Abstract
A subset of stem cells, termed the “side population” (SP), has been identified in several tissues in mammalian species. These cells maintain a high efflux capability for antimitotic drugs. We have investigated whether functionally equivalent stem cells also may be detected in human cancers. We initially examined primary tumor cells from 23 patients with neuroblastoma and cell lines derived from a range of other tumors. A distinct SP was found in neuroblastoma cells from 15 of 23 patients (65%). The SP was capable of sustained expansion ex vivo and showed evidence for asymmetric division, generating both SP and non-SP progeny. These cells also expressed high levels of ABCG2 and ABCA3 transporter genes and had a greater capacity to expel cytotoxic drugs, such as mitoxantrone, resulting in better survival. A SP also was detected in breast cancer, lung cancer, and glioblastoma cell lines, suggesting that this phenotype defines a class of cancer stem cells with inherently high resistance to chemotherapeutic agents that should be targeted during the treatment of malignant disease.
Over the past several years, a primitive CD34low/neg stem cell population has been defined in normal bone marrow. This subset has a unique capacity to efflux lipophilic dyes and, because of its characteristic appearance, has been termed the “side population” (SP). These SP cells have been characterized in the bone marrow and muscle of various species, including humans (1–3). SP cells have shown the capacity to function as stem cells in the tissues from which they were isolated and may be able to transdifferentiate (3, 4). Although its molecular basis remains uncertain, the SP phenotype is clearly distinct from that of the multidrug resistance (MDR)-efflux system, because MDR1-knockout mice have normal numbers of SP cells. Recent evidence suggests that the SP phenotype is associated with high-level expression of the ATP-binding cassette transporter protein ABCG2/Bcrp1 (5).
Solid tumors contain cells that are heterogeneous in phenotype and proliferative potential. Because these malignancies are clonal in origin, it has been suggested that cancer cells in general may undergo processes that are analogous to the self-renewal and differentiation of normal stem cells (6). We therefore reasoned that malignancies also might harbor SP cells, whose intrinsic dye efflux capacity would be expected to be associated with the ability to export many cytotoxic drugs and hence to increase the risk of early relapse. We extended our SP studies to the primary tumor cells of children with neuroblastoma and analyzed established cell lines from several different solid tumors as well. The findings suggest that SP cells represent a previously unrecognized population of human solid tumor cells. Their high drug efflux capacity correlates with the strong expression of drug-transporter proteins (including ABCG2 and ABCA3) distinct from MDR1. Because these malignant SP cells proliferate in a sustained fashion and readily export many cytotoxic drugs, they may be resistant to therapy and contribute to disease relapse.
Materials and Methods
Analysis of Human Tumor Cells and Cell Phenotyping. Human tumor cell lines (SK-N-SH, IMR-32, SK-BR-3, SK-OV-3, NCI-H146, PA-1, Tera-1, SK-N-EP, RD-ES, SaOs2, U87MG, A 204, NCI-H345, and HS 683) were obtained from American Type Culture Collection. The neuroblastoma cell lines LAN-1 and LAN-5 were kindly provided by Robert C. Seeger's laboratory at the University of California, Los Angeles; the neuroblastoma cell line JF was established in our laboratory. Tumor cells were grown in RPMI medium 1640 (GIBCO) containing 10% FBS (HyClone) and 2 mM l-glutamine (BioWhittaker) or in tissue culture medium according to American Type Culture Collection recommendations.
Primary neuroblasts were obtained from 23 neuroblastoma patients with relapsed disease, in accordance with the relevant institutional guidelines of Texas Children's Hospital. Informed consent was obtained from each subject's parent or guardian. At diagnosis, the patients were older than 1 year, had poorly differentiated or undifferentiated neuroblastoma, and were classified as stage IV (n = 17), III (n = 5), or I (n = 1) by International Neuroblastoma Staging System criteria (7).
Neuroblasts were purified and characterized as described in ref. 8 and were grown in RPMI medium 1640 (GIBCO) containing 10% FBS (HyClone) and 2 mM l-glutamine (BioWhittaker). Cells were detached from the cell culture flask with cell dissociation solution (Sigma), and viable cells were counted with trypan blue and stained with the fluorescent dye Hoechst 33342 (Sigma) at a concentration of 5 μg/ml (37°C for 2 h) as described in ref. 2. Cell analysis and sorting were performed on a triple-laser cell sorter (MoFlow, Cytomation, Fort Collins, CO). After excitation of the Hoechst dye at 350 nm and measurement of the fluorescence profile in dualwavelength analysis (405/30 nm and 670/40 nm), the SP was defined as described in refs. 1 and 2. Subsequent antibody incubations were performed at 4°C. Allophycocyanin-, FITC-, or phycoerythrin-labeled antibodies used for phenotyping of the cells included CD45, CD71, CD117 (Becton Dickinson), AC133 (Miltenyi Biotec, Auburn, CA), and GD2 (Pharmingen). P-glycoprotein was identified with antibody MRK16 (Kamiya Biomedical, Seattle) and indirect immunofluorescence staining by using a biotinylated first antibody and a streptavidin–phycoerythrin conjugate (Pharmingen). After the antibody-staining procedure, propidium iodide (2 μg/ml; Sigma) was added to the samples to identify dead cells.
