Abstract
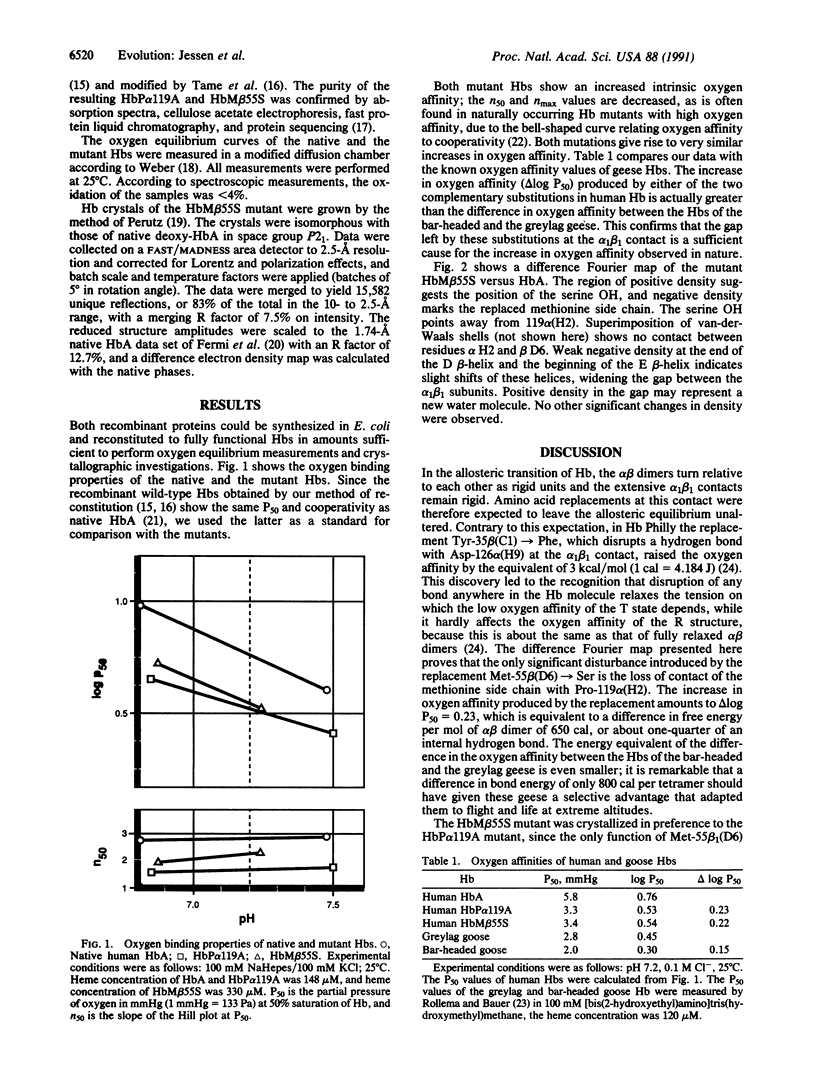
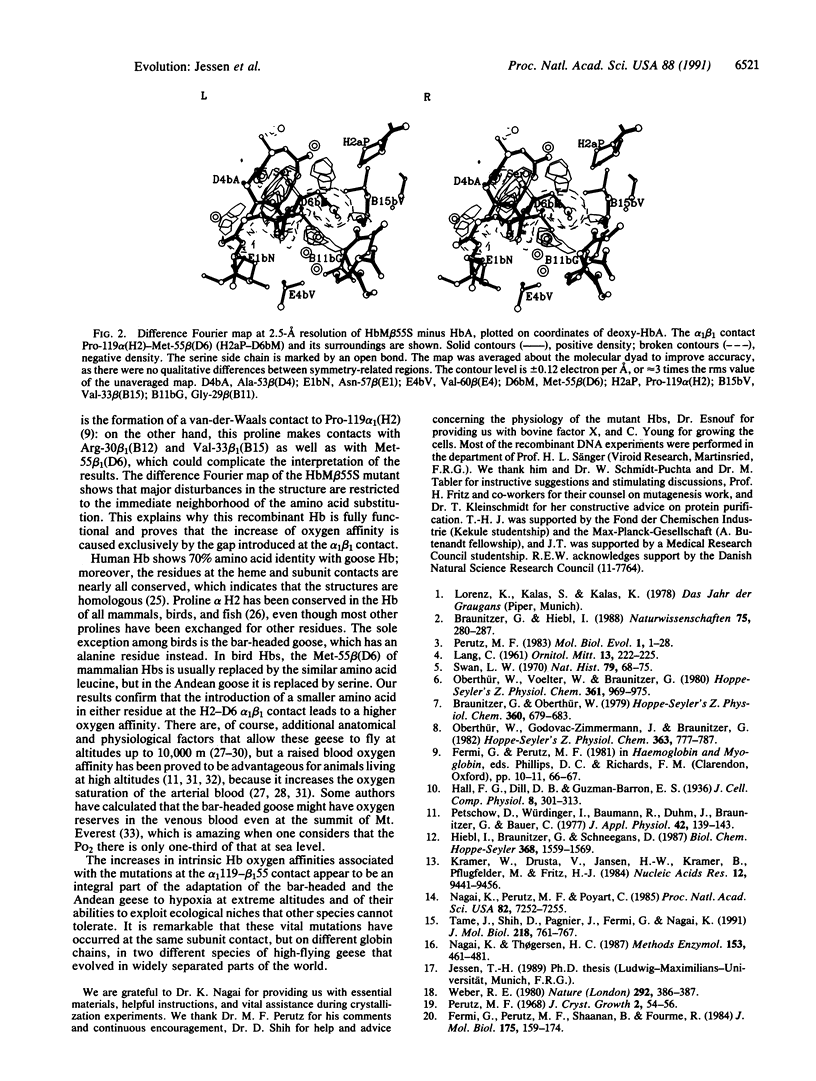
Of two closely related species of geese, one, the greylag goose, lives in the Indian plains all year round, while the other, the bar-headed goose, lives at the Tibetan lakes and migrates across the Himalayas to winter in India. Another species, the Andean goose, lives in the High Andes all year round. Possession of a Hb with high oxygen affinity helps to adapt bar-headed and Andean geese to high altitudes. The Hb amino acid sequences of the bar-headed and the greylag geese differ by four substitutions, of which only one is unique among bird sequences: Pro-119 alpha (H2)----Ala. Perutz proposed that the two-carbon gap left by this substitution at the alpha 1 beta 1 contact raises the oxygen affinity, because it relaxes the tension in the deoxy or T structure [Perutz, M. F. (1983) Mol. Biol. Evol. 1, 1-28]. It was later found that the Hb of the Andean goose has a gap in the same position, due to the complementary substitution Leu-55 beta (D6)----Ser. We have tested Perutz's hypothesis by introducing each of these substitutions into human globin synthesized in Escherichia coli. The reconstituted Hbs combine cooperatively with oxygen. Their oxygen affinities exceed that of normal human Hb by an even larger factor than that found between the high-flying geese and the greylag goose. The mutant Hb Met-55 beta (D6)----Ser was crystallized. Its structure is the same as that of HbA, except in the immediate environment of the gap left by the substitution of the serine for the methionine side chain, which evidently causes the increased oxygen affinity of this Hb.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asakura T., Adachi K., Wiley J. S., Fung L. W., Ho C., Kilmartin J. V., Perutz M. F. Structure and function of haemoglobin Philly (Tyr C1 (35) beta replaced by Phe). J Mol Biol. 1976 Jun 14;104(1):185–195. doi: 10.1016/0022-2836(76)90008-5. [DOI] [PubMed] [Google Scholar]
- Black C. P., Tenney S. M. Oxygen transport during progressive hypoxia in high-altitude and sea-level waterfowl. Respir Physiol. 1980 Feb;39(2):217–239. doi: 10.1016/0034-5687(80)90046-8. [DOI] [PubMed] [Google Scholar]
- Braunitzer G., Hiebl I. Molekulare Aspekte der Höhenatmung von Vögeln. Hämoglobine der Streifengans (Anser indicus), der Andengans (Chloephaga melanoptera) und des Sperbergeiers (Gyps rueppellii). Naturwissenschaften. 1988 Jun;75(6):280–287. doi: 10.1007/BF00367318. [DOI] [PubMed] [Google Scholar]
