Abstract
Synaptic plasticity in the mesolimbic dopamine (DA) system is thought to contribute to the neural adaptations that mediate behavioral sensitization, a model for core aspects of addiction. Recently, it has been demonstrated that multiple classes of drugs of abuse, as well as acute stress, enhance strength at excitatory synapses on midbrain DA neurons. Here, we show that both the cocaine- and stress-induced synaptic enhancement involves an up-regulation of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors. This enhancement requires the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor subunit GluRA as evidenced by its absence in mice lacking this subunit. The cocaine-elicited, but not the stress-elicited, synaptic potentiation in DA neurons was blocked by a D1-like receptor antagonist, indicating that the in vivo triggering mechanisms differ for these forms of experience-dependent synaptic modification. Surprisingly, behavioral sensitization to cocaine was elicited in GluRA(–/–) mice, indicating that potentiation of excitatory synaptic transmission in DA neurons is not necessary for this form of behavioral plasticity. However, GluRA(–/–) mice did not exhibit a conditioned locomotor response when placed in a context previously paired with cocaine, nor did they exhibit conditioned place preference in response to cocaine. We suggest that the drug-induced enhancement of excitatory synaptic transmission in midbrain DA neurons, although not required for behavioral sensitization per se, may contribute to the attribution of incentive value to drug-associated cues.
It is generally accepted that drugs of abuse can produce long-lasting adaptations in critical neural circuits and that these actions contribute to the development and maintenance of drug addiction. One important site implicated in drug experience-dependent plasticity is the mesolimbic dopamine (DA) system, consisting of the ventral tegmental area (VTA) and nucleus accumbens (NAc), which receives projections from DA cells in the VTA (1–3). Drug-induced adaptations in the VTA are thought to play a particularly important role in initiating the cascade of events that lead to behavioral sensitization, an animal model for certain core components of addiction (4–6). Recently, we found that a single in vivo injection of a number of different drugs of abuse cause an increase in the strength of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR)-mediated synaptic transmission at excitatory synapses on midbrain DA neurons (7, 8). Because stress can influence drug-seeking behavior and relapse, and because drugs and stress can cross-sensitize, we also examined the effects of an acute stress and found that this experience also caused a potentiation of excitatory synaptic transmission in the VTA (8).
That distinct classes of drugs of abuse, as well as stress, all caused a similar synaptic adaptation in DA neurons suggests that such plasticity may importantly contribute to the neural circuit adaptations that contribute to addiction. Indeed, several investigators have suggested that potentiation of excitatory synaptic transmission in the VTA is critically important for the development of behavioral sensitization (5, 9–11). It also has recently been shown that excitatory synaptic transmission within the VTA is critical for the development of conditioned place preference (CPP) to cocaine (12). It has been difficult, however, to directly test whether, in fact, the drug-induced enhancement of synaptic transmission in DA neurons is required for sensitization per se and/or other aspects of the drug experience, such as its association with environmental stimuli. Furthermore, much remains unknown about the detailed molecular mechanisms by which drugs of abuse and stress cause synaptic potentiation within the VTA. Here, we first address several key questions about the mechanisms by which cocaine and acute stress elicit this synaptic change. Then, as a test of the functional importance of this synaptic modification, we examine several cocaine-induced behaviors in a mutant mouse line in which the potentiation of synaptic strength in DA neurons normally elicited by cocaine or stress does not occur.
Materials and Methods
Animals and in Vivo Manipulations. C57BL/6 (21–30 days old) or GluRA(–/–) mice (13) (on a C57BL/6 background) were used for all experiments. GluRA(–/–) breeding pairs were provided by P. Seeburg (Max Planck Institute, Heidelberg) and R. Kalb (Children's Hospital, Philadelphia). Mutant animals were produced by heterozygous matings, and littermate, GluRA(+/+) animals were used as controls. Genotype was determined by PCR analysis of tail DNA. Animals were injected i.p. with saline, cocaine (15 mg/kg), SCH23390 (0.5 mg/kg), or eticlopride (0.5 mg/kg). Drugs were obtained from Sigma. Acute stress was elicited with a modified Porsolt forced swim task (8). When experiments required two in vivo manipulations, they were performed 30–45 min apart in the order shown in the figures.
Electrophysiology. Animals were anesthetized with halothane and killed 24–30 h after in vivo manipulations. All remaining procedures were as described in ref. 8. Presumptive DA cells were identified by the presence of large currents evoked by holding cells Ih at –70 mV and stepping to –120 mV in 10 mV increments. Although Ih is present in >80% of DA neurons (14, 15), its presence does not unequivocally identify DA cells; it also may be present in tertiary VTA neurons (16). However, in previous work (7, 8) and the present experiments, this criterion was sufficient to obtain clear differences between control cells and cells from animals that received in vivo manipulations.
A bipolar stimulating electrode was placed 100–300 μm rostral to the recording electrode to stimulate excitatory afferents at 0.1 Hz. Cells were held at –60 mV for 5–15 min to ensure stability and then were depolarized to +40 mV and monitored for 5–15 min, at which point d-2-amino-5-phosphonovaleric acid (D-APV) (50 μM) was applied for 10–20 min. Cells were then returned to –60 mV in D-APV. The ratio of AMPAR- to N-methyl-d-aspartic acid receptor (NMDAR)-mediated excitatory postsynaptic currents (EPSCs) (the AMPA/NMDA ratio) was calculated as described in ref. 8 except for the data in Fig. 2C. Input and series resistances were monitored online throughout each experiment. For application of AMPA, the perfusion rate was increased to ≈15 ml/min, and neurons were held at –60 mV. After a 1-min baseline, the solution was switched to one containing AMPA (1 μM) and cyclothiazide (100 μM) for 30 sec and then switched back to normal artificial cerebrospinal fluid.