Efflux and Survival Studies for Cytotoxic Drug Mitoxantrone. Singlesuspension tumor cells were stained with Hoechst 33342 dye, as described above, for 60 min at 37°C after the addition of mitoxantrone (Sigma) at a final concentration of 100 ng/ml. Cells were centrifuged after 30 min in a prewarmed centrifuge (37°C) and incubated with Hoechst 33342 dye alone for 15 min. The fluorescence emitted from mitoxantrone was detected at 670/40 nm after excitation at 633 nm. To examine whether increased drug efflux results in a survival advantage, neuroblastoma cell lines SK-N-SH and JF were cultured with or without mitoxantrone at a concentration of 1 or 10 ng/ml for 3 days, and the percentage of SP cells was determined subsequently. To analyze the effect of mitoxantrone on the clonogenicity of the tumor cells, the JF line was sorted for SP and non-SP cells directly into 96-well plates in replicates of six at 50 cells per well and cultured with or without 1 ng/ml mitoxantrone. After 7 days, the medium was aspirated and replaced with fresh medium without mitoxantrone. The number of colonies was counted on day 14 of culture.
TaqMan Real-Time RT-PCR Analysis of Gene Expression. RNA was isolated from sorted SP and non-SP cells of neuroblastoma cell lines IMR-32 and JF (RNEasy, Qiagen, Valencia, CA) and analyzed by real-time RT-PCR (TaqMan Master Mix Kit, Applied Biosystems) to detect expression of human MDR1, ABCG2, and ABCA3 transporter proteins. Transporter protein primer and TaqMan probes are listed in Table 1. 18S rRNA was detected by primers and TaqMan probe for rRNA (Applied Biosystems) and was used as the normalizer gene. The RT-PCR reaction consisted of an initial incubation at 48°C for 30 min (RT reaction), followed by incubation at 95°C for 10 min and 40 cycles of 95°C for 15 sec and 55°C for 1 min. Data were collected with the Prism 7700 Sequence Detection System (Applied Biosystems). The level of expression of each transporter protein gene was determined relative to the normalizer RNA gene.
Table 1. Primer and TaqMan probes.
| Protein | Forward primer | Reverse primer | TaqMan probe |
|---|---|---|---|
| MDR1 | TAGTCTCACCTCCAGCGCG | ATCCCGACCTGAAGAGAAACC | VIC-CTGAGGCTCATGCATTTGGCTAATGAGC |
| ABCG2 | CGGGTGACTCATCCCAACAT | CAGGATCTCAGGATGCGTGC | VIC-TACATCCTTAATTGTTAAAGCGCTGCCTCCGA |
| ABC3 | AACCCTGGATCACGTGTTCC | CCTCCGCGTCTCGTAGTTCT | VIC-TGCTGCCCAACCACTGTCTGGG |
In Vitro Cultivation Assay. Neuroblastoma cell lines SK-N-SH, IMR-32, and JF were stained with Hoechst 33342 dye, and SP and non-SP subfractions were separated by a triple-laser sorter. Equal numbers of separated cells were cultured in the appropriate cell medium; fractions of the same cell line were cultured under the same conditions. After 2, 4, and 6 weeks, the cell subpopulations were restained with Hoechst dye and reanalyzed.
Statistical Analysis. Data are expressed as means ± SD. Student's t or Mann–Whitney test were used where appropriate. P ≤ 0.05 was accepted as significant.
Results
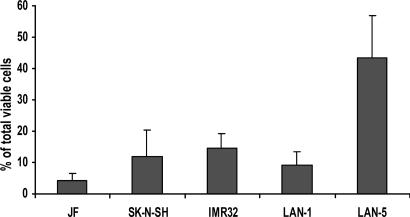
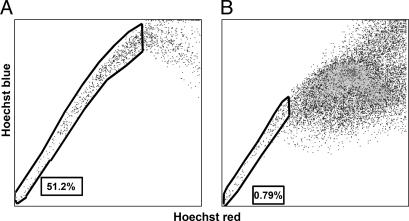
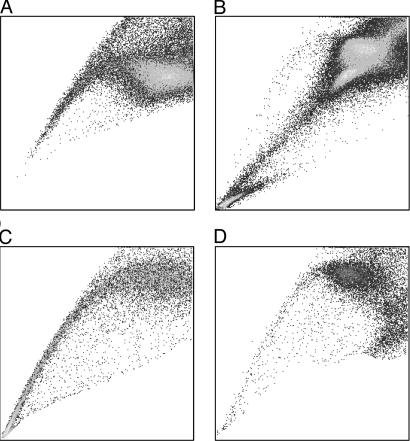
Prevalence of SP Cells in Neuroblastoma. SP cells account for ≈0.03% (range 0.01–0.09%) of all mononuclear cells in normal human bone marrow (1). To investigate the prevalence of SP cells in human neuroblastoma, we stained five neuroblastoma cell lines with Hoechst 33342 dye and identified the SP by its characteristic fluorescent profile in dual-wavelength analysis, as described above. All neuroblastoma cell lines contained SP cells ranging from 4% to 37% (median 10%) of the total viable cells (Fig. 1). To demonstrate that the SP profile was not an artifact associated with the selection of cells capable of prolonged growth in culture, we obtained 23 primary neuroblastoma tumor cell samples, each from a patient with relapsed disease, and separated them from contaminating hematopoietic cells as described in ref. 8. After staining with fluorescent dye Hoechst 33342, a SP was detected in 15 of the 23 samples (65%), and the proportions of SP cells in these positive samples were substantially higher than in human marrow, with a median of 1.9% and a range of 0.8–51% (Fig. 2).
