google Scholar]
- 28.Caux, C., Massacrier, C., Vanbervliet, B., Dubois, B., Van Kooten, C., Durand, I. & Banchereau, J. (1994) J. Exp. Med. 180, 1263–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ridge, J. P., Di Rosa, F. & Matzinger, P. (1998) Nature 393, 474–478. [DOI] [PubMed] [Google Scholar]
- 30.Schoenberger, S. P., Toes, R. E., van der Voort, E. I., Offringa, R. & Melief, C. J. (1998) Nature 393, 480–483. [DOI] [PubMed] [Google Scholar]
- 31.Mekori, Y. A. & Metcalfe, D. D. (2000) Immunol. Rev. 173, 131–140. [DOI] [PubMed] [Google Scholar]
- 32.Baldwin, A. S., Jr. (1996) Annu. Rev. Immunol. 14, 649–683. [DOI] [PubMed] [Google Scholar]
- 33.Ghosh, S., May, M. J. & Kopp, E. B. (1998) Annu. Rev. Immunol. 16, 225–260. [DOI] [PubMed] [Google Scholar]
- 34.Bell, D., Young, J. W. & Banchereau, J. (1999) Adv. Immunol. 72, 255–324. [DOI] [PubMed] [Google Scholar]
- 35.Mintern, J. D., Belz, G., Gerondakis, S., Carbone, F. R. & Heath, W. R. (2002) J. Immunol. 168, 3283–3287. [DOI] [PubMed] [Google Scholar]
- 36.Neumann, M., Fries, H., Scheicher, C., Keikavoussi, P., Kolb-Maurer, A., Brocker, E., Serfling, E. & Kampgen, E. (2000) Blood 95, 277–285. [PubMed] [Google Scholar]
- 37.O'Sullivan, B. J. & Thomas, R. (2002) J. Immunol. 168, 5491–5498. [DOI] [PubMed] [Google Scholar]
- 38.Platzer, C., Fritsch, E., Elsner, T., Lehmann, M. H., Volk, H. D. & Prosch, S. (1999) Eur. J. Immunol. 29, 3098–3104. [DOI] [PubMed] [Google Scholar]
- 39.Tone, M., Tone, Y., Fairchild, P. J., Wykes, M. & Waldmann, H. (2001) Proc. Natl. Acad. Sci. USA 98, 1751–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wardemann, H., Yurasov, S., Schaefer, A., Young, J. W., Meffre, E. & Nussenzweig, M. C. (2003) Science 301, 1374–1377. [DOI] [PubMed] [Google Scholar]
- 41.Cellier, C., Foray, S. & Hermine, O. (2000) N. Engl. J. Med. 342, 1611–1612. [DOI] [PubMed] [Google Scholar]
- 42.Amedei, A., Romagnani, C., Benagiano, M., Azzurri, A., Fomia, F., Torrente, F., Plebani, A., D'Elios, M. M. & Del Prete, G. (2001) Eur. J. Immunol. 31, 1927–1934. [DOI] [PubMed] [Google Scholar]
- 43.Huang, F. P., Platt, N., Wykes, M., Major, J. R., Powell, T. J., Jenkins, C. D. & MacPherson, G. G. (2000) J. Exp. Med. 191, 435–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hawiger, D., Inaba, K., Dorsett, Y., Guo, M., Mahnke, K., Rivera, M., Ravetch, J. V., Steinman, R. M. & Nussenzweig, M. C. (2001) J. Exp. Med. 194, 769–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vermaelen, K. Y., Carro-Muino, I., Lambrecht, B. N. & Pauwels, R. A. (2001) J. Exp. Med. 193, 51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.