Abstract
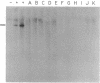
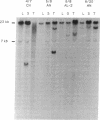
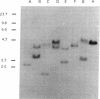
v-abl, the oncogene transduced by Abelson murine leukemia virus, was first characterized by its ability to transform lymphoid cells. bcr-abl, the oncogene formed by a t(9;22) translocation thought to occur in human hematopoietic stem cells, is detectable in almost all cases of chronic myelogenous leukemia (CML), a malignancy of granulocytic cells. bcr-abl also causes a CML-like syndrome in mice whose bone-marrow cells are infected with a retrovirus transducing the gene. More recent reports have suggested that v-abl can, however, cause a disease similar to CML. We demonstrate here that v-abl, when transduced in a helper virus-containing system, causes disease similar to, but distinct from, the CML-like syndrome induced by bcr-abl. Animals whose bone marrow has been infected by v-abl virus develop modest splenomegaly, marked granulocytosis, and malignant disease of several hematopoietic cell types. Unlike animals with CML-like disease resulting from bcr-abl, the polymorphonuclear leukocytes from animals infected with a v-abl construct do not contain the v-abl provirus at a significant frequency. Histopathologic analysis also shows significant differences between the diseases caused by v-abl and bcr-abl.
Full text
PDF

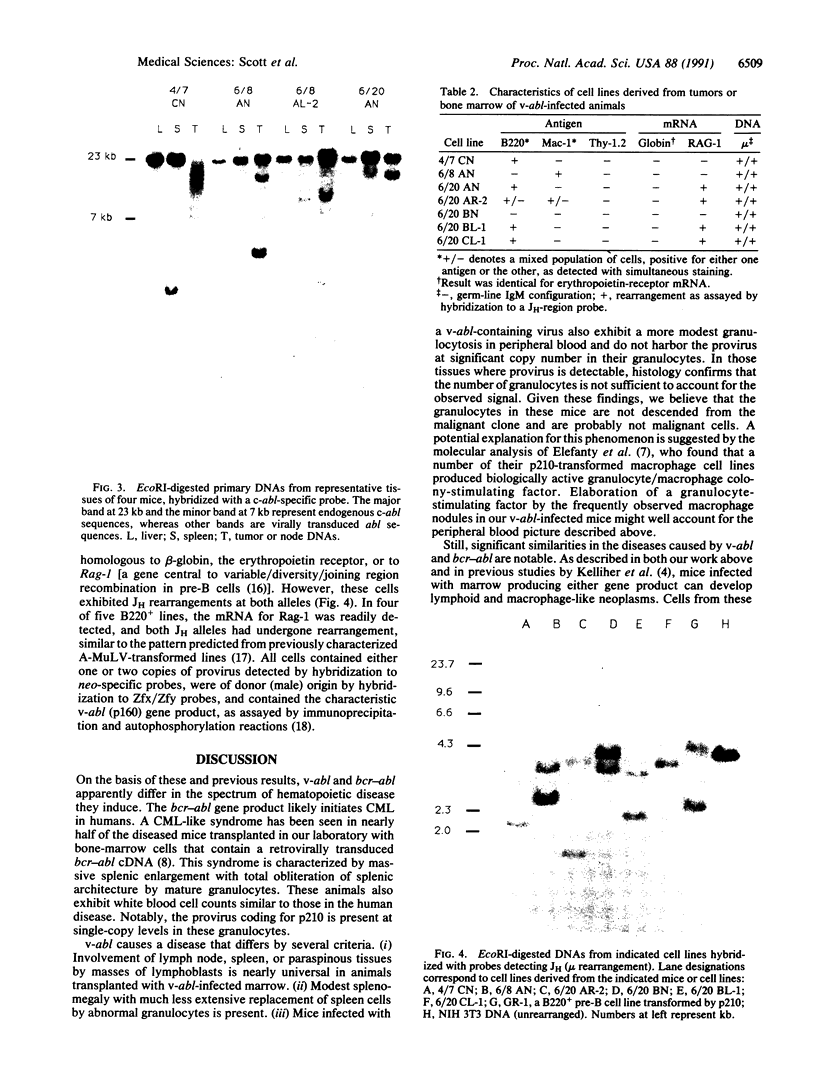

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abelson H. T., Rabstein L. S. Lymphosarcoma: virus-induced thymic-independent disease in mice. Cancer Res. 1970 Aug;30(8):2213–2222. [PubMed] [Google Scholar]
- Barklis E., Mulligan R. C., Jaenisch R. Chromosomal position or virus mutation permits retrovirus expression in embryonal carcinoma cells. Cell. 1986 Nov 7;47(3):391–399. doi: 10.1016/0092-8674(86)90596-9. [DOI] [PubMed] [Google Scholar]
- Champlin R. E., Golde D. W. Chronic myelogenous leukemia: recent advances. Blood. 1985 May;65(5):1039–1047. [PubMed] [Google Scholar]
- Chung S. W., Wong P. M., Durkin H., Wu Y. S., Petersen J. Leukemia initiated by hemopoietic stem cells expressing the v-abl oncogene. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1585–1589. doi: 10.1073/pnas.88.4.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook W. D. Rapid thymomas induced by Abelson murine leukemia virus. Proc Natl Acad Sci U S A. 1982 May;79(9):2917–2921. doi: 10.1073/pnas.79.9.2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Andrea A. D., Lodish H. F., Wong G. G. Expression cloning of the murine erythropoietin receptor. Cell. 1989 Apr 21;57(2):277–285. doi: 10.1016/0092-8674(89)90965-3. [DOI] [PubMed] [Google Scholar]