Fig. 2.
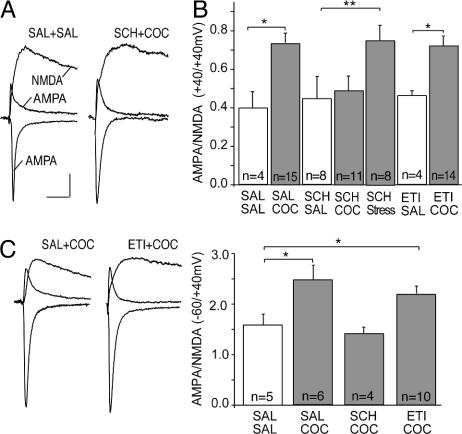
Cocaine, but not stress, enhances synaptic strength via activation of D1-like DA receptors. (A) Sample AMPAR EPSCs and NMDAR EPSCs in DA neurons from mice pretreated as indicated (calibration bars: 30 pA per 20 ms). (B) Summary of AMPA/NMDA ratios (measured at +40 mV) in neurons from mice receiving indicated pretreatments. (C) Sample traces and summary of AMPA/NMDA ratios from mice receiving indicated pretreatments when AMPAR EPSCs were measured at –60 mV and NMDAR EPSCs were measured at +40 mV. SAL, saline; SCH, SCH23390; COC, cocaine; ETI, eticlopride.
Over 75% of the data were collected and analyzed without knowledge of the treatment that the animals had received. There were no differences in the results from blinded and nonblinded experiments, and therefore the results were combined. Control, saline-injected animals were interleaved throughout the course of all experiments, and controls presented in each graph represent the cells obtained during the time period when the manipulations shown in that graph were performed. Two slices were obtained from each animal, and a single cell was examined from each slice. All values are expressed as mean ± SEM. Statistical significance was assessed by using two-tailed Student's t tests.
Behavior. All experiments were performed blind to genotype. Male and female mice were housed separately with free access to food and water on a 12:12-h light/dark cycle. For behavioral sensitization tests, mice were transferred every other day to a testing room for drug injection. Tests consisted of placing an animal into a clear rectangular tub (8.5 inches × 17.5 inches × 9 inches) with a clear plastic insert (2.5 inches × 9 inches × 9 inches) (1 inch = 2.54 cm). Locomotor activity was quantified as “crossovers,” consecutive breaks in infrared photobeams placed across either end of the insert. After a 30-min habituation period, each mouse received an injection of cocaine (15 mg/kg) or saline, and activity was monitored for 60 min. After five every-other-day injections, mice remained in their home cages for 14 days before being returned to the testing room, where each mouse received a saline injection before placement in the monitor cage. Animals then received 5 mg/kg cocaine at 15 min and 10 mg/kg cocaine at 75 min.
For tests of context-dependent locomotion after single cocaine injections, mice were given saline injections on the morning of the training day. Three hours later, animals received cocaine (15 mg/kg), cocaine and SCH23390, or saline and were immediately placed in open field chambers (Med Associates, St. Albans, VT) for 1 h, during which locomotor activity was assessed. Animals were then returned to their home cages for 24 h, at which point all animals were given saline injections and returned to the open field chambers where locomotor activity was assessed. Data were normalized to the activity of saline-injected animals. For CPP experiments, the open field chambers were divided into two compartments with distinct floor and wall patterns. The injection protocol was similar to that used previously (12), except that cocaine was paired with the initially less preferred compartment assayed by an initial 30-min baseline session (17). Three days later, animals received two injections per day (a.m. and p.m.) for 3 days. The experimental group received cocaine paired with the less preferred compartment and saline paired with the more preferred compartment. The control group received saline paired alternatively with the two sides. The day after the last conditioning session, animals were placed in the test apparatus for 30 min, and to quantitate CPP, the time spent in the initially more preferred compartment was subtracted from the time spent in the less preferred compartment. All procedures were approved by the institution's animal care and use committee.
Results
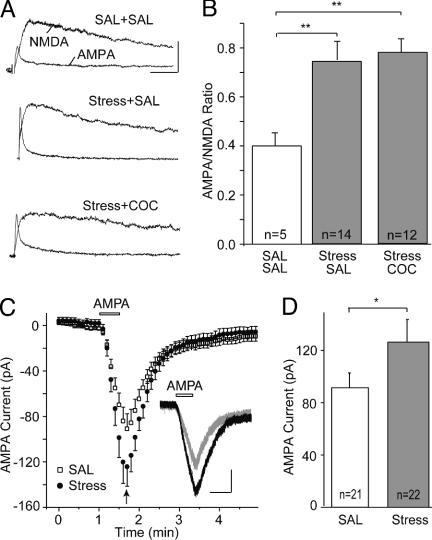
Both Cocaine and Stress Up-Regulate Synaptic AMPARs on Midbrain DA Neurons. Previous results indicated that in vivo administration of cocaine enhanced the strength of excitatory synapses on midbrain DA neurons by increasing the number and/or function of AMPARs (7). To determine whether the synaptic enhancement elicited by stress involves similar mechanisms, we performed occlusion experiments, which examined whether the change in synaptic strength elicited by cocaine linearly added to that caused by stress or rather was occluded by stress. If the two experiences increased synaptic strength by independent mechanisms, the magnitude of the increase should be larger in animals experiencing stress and cocaine compared to animals experiencing stress alone. Conversely, if the mechanisms responsible for the triggering or expression of the synaptic enhancement induced by cocaine or stress are shared (and stress alone causes a maximal effect), administering cocaine and stress together should have no additive effect. As in previous work (7, 8), synaptic strength was measured by calculating the AMPA/NMDA ratio, which normalizes the AMPAR-mediated EPSC to the NMDAR-mediated EPSC. Importantly, the interpretation of this experiment depends on there being a “ceiling effect,” a maximal AMPA/NMDA ratio that can be achieved. As in previous work (8), the AMPA/NMDA ratio was increased 24 h after animals experienced an acute stress plus a saline injection when compared to animals that received only saline injections (Fig. 1 A and B; saline, 0.40 ± 0.05, n = 5; stress, 0.75 ± 0.08, n = 14; P < 0.01). Administering cocaine immediately after the acute stress did not cause any additional increase in the AMPA/NMDA ratio (Fig. 1 A and B; 0.79 ± 0.06, n = 12).