Fig. 1.
Prevalence of Hoechstlow SP cells in neuroblastoma cell lines. Data shown are means ± SD of three to five independent experiments on each line.
Fig. 2.
Characteristic Hoechst 33342 dye staining profiles of two primary neuroblastoma specimens from patients with relapsed disease. (A) Female, 1.4 years old at diagnosis, MYCN-amplified, International Neuroblastoma Staging System stage IV. (B) Male, 7 years old at diagnosis, MYCN-amplified, stage IV. These two tumors illustrate the highest (A) and the lowest (B) proportions of SP cells observed. The SP pattern observed in the remaining patients with intermediate value was identical.
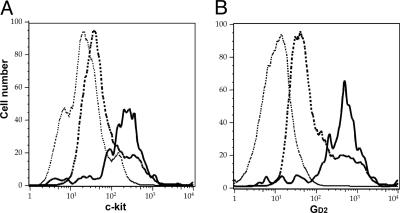
Cell-Surface Phenotype. We next analyzed the surface phenotype of SP cells from fresh neuroblastoma samples. Unlike the majority of SP cells from normal human marrow (1), all tumor SP cells were negative for CD45 (Table 2), a definitive marker of hematopoietic lineages. Compared with non-SP cells, the SP cells showed an increased number and intensity of cells expressing GD2 (ganglioside) and c-kit/CD117 (stem cell factor receptor) (Fig. 3) and low expression of AC 133, CD71, and CD56 (Table 2), compatible with an early position in the phenotypic hierarchy of neural crest progenitor cells as they mature to neuroblasts and subsequently to their differentiated progeny (9–12).
Table 2. Cell-surface markers on human neuroblastoma SP and non-SP cells.
| Cell-surface marker | SP cells | Non-SP cells |
|---|---|---|
| GD2 | ++++ | ++ |
| c-kit | ++++ | ++ |
| CD45 | - | - |
| CD71 | - | +/- |
| CD56 | +/- | +/- |
| AC133 | - | - |
Positivity was assessed by fluorescence-activated cell sorting; the cut-off was measured against an isotype control antibody. Ranking percent positive cells:-, <5%; +/-, 6-10%; +, 10-20%; ++, 21-40%; +++, 41-70%; ++++, >70%.
Fig. 3.
Increased expression of c-kit stem cell factor receptor (A) and GD2 (B) on the SP cells of patients with relapsed neuroblastoma. P ≤ 0.05 for GD2 and c-kit stem cell factor receptor. Solid line, SP; dashed line, non-SP; dotted line, control.
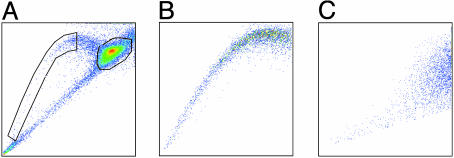
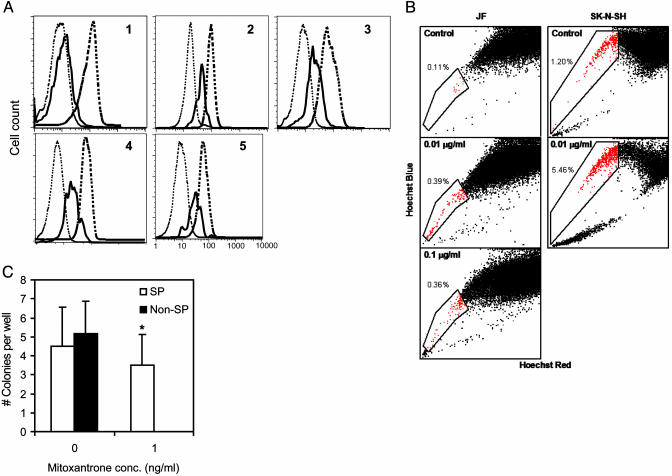
Ex Vivo Growth Characteristics of SP and Non-SP Cells. The growth characteristics of the SP subpopulation also were consistent with the predicted behavior of primitive precursor cells, including a high proliferative rate and self-renewal capacity (13, 14). Hoechstlow SP and Hoechsthigh non-SP cells (SK-N-SH, IMR-32, and JF lines) were separated by flow cytometry, and equal numbers of cells from both subpopulations were cultured in vitro. The cells were restained with Hoechst dye over the 6-week culture period, and the SP and non-SP cell fractions were reanalyzed (illustrated for SK-N-SH in Fig. 4). The SP from all three cell lines rapidly underwent discernible asymmetric division, which generated a SP as well as a non-SP subpopulation (Fig. 4B). The ratio of SP to non-SP cells reached a steady state within 2 weeks and was maintained for at least the entire 6 weeks of culture. Overall, the sorted SP cell fraction expanded 2,200-fold in the JF line (doubling time 84 h), 500-fold in the SK-N-SH line (doubling time 108 h), and 260-fold in IMR-32 line (doubling time 125 h) (see Fig. 9, which is published as supporting information on the PNAS web site).
