Abstract
Carotenoids are orange, yellow, or red photo-protective pigments present in all plastids. The first carotenoid of the pathway is phytoene, a colorless compound that is converted into colored carotenoids through a series of desaturation reactions. Genes coding for carotenoid desaturases have been cloned from microbes but not from plants. We report the cloning of a cDNA for pds1, a soybean (Glycine max) gene that, based on a complementation assay using the photosynthetic bacterium Rhodobacter capsulatus, codes for an enzyme that catalyzes the two desaturation reactions that convert phytoene into zeta-carotene, a yellow carotenoid. The 2281-base-pair cDNA clone analyzed contains an open reading frame with the capacity to code for a 572-residue protein of predicted Mr 63,851. Alignment of the deduced Pds1 peptide sequence with the sequences of fungal and bacterial carotenoid desaturases revealed conservation of several amino acid residues, including a dinucleotide-binding motif that could mediate binding to FAD. The Pds1 protein is synthesized in vitro as a precursor that, upon import into isolated chloroplasts, is processed to a smaller mature form. Hybridization of the pds1 cDNA to genomic blots indicated that this gene is a member of a low-copy-number gene family. One of these loci was genetically mapped using restriction fragment length polymorphisms between Glycine max and Glycine soja. We conclude that pds1 is a nuclear gene encoding a phytoene desaturase enzyme that, as its microbial counterparts, contains sequence motifs characteristic of flavoproteins.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong G. A., Alberti M., Hearst J. E. Conserved enzymes mediate the early reactions of carotenoid biosynthesis in nonphotosynthetic and photosynthetic prokaryotes. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9975–9979. doi: 10.1073/pnas.87.24.9975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong G. A., Alberti M., Leach F., Hearst J. E. Nucleotide sequence, organization, and nature of the protein products of the carotenoid biosynthesis gene cluster of Rhodobacter capsulatus. Mol Gen Genet. 1989 Apr;216(2-3):254–268. doi: 10.1007/BF00334364. [DOI] [PubMed] [Google Scholar]
- Bartholomew D. M., Bartley G. E., Scolnik P. A. Abscisic Acid Control of rbcS and cab Transcription in Tomato Leaves. Plant Physiol. 1991 May;96(1):291–296. doi: 10.1104/pp.96.1.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartley G. E., Schmidhauser T. J., Yanofsky C., Scolnik P. A. Carotenoid desaturases from Rhodobacter capsulatus and Neurospora crassa are structurally and functionally conserved and contain domains homologous to flavoprotein disulfide oxidoreductases. J Biol Chem. 1990 Sep 15;265(26):16020–16024. [PubMed] [Google Scholar]
- Bartley G. E., Scolnik P. A. Carotenoid biosynthesis in photosynthetic bacteria. Genetic characterization of the Rhodobacter capsulatus CrtI protein. J Biol Chem. 1989 Aug 5;264(22):13109–13113. [PubMed] [Google Scholar]
- Berlyn M. B., Last R. L., Fink G. R. A gene encoding the tryptophan synthase beta subunit of Arabidopsis thaliana. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4604–4608. doi: 10.1073/pnas.86.12.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer P., Mayer M., Kleinig H. Molecular oxygen and the state of geometric isomerism of intermediates are essential in the carotene desaturation and cyclization reactions in daffodil chromoplasts. Eur J Biochem. 1989 Sep 1;184(1):141–150. doi: 10.1111/j.1432-1033.1989.tb15000.x. [DOI] [PubMed] [Google Scholar]
- Buckner B., Kelson T. L., Robertson D. S. Cloning of the y1 Locus of Maize, a Gene Involved in the Biosynthesis of Carotenoids. Plant Cell. 1990 Sep;2(9):867–876. doi: 10.1105/tpc.2.9.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamovitz D., Pecker I., Hirschberg J. The molecular basis of resistance to the herbicide norflurazon. Plant Mol Biol. 1991 Jun;16(6):967–974. doi: 10.1007/BF00016069. [DOI] [PubMed] [Google Scholar]