Fig. 1.
Both stress and cocaine up-regulate AMPARs on midbrain DA neurons. (A) Sample AMPAR- and NMDAR-mediated EPSCs from mice pretreated as indicated (calibration bars: 40 pA per 30 ms). SAL, saline; COC, cocaine. (B) Summary of AMPA/NMDA ratios from mice receiving various treatments. (In this and all subsequent figures, n in graphs refers to number of cells examined; **, P < 0.01; *, P < 0.05). (C) Summary of inward current elicited by bath application of AMPA in DA neurons from saline- and stress-pretreated mice. (Inset) Sample AMPA-evoked currents from saline-pretreated (gray) and stress-pretreated (black) mice (calibration bars: 50 pA per 30 s). (D) Peak AMPA current (indicated by arrow in C) was larger in cells from stressed mice.
The lack of additive effects of stress and cocaine on synaptic strength is consistent with the idea that some of the mechanisms responsible for these experience-dependent changes are shared. However, this result does not allow any conclusion as to whether the shared mechanisms involve the initial triggering events and/or the up-regulation of AMPARs. To determine whether, like the effects of cocaine, the stress-induced synaptic enhancement is due to effects on AMPARs we measured the current generated by application of AMPA. The peak AMPA-evoked current was larger in cells from stressed animals when compared with that in cells from control animals (Fig. 1 C and D; saline, 90 ± 13 pA, n = 21; stress, 124 ± 17 pA, n = 22; P < 0.05), suggesting that, like the effects of cocaine, the stress-evoked increase in the AMPA/NMDA ratio is due to an up-regulation of AMPARs.
Cocaine, but Not Stress, Enhances Synaptic Strength via Activation of D1-Like Receptors. How might in vivo cocaine lead to an enhancement of excitatory synaptic transmission in midbrain DA neurons? Activation of NMDARs is required for this synaptic effect of cocaine (7), but because cocaine does not directly activate NMDARs, additional mechanisms must be involved. Given that a major action of cocaine is to block reuptake of DA (2), one possibility is that activation of DA receptors in critical brain areas is also required for the cocaine-induced potentiation. DA receptors are commonly divided into two families, D1- and D2-like receptors (18). To test the role of these DA receptor subtypes, we administered specific D1- or D2-like receptor antagonists before the cocaine injection. Neither the D1 receptor antagonist SCH23390 (0.5 mg/kg) nor the D2 receptor antagonist eticlopride (0.5 mg/kg) had a significant effect on the AMPA/NMDA ratio when administered with saline (Fig. 2 A and B; saline, 0.40 ± 0.09, n = 4; SCH23390, 0.43 ± 0.12, n = 8; eticlopride, 0.46 ± 0.03, n = 4). When administered with cocaine, SCH23390, but not eticlopride, blocked the increase in synaptic strength normally elicited by cocaine (Fig. 2 A and B; cocaine, 0.73 ± 0.05, n = 15; SCH23390 and cocaine, 0.49 ± 0.08, n = 11; eticlopride and cocaine, 0.72 ± 0.04, n = 14; P < 0.05).
Having established that D1-like receptors are required for the synaptic enhancement elicited by cocaine, we next asked whether the effects of stress also require this receptor. Administration of SCH23390 before the acute stress, however, did not block the subsequent increase in the AMPA/NMDA ratio (Fig. 2B; SCH23390 and stress, 0.74 ± 0.08, n = 8). Thus, even though cocaine and stress enhance synaptic strength in DA neurons via a shared expression mechanism, the events involved in the initial triggering of these changes are different. This conclusion is further supported by the finding that administration of a glucocorticoid receptor antagonist blocks stress-elicited, but not cocaine-elicited, synaptic modification in DA neurons (8).
Our protocol for measuring the AMPA/NMDA ratios involves holding cells at +40 mV and applying d-2-amino-5-phosphonovaleric acid (D-APV). A limitation of this approach is that if inwardly rectifying AMPARs normally contribute to synaptic transmission, they would not contribute to the EPSCs measured at +40 mV (19). Conceivably, a replacement of inwardly rectifying AMPARs with nonrectifying AMPARs could account for the observed increased in the AMPA/NMDA ratio without affecting synaptic strength at hyperpolarized membrane potentials. To address this possibility, we calculated AMPA/NMDA ratios by measuring AMPAR EPSCs at –60 mV and NMDAR EPSCs at +40 mV. Cocaine administration still increased the AMPA/NMDA ratio (Fig. 2C; saline, 1.75 ± 0.37, n = 5; cocaine, 2.50 ± 0.24, n = 6), an effect that was blocked by SCH23390 but not by eticlopride (SCH23390 and cocaine, 1.45 ± 0.13, n = 4; eticlopride, 2.20 ± 0.15, n = 10). These results confirm that the increase in AMPA/NMDA ratio measured at +40 mV reflects an increase in synaptic strength and not a change in rectification properties of the AMPARs.