Fig. 4.
SP cells from neuroblastoma cell lines become SP and non-SP cells, whereas non-SP cells remain non-SP (illustrated for SK-N-SH). Hoechst 33342 dye-stained neuroblastoma cells (A) were sorted for the SP and non-SP fraction, and each subpopulation was cultivated. After 6 weeks, the SP (B) and non-SP (C) were stained with Hoechst dye and reanalyzed.
By contrast, the sorted Hoechsthigh non-SP cells remained exclusively non-SP (Fig. 4C) and multiplied at a lower rate than SP cells, expanding 12.3-fold in JF (doubling time 12 days) and 2.9-fold in SK-N-SH (doubling time 28 days). Non-SP cells from the IMR-32 population failed to double over the 6-week period. These differences are not likely to be a consequence of longer retention of potentially toxic Hoechst dye by non-SP compared with SP cells, because the viability of all cells was identical after sorting (>90%) and remained so throughout the study period.
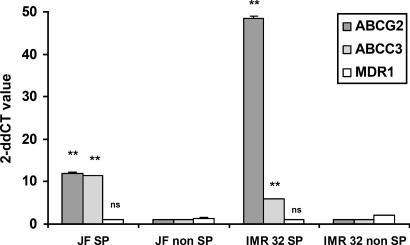
Gene Expression Analysis. The SP phenotype in marrow, liver, and other tissues has been associated with the expression of drug transporter genes and the capacity to export many cytotoxic drugs (5). If neuroblastoma SP cells share this capacity, it would have important implications for resistance to therapy. We therefore isolated RNA from sorted SP and non-SP cells of the neuroblastoma cell lines IMR-32 and JF and used a real-time RT-PCR assay to quantify the relative expression of the ABCG2 transporter, the gene product currently believed to be most closely associated with the SP phenotype (5). All SP fractions expressed higher levels of the ABCG2 transporter gene than did non-SP fractions (Fig. 5). We also measured the expression levels of two other ABC transporter genes, ABCA3 and MDR1. The first gene was clearly expressed at higher concentrations in SP compared with non-SP cells, whereas the second gene was not.
Fig. 5.
Relative expression of ATP-binding cassette transporter genes in neuroblastoma SP and non-SP cells by quantitative real-time RT-PCR amplification analysis. For quantification of gene expression, amplification of 18S ribosomal RNA has been performed as an endogenous control to standardize the amount of sample. The amount of target, normalized to the endogenous reference and relative to a calibrator, is given by 2–ΔΔCT. [The threshold cycle (CT) indicates the fractional cycle number at which the amount of amplified target reaches a fixed threshold.] Shown are the representative results of three different experiments. SD < 5%. For ABCG2 and ABCA3, P = 0.01, whereas the P value for MDR1 was not significant.
Efflux of Mitoxantrone from Neuroblastoma SP Cells. The presence of high levels of ABC transporter proteins indicates that the SP should have an enhanced efflux capacity not only for Hoechst 33342 dyes but also for lipophilic antineoplastic drugs, including those used for the treatment of neuroblastoma. To investigate the cotransport of these agents with Hoechst dye, we simultaneously incubated cells from five neuroblastoma patients and the IMR-32 and SK-N-SH neuroblastoma cell lines with Hoechst dye and mitoxantrone. The drug efflux from the SP and non-SP cells was measured by specific emission fluorescence at 670/40 nm (mitoxantrone). In each of the patients' cells and in both cell lines, increased efflux of mitoxantrone with Hoechst dye could be demonstrated in the SP cell fraction compared with non-SP cells (Fig. 6A). We used two techniques to determine whether drug efflux provides SP cells with a survival advantage. In the first, we looked at bulk cultures of JF and SK-N-SH cells and added 0, 1, or 10 ng/ml mitoxantrone. By day 3, there was an increase in the proportion of SP cells present (Fig. 6B), indicating selection of this population. We next looked at clonal growth, sorting SP and non-SP cells from the JF line. SP and non-SP cells both formed colonies by day 14 in the absence of mitoxantrone, forming 4.5 ± 2.1 and 5.2 ± 1.7 colonies per well, respectively. However, in the presence of 1 ng/ml mitoxantrone, non-SP cells formed no colonies at all, whereas the number of colonies formed by the SP cells was unchanged at 3.5 ± 1.6 colonies per well (Fig. 6C). These data indicate that in the presence of mitoxantrone, the drug efflux capacity of SP cells affords them a survival advantage.