- Braunitzer G., Oberthür W. Die primärstruktur des Hämoglobins der Graugans (Anser anser) Die ungleiche Evolution der beta-Ketten (Versuch einer biochemischen Analyse des Verhaltens). Hoppe Seylers Z Physiol Chem. 1979 May;360(5):679–683. [PubMed] [Google Scholar]
- Chothia C., Lesk A. M. The relation between the divergence of sequence and structure in proteins. EMBO J. 1986 Apr;5(4):823–826. doi: 10.1002/j.1460-2075.1986.tb04288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton J. W., Skelton T. D., Berger E. Survival at extreme altitude: protective effect of increased hemoglobin-oxygen affinity. Science. 1974 Feb 22;183(4126):743–744. doi: 10.1126/science.183.4126.743. [DOI] [PubMed] [Google Scholar]
- Faraci F. M., Fedde M. R. Regional circulatory responses to hypocapnia and hypercapnia in bar-headed geese. Am J Physiol. 1986 Mar;250(3 Pt 2):R499–R504. doi: 10.1152/ajpregu.1986.250.3.R499. [DOI] [PubMed] [Google Scholar]
- Faraci F. M., Kilgore D. L., Jr, Fedde M. R. Blood flow distribution during hypocapnic hypoxia in Pekin ducks and bar-headed geese. Respir Physiol. 1985 Jul;61(1):21–30. doi: 10.1016/0034-5687(85)90025-8. [DOI] [PubMed] [Google Scholar]
- Fermi G., Perutz M. F., Shaanan B., Fourme R. The crystal structure of human deoxyhaemoglobin at 1.74 A resolution. J Mol Biol. 1984 May 15;175(2):159–174. doi: 10.1016/0022-2836(84)90472-8. [DOI] [PubMed] [Google Scholar]
- Hiebl I., Braunitzer G., Schneeganss D. The primary structures of the major and minor hemoglobin-components of adult Andean goose (Chloephaga melanoptera, Anatidae): the mutation Leu----Ser in position 55 of the beta-chains. Biol Chem Hoppe Seyler. 1987 Dec;368(12):1559–1569. doi: 10.1515/bchm3.1987.368.2.1559. [DOI] [PubMed] [Google Scholar]
- Kleinschmidt T., Sgouros J. G. Hemoglobin sequences. Biol Chem Hoppe Seyler. 1987 Jun;368(6):579–615. [PubMed] [Google Scholar]
- Kramer W., Drutsa V., Jansen H. W., Kramer B., Pflugfelder M., Fritz H. J. The gapped duplex DNA approach to oligonucleotide-directed mutation construction. Nucleic Acids Res. 1984 Dec 21;12(24):9441–9456. doi: 10.1093/nar/12.24.9441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai K., Perutz M. F., Poyart C. Oxygen binding properties of human mutant hemoglobins synthesized in Escherichia coli. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7252–7255. doi: 10.1073/pnas.82.21.7252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai K., Thøgersen H. C. Synthesis and sequence-specific proteolysis of hybrid proteins produced in Escherichia coli. Methods Enzymol. 1987;153:461–481. doi: 10.1016/0076-6879(87)53072-5. [DOI] [PubMed] [Google Scholar]