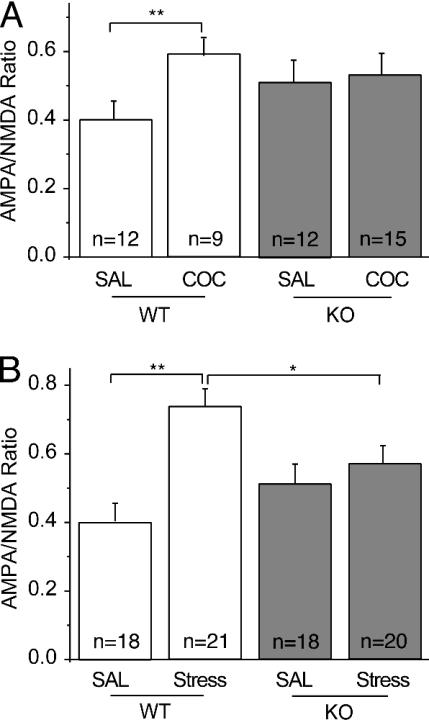
Cocaine and Stress Do Not Potentiate Synaptic Strength in GluRA(–/–) Mice. We have demonstrated that the increase in synaptic strength elicited by cocaine or stress involves an up-regulation of AMPARs. Long-term potentiation (LTP) in the CA1 region of the hippocampus also involves AMPAR modifications (20), raising the possibility that the two forms of plasticity share some underlying mechanisms. As an initial test of this hypothesis, we examined the effects of cocaine and stress on mice lacking the AMPAR subunit GluRA (GluR1) (13). Several lines of evidence suggest that GluRA is required for NMDAR-dependent LTP (21). Most importantly, LTP is dramatically impaired or blocked in GluRA(–/–) mice beginning at 4–5 weeks of age (13, 22). As expected, administration of cocaine to WT animals enhanced the AMPA/NMDA ratio (Fig. 3A; saline, 0.40 ± 0.06, n = 12; cocaine, 0.59 ± 0.05, n = 9). In contrast, in GluRA(–/–) mice, cocaine had no significant effect (Fig. 3A; saline 0.51 ± 0.06, n = 12; cocaine, 0.52 ± 0.05, n = 15). However, there was a trend for basal AMPA/NMDA ratios to be higher in the GluRA(–/–) mice that received saline injections.
Fig. 3.
Cocaine and stress do not potentiate synaptic strength in DA neurons from GluRA (–/–) mice. (A) Summary of AMPA/NMDA ratios in neurons from WT and GluRA(–/–) (KO) mice that received saline or cocaine injections. (B) Summary of AMPA/NMDA ratios in WT and knockout mice that were acutely stressed.
We next examined the effects of acute stress in the GluRA(–/–) mice. Similar to the cocaine results, stress increased the AMPA/NMDA ratio in WT mice but not in the knockout mice [Fig. 3B; WT saline, 0.40 ± 0.06, n = 18; WT stress, 0.74 ± 0.05, n = 21; GluRA(–/–) saline, 0.51 ± 0.06, n = 18; GluRA(–/–) stress, 0.54 ± 0.05, n = 20]. Again, there was a trend for the knockout mice to exhibit higher AMPA/NMDA ratios and when the animals from the two sets of experiments were combined, the increase became significant [WT saline, 0.40 ± 0.03, n = 30; GluRA(–/–) saline, 0.52 ± 0.04, n = 30; P < 0.05]. This increase likely reflects developmental compensations at synapses on DA neurons. Nonetheless, neither cocaine nor stress caused a further increase in synaptic strength, suggesting that GluRA is required for this in vivo synaptic modification.
Cocaine-Induced Behaviors in GluRA(–/–) Mice. A powerful strategy for examining the functional importance of synaptic plasticity has been to study the behavior of mutant mice in which synaptic plasticity is altered (23–25). Although this approach alone does not permit direct causal relationships between synaptic changes and behavior to be firmly established, it does allow important correlations to be made. Mutant animals also facilitate direct tests of hypotheses that invoke functional relevance to synaptic plasticity mechanisms. Having established that the synaptic enhancement caused by cocaine is absent in the GluRA(–/–) mice, we therefore were able to use these mice to test the hypothesis that this synaptic modification is necessary for various behaviors elicited by cocaine. We initially focused on behavioral sensitization, because it has been proposed that synaptic plasticity (5, 9, 10) and an increase in the level of GluRA (11) in the VTA are crucial for the development of sensitization.