Fig. 6.
Efflux and survival of mitoxantrone from neuroblastoma SP cells. (A) Efflux of mitoxantrone in primary neuroblastoma samples from five patients (nos. 1–5) with relapsed disease after incubation with Hoechst 33342 dye and mitoxantrone. Fluorescence was plotted for three gated cell populations: SP (solid line), non-SP (dashed line), and control (dotted line) (P < 0.05). (B) JF and SK-N-SH lines were cultured in the presence of 0, 1, or 10 ng/ml mitoxantrone for 3 days, then analyzed for the SP cells. (C) Fifty SP or non-SP cells were sorted in six replicates into 96-well plates and cultured with 1 ng/ml mitoxantrone. After 14 days of culture, the number of colonies formed in each well was counted (*, P < 0.05).
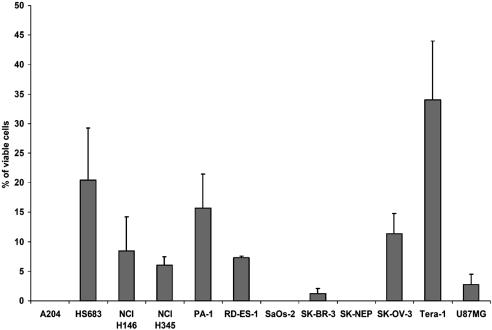
SP Cells in Other Tumor Cell Lines. Finally, we determined whether a SP could be detected in tumors other than neuroblastoma. As shown in Fig. 7, a Hoechstlow SP was identified in small-cell lung cancer, breast adenocarcinoma, Ewing sarcoma, and teratocarcinoma and in a range of other tumors as well (Fig. 8 and Table 3). No such population was found in cell lines derived from Wilms tumor, rhabdomyosarcoma, or osteosarcoma (Fig. 8 and Table 3).
Fig. 7.
Characteristic Hoechst 33342 dye SPs discerned in four tumor cell lines. Plots for Ewing sarcoma RD-ES (A), teratocarcinoma PA-1 (B), breast adenocarcinoma SK-BR-3 (C), and small-cell lung cancer NCI-H146 (D) are shown.
Fig. 8.
Overall prevalence of Hoechstlow SP cells in 12 tumor cell lines. Data shown are means ± SD of three to five independent experiments. See Table 3 for the key to the origin of the cell lines.
Table 3.
Key to origin of cell lines in Fig. 8
| Cell line | Tumor | Morphology/stage | Patient age, years |
|---|---|---|---|
| SK-BR-3 | Adenocarcinoma mammary gland, malignant pleural effusion | Epithelial | 43 |
| SK-OV-3 | Adenocarcinoma, ovary, ascites | Epithelial | 64 |
| HCl-H345 | Small-cell carcinoma of the lung, metastatic side: bone marrow | Floating gland-like aggregates | 64 |
| PA-1 | Teratocarcinoma, ovary, ascitic fluid | Epithelial | 12 |
| Tera-1 | Embryonal carcinoma, metastatic site: lung | Epithelial | 47 |
| SK-NEP-1 | Wilms tumor, kidney, pleural effusion | Epithelial-like | 25 |
| RD-ES | Ewing sarcoma | Epithelial | 19 |
| SaO2 | Osteogenic sarcoma | Epithelial | 11 |
| U87MG | Glioblastoma, astrocytoma | Epithelial | 44 |
| HS 683 | Brain glioma | Fibroblast | 76 |
| A 204 | Rhabdomyosarcoma | Epithelial | 1 |
| NCl-H146 | Small-cell carcinoma of the lung, pleural effusion cells | Floating aggregates | 59 |
Discussion
Stem cells have an extensive capacity to proliferate, differentiate, and self-renew, enabling them to repopulate recipients after transplantation (15–17). Stem cell subpopulations have been defined in many mammals, including humans, by using the fluorescent dyes rhodamine 123 and Hoechst 33342 (10, 18–20). An adult stem cell subpopulation has been identified that can rapidly efflux the Hoechst dye to produce a characteristic SP profile based on fluorescence-activated flow cytometric analysis. SP cells obtained from normal marrow are characterized by a CD34low/neg phenotype and a capacity to repopulate lethally irradiated mice (1, 2). A similar SP has been described in monkeys and will generate CD34+ cells ex vivo (2). Wulf et al. (21) detected a malignant CD45+CD34low/neg SP cell subset in >80% of the acute myeloid leukemia patients they studied. These cells generated CD45+CD34+ malignant hematopoietic stem cells as well as committed myeloid progenitors. Because the cells also expelled lipophilic antileukemic drugs, the authors concluded that they might be candidate leukemic stem cells capable of contributing to relapse (21).