- Cline K., Werner-Washburne M., Lubben T. H., Keegstra K. Precursors to two nuclear-encoded chloroplast proteins bind to the outer envelope membrane before being imported into chloroplasts. J Biol Chem. 1985 Mar 25;260(6):3691–3696. [PubMed] [Google Scholar]
- Giuliano G., Pollock D., Scolnik P. A. The gene crtI mediates the conversion of phytoene into colored carotenoids in Rhodopseudomonas capsulata. J Biol Chem. 1986 Oct 5;261(28):12925–12929. [PubMed] [Google Scholar]
- Giuliano G., Scolnik P. A. Transcription of Two Photosynthesis-Associated Nuclear Gene Families Correlates with the Presence of Chloroplasts in Leaves of the Variegated Tomato ghost Mutant. Plant Physiol. 1988 Jan;86(1):7–9. doi: 10.1104/pp.86.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lütcke H. A., Chow K. C., Mickel F. S., Moss K. A., Kern H. F., Scheele G. A. Selection of AUG initiation codons differs in plants and animals. EMBO J. 1987 Jan;6(1):43–48. doi: 10.1002/j.1460-2075.1987.tb04716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misawa N., Nakagawa M., Kobayashi K., Yamano S., Izawa Y., Nakamura K., Harashima K. Elucidation of the Erwinia uredovora carotenoid biosynthetic pathway by functional analysis of gene products expressed in Escherichia coli. J Bacteriol. 1990 Dec;172(12):6704–6712. doi: 10.1128/jb.172.12.6704-6712.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogen B. D., MacDonald M. H., Graybosch R., Hunt A. G. Upstream sequences other than AAUAAA are required for efficient messenger RNA 3'-end formation in plants. Plant Cell. 1990 Dec;2(12):1261–1272. doi: 10.1105/tpc.2.12.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möller W., Amons R. Phosphate-binding sequences in nucleotide-binding proteins. FEBS Lett. 1985 Jul 1;186(1):1–7. doi: 10.1016/0014-5793(85)81326-0. [DOI] [PubMed] [Google Scholar]
- Pollock D., Bauer C. E., Scolnik P. A. Transcription of the Rhodobacter capsulatus nifHDK operon is modulated by the nitrogen source. Construction of plasmid expression vectors based on the nifHDK promoter. Gene. 1988 May 30;65(2):269–275. doi: 10.1016/0378-1119(88)90463-5. [DOI] [PubMed] [Google Scholar]
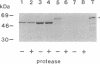
- Reed J. E., Cline K., Stephens L. C., Bacot K. O., Viitanen P. V. Early events in the import/assembly pathway of an integral thylakoid protein. Eur J Biochem. 1990 Nov 26;194(1):33–42. doi: 10.1111/j.1432-1033.1990.tb19423.x. [DOI] [PubMed] [Google Scholar]
- Reichardt J. K., Berg P. Conservation of short patches of amino acid sequence amongst proteins with a common function but evolutionarily distinct origins: implications for cloning genes and for structure-function analysis. Nucleic Acids Res. 1988 Sep 26;16(18):9017–9026. doi: 10.1093/nar/16.18.9017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidhauser T. J., Lauter F. R., Russo V. E., Yanofsky C. Cloning, sequence, and photoregulation of al-1, a carotenoid biosynthetic gene of Neurospora crassa. Mol Cell Biol. 1990 Oct;10(10):5064–5070. doi: 10.1128/mcb.10.10.5064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. F., Smith T. F. Automatic generation of primary sequence patterns from sets of related protein sequences. Proc Natl Acad Sci U S A. 1990 Jan;87(1):118–122. doi: 10.1073/pnas.87.1.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
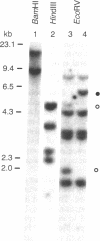
- Viitanen P. V., Doran E. R., Dunsmuir P. What is the role of the transit peptide in thylakoid integration of the light-harvesting chlorophyll a/b protein? J Biol Chem. 1988 Oct 15;263(29):15000–15007. [PubMed] [Google Scholar]