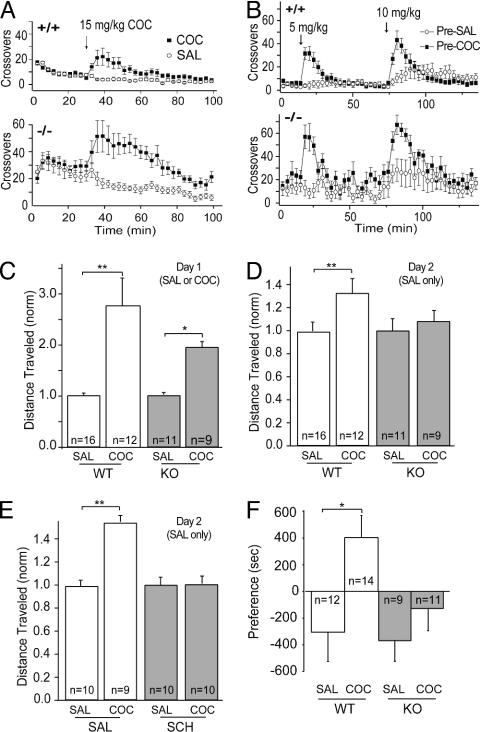
To examine sensitization, GluRA(–/–) mice and littermate controls were given either a saline or cocaine (15 mg/kg) injection every other day for 5 days. In response to saline, GluRA(–/–) mice exhibited higher locomotor activity than WT animals [Fig. 4A; WT, n = 10; GluRA(–/–), n = 9]. Nonetheless, both sets of mice showed enhancement of locomotor activity in response to cocaine (Fig. 4A) for each of the 5 days of injection. Both cocaine- and saline-pretreated animals were then tested with two different doses of cocaine 2 weeks after the final pretreatment injections. Both WT (n = 10 for each group) and GluRA(–/–) (n = 8 for each group) mice showed robust sensitization, which was most evident with the lower dose (Fig. 4B).
Fig. 4.
Cocaine-induced behaviors in GluRA(–/–) mice. (A) Locomotor activity response (3-min bins) to an initial cocaine injection in WT (+/+)(n = 10) and knockout (–/–) (n = 9) mice. (B) Locomotor activity in WT and knockout mice tested 2 weeks after last saline or cocaine pretreatment. Animals received a low dose followed by a higher dose of cocaine. (C) Locomotor activity response to a single injection of saline or cocaine on day 1 in WT and knockout mice. Data were normalized to the distance traveled by the saline treated mice. (D) Locomotor activity response to saline injections only on day 2. (E) Locomotor activity response to saline injections on day 2 in WT mice who received either saline or SCH23390 immediately before cocaine or saline injections on day 1. (F) CPP is absent in GluRA(–/–) mice. Time spent in initially less preferred compartment minus time spent in more preferred compartment in saline- and cocaine-pretreated WT and knockout animals.
Although these results demonstrate that GluRA is not required for the sensitization triggered by cocaine, sensitization is a complex phenomenon that is influenced by several variables. When animals are given an injection in the context in which they previously received drug, part of the enhanced behavioral response is due to “sensitization” (i.e., a nonassociative increase in responsiveness), but part is due to drug-context conditioning (i.e., a conditioned locomotor response) (4, 6, 26–28). To test whether GluRA(–/–) mice learned to associate context with the drug experience, we initially used a protocol in which on day 1 the animals received cocaine or saline and were placed in the test apparatus, but on day 2 both groups received only saline before being tested. Again, cocaine increased locomotor activity in both WT and mutant animals [Fig. 4C; WT saline, 1.0 ± 0.08, n = 16; WT cocaine 2.79 ± 0.53, n = 12; GluRA(–/–) saline, 1.0 ± 0.04, n = 11; GluRA(–/–) cocaine, 1.98 ± 0.13, n = 9]. However, only WT mice that received cocaine on day 1 showed a conditioned increase in locomotor activity on day 2; the GluRA(–/–) mice that received cocaine on day 1 showed no such memory of the drug experience [Fig. 4D; WT saline pretreated, 1.0 ± 0.08; WT cocaine pretreated, 1.32 ± 0.13; GluRA(–/–) saline pretreated, 1.0 ± 0.11; GluRA(–/–) cocaine pretreated, 1.08 ± 0.10]. To further test whether the cocaine-induced synaptic enhancement in DA neurons is required for this conditioned locomotor response, we administered SCH23390 to WT mice shortly before the cocaine on day 1. This also prevented the conditioned locomotor response on day 2 (Fig. 4E; saline/saline-pretreated, 1.0 ± 0.07, n = 10; saline/cocaine-pretreated, 1.46 ± 0.09, n = 9; SCH23390/saline-pretreated, 1.0 ± 0.13, n = 10; SCH23390/cocaine-pretreated, 1.01 ± 0.07, n = 10).
These results suggest that animals in which cocaine does not potentiate synaptic strength on DA neurons are unable to form an association between environmental stimuli and cocaine exposure. To more specifically test whether this cocaine-induced synaptic modification may also be required for animals to learn to associate environmental cues with the reinforcing properties of cocaine, we examined CPP in the GluRA(–/–) mice. CPP has previously been shown to be impaired by D1-like receptor antagonists (29, 30) and glutamate receptor antagonists injected into the VTA (12). Consistent with these results, WT mice exhibited robust CPP, whereas GluRA(–/–) mice did not [Fig. 4F; WT saline, –305 ± 219 s, n = 12; WT cocaine, 403 ± 166 s, n = 14; GluRA(–/–) saline, –378 ± 159 s, n = 9; GluRA(–/–) cocaine –134 ± 160 s, n = 11].