Although the presence of SP cells in a leukemic population is perhaps predictable from the known hierarchy of normal and malignant hematopoietic stem cells, the presence of such a population in a malignant solid tumor is less expected. Their presence may allow identification of cancer stem cells and indicate a mechanism of oncogenesis and of drug resistance. Our interest in neuroblastoma (8, 22–24) initially led us to focus on this disease. All five neuroblastoma cell lines analyzed and 15 of 23 primary neuroblastomas contained CD45–CD71– GD2+ SP cells. Although we cannot precisely assign a position to SP cells in the developmental hierarchy of neuroblastoma cells, the phenotypic characteristics of our SP subset, such as high expression of GD2 and stem cell factor receptor, together with their greater replicative potential in culture, are at least consistent with proximity to an early cell in the hierarchy of neural crest progenitor development. Moreover, there was evidence for asymmetric division: Hoechstlow cells generated both additional Hoechstlow and Hoechsthigh cells, whereas Hoechsthigh cells produced only other Hoechsthigh progeny. However, we cannot formally exclude the possibility that the apparent difference in proliferative and differentiating capacity between SP and non-SP cells is a consequence of damage induced by the Hoechst dye, which is retained longer in the non-SP (Hoechsthigh) subpopulation (18). Arguing against this interpretation is our observation that the viability of the two populations remained identical.
Independent of whether SP neuroblasts are truly a tumor “stem cell” population, their high expression of drug efflux transporter genes and their associated high capacity to efflux lipophilic drugs may have a significant influence on treatment outcome. Increased expression of the MDR-associated transporter p-glycoprotein, the product of the MDR1 gene (25, 26), has been suggested as a potential cause of drug resistance in refractory and relapsed neuroblastoma (27). MDR1 also has been described as a differentiation marker in neuroblastoma (28) and may be an important prognostic indicator (26), although this link is not conclusive (29). We found that two other transporter proteins, ABCA3 and ABCG2, are both expressed at higher levels in neuroblastoma SP cells than in non-SP cells. ABCA3, a member of the “C” subfamily of the ABC transporter family, is an intracellular membrane-associated protein that seems to be involved in vesicular transport and surfactant secretion of alveolar cells type II (30), whereas ABCG2 has been identified in breast cancer (31–33), lung cancer (34), SP cells derived from adult human pancreatic islet of Langerhans (35), and normal hematopoietic SP cells (4, 36, 37). The latter protein can confer cellular resistance to antineoplastic drugs, such as mitoxantrone, and has been shown to be expressed in some acute myeloid leukemic blasts (14). How important is this expression of a multidrug-resistance phenotype to the pathophysiology of neuroblastoma and its response to treatment? Because our clinical samples of neuroblastoma were obtained from patients in relapse, the SP cells may have been selected in vivo by drug treatment, reiterating the selection of stem cells transduced to express even a single drug-resistance gene (38, 39). Certainly, we can demonstrate such selection when we culture SP and non-SP cells in the presence of cytotoxic drugs in vitro. Hence, the proportion of SP cells in newly diagnosed neuroblastoma may be much lower than in relapsed disease. Nonetheless, their presence at any stage of the disease may affect the ultimate outcome of therapy. These drug-resistant cells are evidently capable of rapid, long-term proliferation, making them potentially important contributors to early relapse.
At present, MYCN and the MDR-associated protein (MRP) gene are perhaps the best molecular predictors of a poor outcome in neuroblastoma (7, 26, 40, 41). In our small series, MYCN expression seemed to be unrelated to the SP subset within samples, because essentially the same proportion of patients indicated an amplified MYCN gene whether the analysis was done in the group as a whole (14 of 19, 74%) or in patients with a SP of tumor cells (10 of 15, 71%). If evaluation of a larger series of tumors confirms that SP and MYCN expression are independent variables, it will be important to test prospectively whether the presence of a SP tumor cell population is an independent prognostic marker.
Finally, it may be possible to extend these observations in neuroblastoma to certain other solid tumors of children and adults. Several tumors failed to demonstrate SP cells, either because of inappropriate culture conditions or because they lack a stem cell population that can be so defined. Nevertheless, our discovery of a stable SP in cell lines derived from Ewing sarcoma, teratocarcinoma, small-cell lung cancer, breast adenocarcinoma, and several other tumors including glioblastoma (Fig. 7) suggests that the SP profile may be a more general feature of malignant disease than previously suspected.
Supplementary Material
Acknowledgments
We thank Michael L. Cubbage, Brian S. Newsom, and Christopher L. Threeton for assistance with flow cytometry and Zhuyong Mei for assistance with neuroblastoma cell culture. This work was supported by National Institutes of Health Grant RO1 CA78792 (to M.K.B.) and Deutsche Krebshilfe Grants D/98/27078 and 10-1823 (to C.H.-J.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: MDR, multidrug resistance; SP, side population (Hoechst 33342-effluxing cells).