- Daley G. Q., Van Etten R. A., Baltimore D. Induction of chronic myelogenous leukemia in mice by the P210bcr/abl gene of the Philadelphia chromosome. Science. 1990 Feb 16;247(4944):824–830. doi: 10.1126/science.2406902. [DOI] [PubMed] [Google Scholar]
- Elefanty A. G., Hariharan I. K., Cory S. bcr-abl, the hallmark of chronic myeloid leukaemia in man, induces multiple haemopoietic neoplasms in mice. EMBO J. 1990 Apr;9(4):1069–1078. doi: 10.1002/j.1460-2075.1990.tb08212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fialkow P. J., Jacobson R. J., Papayannopoulou T. Chronic myelocytic leukemia: clonal origin in a stem cell common to the granulocyte, erythrocyte, platelet and monocyte/macrophage. Am J Med. 1977 Jul;63(1):125–130. doi: 10.1016/0002-9343(77)90124-3. [DOI] [PubMed] [Google Scholar]
- Goff S. P., Gilboa E., Witte O. N., Baltimore D. Structure of the Abelson murine leukemia virus genome and the homologous cellular gene: studies with cloned viral DNA. Cell. 1980 Dec;22(3):777–785. doi: 10.1016/0092-8674(80)90554-1. [DOI] [PubMed] [Google Scholar]
- Jackson P., Baltimore D. N-terminal mutations activate the leukemogenic potential of the myristoylated form of c-abl. EMBO J. 1989 Feb;8(2):449–456. doi: 10.1002/j.1460-2075.1989.tb03397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelliher M. A., McLaughlin J., Witte O. N., Rosenberg N. Induction of a chronic myelogenous leukemia-like syndrome in mice with v-abl and BCR/ABL. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6649–6653. doi: 10.1073/pnas.87.17.6649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konkel D. A., Tilghman S. M., Leder P. The sequence of the chromosomal mouse beta-globin major gene: homologies in capping, splicing and poly(A) sites. Cell. 1978 Dec;15(4):1125–1132. doi: 10.1016/0092-8674(78)90040-5. [DOI] [PubMed] [Google Scholar]
- Konopka J. B., Witte O. N. Detection of c-abl tyrosine kinase activity in vitro permits direct comparison of normal and altered abl gene products. Mol Cell Biol. 1985 Nov;5(11):3116–3123. doi: 10.1128/mcb.5.11.3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W. J., Dreazen O., Kloetzer W., Gale R. P., Arlinghaus R. B. Characterization of bcr gene products in hematopoietic cells. Oncogene. 1989 Feb;4(2):127–138. [PubMed] [Google Scholar]
- Mardon G., Luoh S. W., Simpson E. M., Gill G., Brown L. G., Page D. C. Mouse Zfx protein is similar to Zfy-2: each contains an acidic activating domain and 13 zinc fingers. Mol Cell Biol. 1990 Feb;10(2):681–688. doi: 10.1128/mcb.10.2.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer B. J., Jackson P. K., Baltimore D. The noncatalytic src homology region 2 segment of abl tyrosine kinase binds to tyrosine-phosphorylated cellular proteins with high affinity. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):627–631. doi: 10.1073/pnas.88.2.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prywes R., Foulkes J. G., Baltimore D. The minimum transforming region of v-abl is the segment encoding protein-tyrosine kinase. J Virol. 1985 Apr;54(1):114–122. doi: 10.1128/jvi.54.1.114-122.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prywes R., Foulkes J. G., Rosenberg N., Baltimore D. Sequences of the A-MuLV protein needed for fibroblast and lymphoid cell transformation. Cell. 1983 Sep;34(2):569–579. doi: 10.1016/0092-8674(83)90389-6. [DOI] [PubMed] [Google Scholar]
- Prywes R., Hoag J., Rosenberg N., Baltimore D. Protein stabilization explains the gag requirement for transformation of lymphoid cells by Abelson murine leukemia virus. J Virol. 1985 Apr;54(1):123–132. doi: 10.1128/jvi.54.1.123-132.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy E. P., Smith M. J., Srinivasan A. Nucleotide sequence of Abelson murine leukemia virus genome: structural similarity of its transforming gene product to other onc gene products with tyrosine-specific kinase activity. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3623–3627. doi: 10.1073/pnas.80.12.3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg N., Witte O. N. The viral and cellular forms of the Abelson (abl) oncogene. Adv Virus Res. 1988;35:39–81. doi: 10.1016/s0065-3527(08)60708-3. [DOI] [PubMed] [Google Scholar]
- Schatz D. G., Oettinger M. A., Baltimore D. The V(D)J recombination activating gene, RAG-1. Cell. 1989 Dec 22;59(6):1035–1048. doi: 10.1016/0092-8674(89)90760-5. [DOI] [PubMed] [Google Scholar]
- Schlissel M. S., Baltimore D. Activation of immunoglobulin kappa gene rearrangement correlates with induction of germline kappa gene transcription. Cell. 1989 Sep 8;58(5):1001–1007. doi: 10.1016/0092-8674(89)90951-3. [DOI] [PubMed] [Google Scholar]
- Scott M. L., Davis M. M., Feinberg M. B. Transformation of T-lymphoid cells by Abelson murine leukemia virus. J Virol. 1986 Aug;59(2):434–443. doi: 10.1128/jvi.59.2.434-443.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidmarsh G. F., Heimfeld S., Whitlock C. A., Weissman I. L., Müller-Sieburg C. E. Identification of a novel bone marrow-derived B-cell progenitor population that coexpresses B220 and Thy-1 and is highly enriched for Abelson leukemia virus targets. Mol Cell Biol. 1989 Jun;9(6):2665–2671. doi: 10.1128/mcb.9.6.2665. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Van Etten R. A., Jackson P., Baltimore D. The mouse type IV c-abl gene product is a nuclear protein, and activation of transforming ability is associated with cytoplasmic localization. Cell. 1989 Aug 25;58(4):669–678. doi: 10.1016/0092-8674(89)90102-5. [DOI] [PubMed] [Google Scholar]