Discussion
It is generally accepted that drug-induced adaptations in the VTA are required for the induction, but not the expression, of behavioral sensitization, a form of behavioral plasticity that is associated with an increase in the incentive value of drugs and associated cues (4, 6, 31). Thus, critical questions, in terms of understanding the neural mechanisms of addiction, are these: What adaptations occur in the VTA in response to drugs of abuse, and which of these are critical for mediating changes in behavior? Injection of glutamate receptor antagonists into the VTA at the time of drug exposure prevents the development of sensitization (32–34), the increase in cocaine self-administration elicited by prior intra-VTA injections of amphetamine (35), and cocaine-elicited CPP (12). Chronic psychostimulant administration also increases the burst firing of DA neurons in response to prefrontal cortex stimulation in vivo (36) and their single unit responses in vivo to iontophoretically applied AMPA (37, 38). Together, these finding suggest that modifications of excitatory synaptic transmission within the VTA may be important for drug-induced behavioral plasticity (5, 9–11).
To test this hypothesis, we measured excitatory synaptic strength in midbrain slices and found that a single in vivo injection of several classes of drugs of abuse, as well as an acute stress, potentiated the strength of excitatory synapses on midbrain DA neurons (7, 8). Here, we extend these findings by demonstrating that stress and cocaine both enhance synaptic strength via a common expression mechanism involving an up-regulation of AMPARs. However, pharmacological manipulations indicate that cocaine and stress use different mechanisms in vivo to trigger this synaptic modification. Cocaine-induced, but not stress-induced, potentiation was blocked by a D1-like receptor antagonist, whereas, in previous work, we found that a glucocorticoid receptor antagonist blocked only the stress-induced synaptic enhancement (8). These experiments do not allow us to determine where in the brain D1-like receptors are required to trigger the cocaine-induced potentiation. Possibilities include medial prefrontal cortex, in which D1 receptors increase the excitability of pyramidal cells (39), and DA neurons themselves, in which D1-like receptors may transiently enhance NMDAR EPSCs (A. Bonci, personal communication).
Because the AMPAR subunit GluRA is important for NMDAR-dependent LTP in the hippocampus (21), we examined the effects of cocaine and stress in mice lacking GluRA (13). Both the cocaine- and stress-induced synaptic enhancement in midbrain DA neurons were absent in GluRA(–/–) mice, providing further evidence that this synaptic modification involves up-regulation of AMPARs. One caveat, however, to this conclusion is that basal properties of excitatory synapses on DA neurons in the GluRA(–/–) mouse were not normal; the basal AMPA/NMDA ratio was slightly increased, albeit not to the same extent as the increase caused by cocaine or stress. The mechanisms responsible for this increase are unknown but likely include some sort of developmental compensations.
It has been proposed that an LTP-like process at excitatory synapses on DA neurons is required for the development of behavioral sensitization (5, 9–11). Increases in GluRA have been proposed to be particularly important (11) based on several lines of evidence; notably, that overexpression of GluRA in VTA neurons sensitizes animals to the behavioral effects of morphine (40). The GluRA(–/–) mice allowed us to test the importance of both the potentiation of synaptic strength on DA neurons and GluRA itself for the induction of sensitization. Although the GluRA(–/–) mice were more active than WT controls, they exhibited long-lasting sensitization in response to repeated exposure to cocaine. This result is consistent with previous work reporting that GluRA(–/–) mice exhibit sensitization to morphine and amphetamine (41), as well as with a preliminary report in which cocaine sensitization was examined (42). These results demonstrate that changes in GluRA levels and LTP in the VTA are not necessary for the development of sensitization and that other neural adaptations within the VTA must play a critical role. These adaptations could include changes in the intrinsic excitability of DA neurons (36–38), the presynaptic release of glutamate (5), and/or inhibitory synaptic transmission (43), all of which could profoundly affect VTA function. However, it also is important to consider that because GluRA was deleted early in development, the animals' responses to drugs of abuse may be altered such that mechanisms not normally in place might be sufficient to induce sensitization (44). Furthermore, GluRA is absent throughout the brain in the GluRA(–/–) mice, not just in the VTA, and the role of this protein may differ in different brain regions (45). Thus, a definitive test of the importance of LTP and GluRA in the VTA for sensitization will require temporal control of GluRA expression in a spatially restricted manner so that only VTA DA neurons are affected.
Behavioral sensitization is context-dependent because a portion of the enhanced behavioral response is due to drug-environment conditioning. Mere placement of an animal into the drug-paired environment elicits a conditioned locomotor response (4, 6, 26, 27), indicating that animals learn to associate environmental cues with the drug experience. To test whether GluRA(–/–) mice were able to make such associations, we examined their conditioned locomotor response after a single cocaine exposure. WT mice showed an increase in conditioned locomotor activity when placed in the cocaine-paired context, whereas the GluRA(–/–) mice did not (Fig. 4D). Administering the D1-like receptor antagonist that blocked the cocaine-induced synaptic potentiation also prevented this conditioned locomotor response. Furthermore, GluRA(–/–) mice did not exhibit CPP in response to repeated pairings of cocaine with environmental cues.
Based on these findings, we suggest that the cocaine-induced enhancement of excitatory synapses on DA neurons contributes either to learning the association between context and the drug experience or to attributing motivational significance to the experience. Consistent with this hypothesis, injection of glutamate receptor antagonists into the VTA prevents cocaine-elicited CPP (12), and GluRA(–/–) mice fail to exhibit conditioned reinforcement (46). The latter finding was interpreted as indicating that the mutant mice could not attribute affective (i.e., reinforcing) properties to a cue previously associated with a primary reinforcer (46). However, the results obtained from the GluRA(–/–) mice in this study (46) were similar to those in rats after lesions of the basolateral nucleus of the amygdala (47); thus, we cannot rule out that impairments in this or other structures contributed to the behaviors measured here. It is also possible that the lack of a conditioned locomotor response after a single exposure to cocaine in the GluRA(–/–) mice might be a consequence of their heightened basal locomotor activity somehow masking the conditioned response. However, the animals show a robust locomotor response to cocaine, suggesting that a “ceiling” effect cannot explain their lack of conditioned responding.