References
- 1.Goodell, M. A., Brose, K., Paradis, G., Conner, A. S. & Mulligan, R. C. (1996) J. Exp. Med. 183, 1797–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goodell, M. A., Rosenzweig, M., Kim, H., Marks, D. F., DeMaria, M., Paradis, G., Grupp, S. A., Sieff, C. A., Mulligan, R. C. & Johnson, R. P. (1997) Nat. Med. 3, 1337–1345. [DOI] [PubMed] [Google Scholar]
- 3.Gussoni, E., Soneoka, Y., Strickland, C. D., Buzney, E. A., Khan, M. K., Flint, A. F., Kunkel, L. M. & Mulligan, R. C. (1999) Nature 401, 390–394. [DOI] [PubMed] [Google Scholar]
- 4.Jackson, K. A., Mi, T. & Goodell, M. A. (1999) Proc. Natl. Acad. Sci. USA 96, 14482–14486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou, S., Schuetz, J. D., Bunting, K. D., Colapietro, A.-M., Sampath, J., Morris, J. J., Lagutina, I., Grosveld, G. C., Osawa, M., Nakauchi, H. & Sorrentino, B. P. (2001) Nat. Med. 7, 1028–1034. [DOI] [PubMed] [Google Scholar]
- 6.Reya, T., Morrison, S. J., Clarke, M. F. & Weissman, I. (2001) Nature 414, 105–111. [DOI] [PubMed] [Google Scholar]
- 7.Brodeur, G. M., Seeger, R. C., Barrett, A., Berthold, F., Castleberry, R. P., D'Angio, G., De Bernardi, B., Evans, A. E., Favrot, M., Freeman, A. I., et al. (1988) J. Clin. Oncol. 6, 1874–1881. [DOI] [PubMed] [Google Scholar]
- 8.Bowman, L., Grossmann, M., Rill, D., Brown, M., Zhong, W.-Y., Alexander, B., Leimig, T., Coustain-Smith, E., Campana, D., Jenkins, J., et al. (1998) Blood 92, 1941–1949. [PubMed] [Google Scholar]
- 9.Svennerholm, L., Bostrom, K., Fredman, P., Mansson, J. E., Rosengren, B. & Rynmark, B. M. (1989) Biochim. Biophys. Acta 1005, 109–117. [DOI] [PubMed] [Google Scholar]
- 10.Wolf, N. S., Kone, A., Priestley, G. V. & Bartelmez, S. H. (1993) Exp. Hematol. 21, 614–622. [PubMed] [Google Scholar]
- 11.Asman, L. K., Cambereri, A. C., To, L. B., Levinsky, R. J. & Juttner, C. A. (1991) Blood 78, 30–37. [PubMed] [Google Scholar]
- 12.Papayannopoulou, T., Brice, M., Broudy, V. C. & Zsebo, K. M. (1991) Blood 78, 1403–1412. [PubMed] [Google Scholar]
- 13.Lin, H. & Schagat, T. (1997) Trends Genet. 13, 33–39. [DOI] [PubMed] [Google Scholar]
- 14.Rhyu, M. & Knoblich, J. A. (1995) Cell 52, 523–526. [DOI] [PubMed] [Google Scholar]
- 15.Morrison, S. J., Uchida, N. & Weissman, I. L. (1995) Annu. Rev. Cell Dev. Biol. 11, 35–71. [DOI] [PubMed] [Google Scholar]
- 16.Ogawa, M. (1993) Blood 81, 2844–2853. [PubMed] [Google Scholar]
- 17.Orlic, D., Anderson, S. & Bodine, D. M. (1994) Blood Cells 20, 107–117. [PubMed] [Google Scholar]
- 18.Leemhuis, T., Yoder, M. C., Grigsby, S., Aguero, B., Eder, P. & Srow, E. F. (1996) Exp. Hematol. 24, 1215–1224. [PubMed] [Google Scholar]
- 19.Bradford, G. B., Williams, B., Rossi, R. & Bertoncello, I. (1997) Exp. Hematol. 25, 445–453. [PubMed] [Google Scholar]
- 20.Bertolini, F., Battaglia, M., Lanza, A., Gibelli, N., Palermo, B., Pavesi, L., Caprotti, M. & Robustelli della Cuna, G. (1997) Blood 90, 3027–3036. [PubMed] [Google Scholar]
- 21.Wulf, G. G., Wang, R.-Y., Kuehnle, I., Weidner, D., Marini, F., Brenner, M. K., Andreeff, M. & Goodell, M. A. (2001) Blood 98, 1166–1173. [DOI] [PubMed] [Google Scholar]
- 22.Bowman, L. C., Grossman, M., Rill, D., Brown, M., Zhong, W. Y., Alexander, B., Leimig, T., Coustan-Smith, E., Campana, D., Jenkins, J., et al. (1998) Hum. Gene Ther. 9, 1303–1311. [DOI] [PubMed] [Google Scholar]