- Oberthür W., Godovac-Zimmermann J., Braunitzer G., Wiesner H. The amino acid sequence of Canada goose (Branta canadensis) and mute swan (Cygnus olor) hemoglobins. Two different species with identical beta-chains. Hoppe Seylers Z Physiol Chem. 1982 Aug;363(8):777–787. doi: 10.1515/bchm2.1982.363.2.777. [DOI] [PubMed] [Google Scholar]
- Oberthür W., Voelter W., Braunitzer G. Die Sequenz der Hämoglobine von Streifengans (Anser indicus) und Strauss (Struthio camelus). Inositpentaphosphat als Modulator der Evolutionsgeschwindigkeit: Die überraschende Sequenz alpha 63 (E12) Valin. Hoppe Seylers Z Physiol Chem. 1980;361(6):969–975. [PubMed] [Google Scholar]
- Olson J. S., Mathews A. J., Rohlfs R. J., Springer B. A., Egeberg K. D., Sligar S. G., Tame J., Renaud J. P., Nagai K. The role of the distal histidine in myoglobin and haemoglobin. Nature. 1988 Nov 17;336(6196):265–266. doi: 10.1038/336265a0. [DOI] [PubMed] [Google Scholar]
- Perutz M. F. Species adaptation in a protein molecule. Mol Biol Evol. 1983 Dec;1(1):1–28. doi: 10.1093/oxfordjournals.molbev.a040299. [DOI] [PubMed] [Google Scholar]
- Petschow D., Würdinger I., Baumann R., Duhm J., Braunitzer G., Bauer C. Causes of high blood O2 affinity of animals living at high altitude. J Appl Physiol Respir Environ Exerc Physiol. 1977 Feb;42(2):139–143. doi: 10.1152/jappl.1977.42.2.139. [DOI] [PubMed] [Google Scholar]
- Rollema H. S., Bauer C. The interaction of inositol pentaphosphate with the hemoglobins of highland and lowland geese. J Biol Chem. 1979 Dec 10;254(23):12038–12043. [PubMed] [Google Scholar]
- Scheid P. Mechanisms of gas exchange in bird lungs. Rev Physiol Biochem Pharmacol. 1979;86:137–186. doi: 10.1007/BFb0031533. [DOI] [PubMed] [Google Scholar]
- Tame J., Shih D. T., Pagnier J., Fermi G., Nagai K. Functional role of the distal valine (E11) residue of alpha subunits in human haemoglobin. J Mol Biol. 1991 Apr 20;218(4):761–767. doi: 10.1016/0022-2836(91)90264-7. [DOI] [PubMed] [Google Scholar]
- Turek Z., Kreuzer F., Ringnalda B. E. Blood gases at several levels of oxygenation in rats with a left-shifted blood oxygen dissociation curve. Pflugers Arch. 1978 Aug 25;376(1):7–13. doi: 10.1007/BF00585241. [DOI] [PubMed] [Google Scholar]