The previous study that examined sensitization to morphine or amphetamine in GluRA(–/–) mice found that a context-dependent sensitization was still present in the mutant animals, whereas “context-independent” sensitization was not (41). There are several explanations for this apparent discrepancy in results. Importantly, we studied the effects of a single injection of cocaine, not the effects of repeated administration of morphine or amphetamine. Previous research has shown that the influence of context on sensitization depends on both the dose and the number of cocaine injections given (28). It is conceivable and even likely that, depending on the specific drug and extent of its use, different neural adaptations may come into play. Furthermore, we used much younger animals compared with this previous work (41). This too may have an influence on the behavioral responses of these animals, especially because the mechanisms of synaptic plasticity can change during postnatal development (22, 48).
In summary, we have demonstrated that the cocaine- and stress-induced potentiations of excitatory synaptic transmission in midbrain DA neurons share an expression mechanism that involves the AMPAR subunit GluRA. These same synaptic enhancements occur even though their initial in vivo triggering mechanisms differ. Further work will be necessary to determine whether the intracellular signaling cascades leading to the GluRA-dependent up-regulation of AMPARs in DA neurons are similar to those involved in LTP in the mature hippocampus. That they may differ is suggested by the finding that long-term depression (LTD) in midbrain DA neurons, like LTD in the hippocampus, involves endocytosis of AMPARs but uses a different signaling cascade to trigger this event (49). We have also demonstrated that although GluRA(–/–) mice can develop behavioral sensitization to repeated injections of cocaine, they do not show a conditioned locomotor response after a single injection, nor do they exhibit CPP in response to cocaine. These results provide important correlations and possible links between the drug-induced potentiation of synaptic transmission in midbrain DA neurons and some key behavioral consequences of drug exposure.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; AMPAR, AMPA receptor; CPP, conditioned place preference; DA, dopamine; EPSC, excitatory postsynaptic current; LTP, long-term potentiation; NMDA, N-methyl-d-aspartic acid; NMDAR, NMDA receptor; VTA, ventral tegmental area.
References
- 1.Koob, G. F., Sanna, P. P. & Bloom, F. E. (1998) Neuron 21, 467–476. [DOI] [PubMed] [Google Scholar]
- 2.Nestler, E. J. (2001) Nat. Rev. Neurosci. 2, 119–128. [DOI] [PubMed] [Google Scholar]
- 3.Hyman, S. E. & Malenka, R. C. (2001) Nat. Rev. Neurosci. 2, 695–703. [DOI] [PubMed] [Google Scholar]
- 4.Everitt, B. J. & Wolf, M. E. (2002) J. Neurosci. 22, 3312–3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vanderschuren, L. J. & Kalivas, P. W. (2000) Psychopharmacology (Berlin) 151, 99–120. [DOI] [PubMed] [Google Scholar]
- 6.Robinson, T. E. & Berridge, K. C. (2003) Annu. Rev. Psychol. 54, 25–53. [DOI] [PubMed] [Google Scholar]
- 7.Ungless, M. A., Whistler, J. L., Malenka, R. C. & Bonci, A. (2001) Nature 411, 583–587. [DOI] [PubMed] [Google Scholar]
- 8.Saal, D., Dong, Y., Bonci, A. & Malenka, R. C. (2003) Neuron 37, 577–582. [DOI] [PubMed] [Google Scholar]
- 9.Clark, D. & Overton, P. G. (1998) Addict. Biol. 3, 109–135. [DOI] [PubMed] [Google Scholar]
- 10.Wolf, M. E. (1998) Prog. Neurobiol. 54, 679–720. [DOI] [PubMed] [Google Scholar]
- 11.Carlezon, W. A., Jr., & Nestler, E. J. (2002) Trends Neurosci. 25, 610–615. [DOI] [PubMed] [Google Scholar]
- 12.Harris, G. C. & Aston-Jones, G. (2003) Neuropsychopharmacology 28, 73–76. [DOI] [PubMed] [Google Scholar]
- 13.Zamanillo, D., Sprengel, R., Hvalby, O., Jensen, V., Burnashev, N., Rozov, A., Kaiser, K. M., Köster, H. J., Borchardt, T., Worley, P., et al. (1999) Science 284, 1805–1811. [DOI] [PubMed] [Google Scholar]
- 14.Cameron, D. L., Wessendorf, M. W. & Williams, J. T. (1997) Neuroscience 77, 155–166. [DOI] [PubMed] [Google Scholar]
- 15.Neuhoff, H., Neu, A., Liss, B. & Roeper, J. (2002) J. Neurosci. 22, 1290–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Margolis, E. B., Hjelmstad, G. O., Bonci, A. & Fields, H. L. (2003) J. Neurosci. 23, 9981–9986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bardo, M. T., Rowlett, J. K. & Harris, M. J. (1995) Neurosci. Biobehav. Rev. 19, 39–51. [DOI] [PubMed] [Google Scholar]