- 23.Brenner, M. K., Heslop, H., Krance, R., Horowitz, M., Strother, D., Nuchtern, J., Grilley, B., Martingango, E. & Cooper, K. (2000) Hum. Gene Ther. 11, 1477–1488. [DOI] [PubMed] [Google Scholar]
- 24.Rossig, C., Bollard, C. M., Nuchtern, J. G., Merchant, D. A. & Brenner, M. K. (2001) Int. J. Cancer 94, 228–236. [DOI] [PubMed] [Google Scholar]
- 25.Cowie, F. J., Pritchard-Jones, K., Renshaw, J. & Pinkerton, C. R. (1998) Int. J. Oncol. 12, 1143–1149. [DOI] [PubMed] [Google Scholar]
- 26.Haber, M., Bordow, S. B., Haber, P. S., Marshall, G. M., Stewart, B. W. & Norris, M. D. (1997) Eur. J. Cancer 33, 2031–2036. [DOI] [PubMed] [Google Scholar]
- 27.Goldstein, L. J., Fojo, A. T., Ueda, K., Crist, W., Green, A., Broderu, G., Pastan, I. & Gottesman, M. M. (1990) J. Clin. Oncol. 8, 128–136. [DOI] [PubMed] [Google Scholar]
- 28.Kim, C. J., Lee, Y. A., Ahn, H. S., Yun, C. K., Kim, C. W. & Chi, J. G. (1995) Pathol. Res. Pract. 191, 1010–1015. [DOI] [PubMed] [Google Scholar]
- 29.Dhooge, C. R., De Moerloose, B. M., Benoit, Y. C. M., Van Roy, N., Philippe, J. & Laureys, G. G. (1997) Cancer 80, 1250–1257. [DOI] [PubMed] [Google Scholar]
- 30.Mulugeta, S., Gray, J. M., Notarfrancesco, K. L., Gonzales, L. W., Koval, M., Feinstein, S. I., Ballard, P. L., Fisher, A. B. & Shuman, H. (2002) J. Biol. Chem. 277, 22147–22155. [DOI] [PubMed] [Google Scholar]
- 31.Doyle, L. A., Yang, W., Abruzzo, L. V., Krogmann, T., Gao, Y., Rishi, A. K. & Ross, D. D. (1998) Proc. Natl. Acad. Sci. USA 95, 15665–15670, and erratum (1999) 96, 2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Allikmets, R., Schriml, L. M., Hutchinson, A., Romano-Spica, V. & Dean, M. (1998) Cancer Res. 58, 5337–5339. [PubMed] [Google Scholar]
- 33.Miyake, K., Mickley, L., Litman, T., Zhan, Z., Robey, R., Cristensen, B., Brangi, M., Greenberger, L., Dean, M., Fojo, T. & Bates, S. E. (1999) Cancer Res. 59, 8–13. [PubMed] [Google Scholar]
- 34.Kawabata S., Oka, M., Soda, H., Shiozawa, K., Nakatomi, K., Tsurutani, J., Nakamura, Y., Doi, S., Kitazaki, T., Suguhara, K., et al. (2003) Clin. Cancer Res. 9, 3052–3057. [PubMed] [Google Scholar]
- 35.Lechner, A., Leech, C. A., Abraham, E. J., Nolan, A. L. & Habener, J. F. (2002) Biochem. Biophys. Res. Commun. 293, 670–674. [DOI] [PubMed] [Google Scholar]
- 36.Kim, T., Turnquist, H., Jackson, J., Sgagias, M., Yan, Y., Gong, M., Dean, M., Sharp, J. G. & Cowan, K. (2002) Clin. Cancer Res. 8, 22–28. [PubMed] [Google Scholar]
- 37.Scharenberg, C. W., Harkey, M. A. & Torok-Storb, B. (2002) Blood 99, 507–512. [DOI] [PubMed] [Google Scholar]
- 38.Allay, J. A., Persons, D. A., Galipeau, J., Riberdy, J. M., Ashmun, R. A., Blakley, R. L. & Sorrentino, B. P. (1998) Nat. Med. 4, 1136–1143. [DOI] [PubMed] [Google Scholar]
- 39.Sawai, N., Zhou, S., Vanin, E. F., Houghton, P., Brent, T. P. & Sorrentino, B. P. (2001) Mol. Ther. 3, 78–87. [DOI] [PubMed] [Google Scholar]
- 40.Norris, M. D., Bordow, S. B., Marshall, G. M., Haber, P. S., Cohn, S. L. & Haber, M. (1996) N. Engl. J. Med. 334, 231–238. [DOI] [PubMed] [Google Scholar]
- 41.Norris, M. D., Bordow, S. B., Haber, P. S., Marshall, G. M., Kavallaris, M., Madafiglio, J., Cohn, S. L., Salwen, H., Schmidt, M. L., Hipfner, D. R., et al. (1997) Eur. J. Cancer 33, 1911–1916. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.