- 18.Civelli, O., Bunzow, J. R. & Grandy, D. K. (1993) Annu. Rev. Pharmacol. Toxicol. 33, 281–307. [DOI] [PubMed] [Google Scholar]
- 19.Dingledine, R., Borges, K., Bowie, D. & Traynelis, S. F. (1999) Pharmacol. Rev. 51, 7–61. [PubMed] [Google Scholar]
- 20.Malenka, R. C. & Nicoll, R. A. (1999) Science 285, 1870–1874. [DOI] [PubMed] [Google Scholar]
- 21.Malinow, R. & Malenka, R. C. (2002) Annu. Rev. Neurosci. 25, 103–126. [DOI] [PubMed] [Google Scholar]
- 22.Jensen, V., Kaiser, K. M., Borchardt, T., Adelmann, G., Rozov, A., Burnashev, N., Brix, C., Frotscher, M., Andersen, P., Hvalby, O., et al. (2003) J. Physiol. 553, 843–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Silva, A., Simposon, E., Takahashi, J. S., Lipp, H.-P., Nakanishi, S., Wehner, J. M., Giese, K. P., Tully, T., Abel, T., Chapman, P. F., et al. (1997) Neuron 19, 755–759.9354323 [Google Scholar]
- 24.Mayford, M. & Kandel, E. R. (1999) Trends Genet. 15, 463–470. [DOI] [PubMed] [Google Scholar]
- 25.Martin, S. J., Grimwood, P. D. & Morris, R. G. (2000) Annu. Rev. Neurosci. 23, 649–711. [DOI] [PubMed] [Google Scholar]
- 26.Badiani, A., Browman, K. E. & Robinson, T. E. (1995) Brain Res. 674, 291–298. [DOI] [PubMed] [Google Scholar]
- 27.Anagnostaras, S. G. & Robinson, T. E. (1996) Behav. Neurosci. 110, 1397–1414. [DOI] [PubMed] [Google Scholar]
- 28.Pert, A., Post, R. & Weiss, S. R. (1990) NIDA Res. Monogr. 97, 208–241.241 [PubMed] [Google Scholar]
- 29.Shippenberg, T. S. & Heidbreder, C. (1995) J. Pharmacol. Exp. Ther. 273, 808–815. [PubMed] [Google Scholar]
- 30.Pruitt, D. L., Bolanos, C. A. & McDougall, S. A. (1995) Eur. J. Pharmacol. 283, 125–131. [DOI] [PubMed] [Google Scholar]
- 31.Vezina, P., Lorrain, D. S., Arnold, G. M., Austin, J. D. & Suto, N. (2002) J. Neurosci. 22, 4654–4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kalivas, P. W. & Alesdatter, J. E. (1993) J. Pharmacol. Exp. Ther. 267, 486–495. [PubMed] [Google Scholar]
- 33.Vezina, P. & Queen, A. L. (2000) Psychopharmacology (Berlin) 151, 184–191. [DOI] [PubMed] [Google Scholar]
- 34.Li, Y. & Wolf, M. E. (1999) Psychopharmacology (Berlin) 141, 351–361. [DOI] [PubMed] [Google Scholar]
- 35.Suto, N., Tanabe, L. M., Austin, J. D., Creekmore, E. & Vezina, P. (2003) Neuropsychopharmacology 28, 629–639. [DOI] [PubMed] [Google Scholar]
- 36.Tong, Z. Y., Overton, P. G. & Clark, D. (1995) Brain Res. 674, 63–74. [DOI] [PubMed] [Google Scholar]
- 37.White, F. J., Hu, X. T., Zhang, X. F. & Wolf, M. E. (1995) J. Pharmacol. Exp. Ther. 273, 445–454. [PubMed] [Google Scholar]
- 38.Zhang, X. F., Hu, X. T., White, F. J. & Wolf, M. E. (1997) J. Pharmacol. Exp. Ther. 281, 699–706. [PubMed] [Google Scholar]
- 39.Dong, Y. & White, F. J. (2003) J. Neurosci. 23, 2686–2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carlezon, W. A., Jr., Boundy, V. A., Haile, C. N., Lane, S. B., Kalb, R. G., Neve, R. L. & Nestler, E. J. (1997) Science 277, 812–814. [DOI] [PubMed] [Google Scholar]
- 41.Vekovischeva, O. Y., Zamanillo, D., Echenko, O., Seppala, T., Uusi-Oukari, M., Honkanen, A., Seeburg, P. H., Sprengel, R. & Korpi, E. R. (2001) J. Neurosci. 21, 4451–4459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stephens, D. N. & Mead, A. N. (2003) Trends Neurosci. 26, 181–182. [DOI] [PubMed] [Google Scholar]
- 43.Bonci, A. & Williams, J. T. (1996) Neuron 16, 631–639. [DOI] [PubMed] [Google Scholar]
- 44.Carlezon, W. A. & Nestler, E. J. (2003) Trends Neurosci. 26, 182–183. [Google Scholar]
- 45.Kelz, M. B., Chen, J., Carlezon, W. A., Jr., Whisler, K., Gilden, L., Beckmann, A. M., Steffen, C., Zhang, Y. J., Marotti, L., Self, D. W., et al. (1999) Nature 401, 272–276. [DOI] [PubMed] [Google Scholar]
- 46.Mead, A. N. & Stephens, D. N. (2003) J. Neurosci. 23, 1041–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Everitt, B., Cardinal, R., Hall, J., Parkinson, J. & Robbins, T. (2000) in The Amygdala, ed. Aggleton, J. (Oxford Univ. Press, New York), pp. 353–390.
- 48.Yasuda, H., Barth, A. L., Stellwagen, D. & Malenka, R. C. (2003) Nat. Neurosci. 6, 15–16. [DOI] [PubMed] [Google Scholar]
- 49.Gutlerner, J. L., Penick, E. C., Snyder, E. M. & Kauer, J. A. (2002) Neuron 36, 921–931. [DOI] [PubMed] [Google Scholar]