Abstract
Cloning by nuclear transfer using mammalian somatic cells has enormous potential application. However, somatic cloning has been inefficient in all species in which live clones have been produced. High abortion and fetal mortality rates are commonly observed. These developmental defects have been attributed to incomplete reprogramming of the somatic nuclei by the cloning process. Various strategies have been used to improve the efficiency of nuclear transfer, however, significant breakthroughs are yet to happen. In this review we will discuss studies conducted, in our laboratories and those of others, to gain a better understanding of nuclear reprogramming. Because cattle are a species widely used for nuclear transfer studies, and more laboratories have succeeded in cloning cattle than any other specie, this review will be focused on somatic cell cloning of cattle.
Keywords: nuclear transfer, donor cell types, donor age, serum starvation, cell passage
Introduction
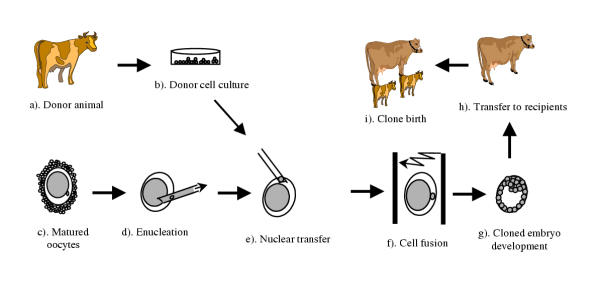
Somatic cell cloning (cloning or nuclear transfer) is a technique in which the nucleus (DNA) of a somatic cell is transferred into an enucleated metaphase-II oocyte for the generation of a new individual, genetically identical to the somatic cell donor (Figure 1). The success of cloning an entire animal, Dolly, from a differentiated adult mammary epithelial cell [1] has created a revolution in science. It demonstrated that genes inactivated during tissue differentiation can be completely re-activated by a process called nuclear reprogramming: the reversion of a differentiated nucleus back to a totipotent status. Somatic cloning may be used to generate multiple copies of genetically elite farm animals, to produce transgenic animals for pharmaceutical protein production or xeno-transplantation [2-5], or to preserve endangered species. With optimization, it also promises enormous biomedical potential for therapeutic cloning and allo-transplantation [6]. In addition to its practical applications, cloning has become an essential tool for studying gene function [7], genomic imprinting [8], genomic re-programming [9-12], regulation of development, genetic diseases, and gene therapy, as well as many other topics.
Figure 1.
Schematic diagram of the somatic cloning process. Cells are collected from donor (a) and cultured in vitro (b). A matured oocyte (c) is then enucleated (d) and a donor cell is transferred into the enucleated oocyte (e). The somatic cell and the oocyte is then fused (f) and the embryos is allowed to develop to a blastocyst in vitro (g). The blastocyst can then be transferred to a recipient (h) and cloned animals are born after completion of gestation (i).
One of the most difficult challenges faced, however, is cloning's low efficiency and high incidence of developmental abnormalities [13-19]. Currently, the efficiency for nuclear transfer is between 0–10%, i.e., 0–10 live births after transfer of 100 cloned embryos. Developmental defects, including abnormalities in cloned fetuses and placentas, in addition to high rates of pregnancy loss and neonatal death have been encountered by every research team studying somatic cloning. It has been proposed that low cloning efficiency may be largely attributed to the incomplete reprogramming of epigenetic signals [20-23].
Factors affecting nuclear reprogramming
Various strategies have been employed to modify donor cells and the nuclear transfer procedure in attempts to improve the efficiency of nuclear transfer. Most of these efforts are focused on donor cells. These include: a) synchrony of the cell cycle stage of donor cells [24-26], as well as synchrony between donor cells and recipient oocytes [27,28]; b) using somatic cells from donors of various ages [29-33], tissue origins [26,34-39], passages [16,40,41] and culture conditions [42]; c) transfer of stem cells with low levels of epigenetic marks [43-48]; and d) modifying epigenetic marks of donor cells with drugs [49-51]. Although the efficiency of nuclear transfer has been dramatically improved from the initial success rate of one live clone born from 277 embryo transfers [1], none of the aforementioned efforts abolished the common problems associated with nuclear transfer. These observations suggest that further studies on nuclear reprogramming are needed in order to understand the underlying mechanisms of reprogramming and significantly improve the ability of the differentiated somatic nuclei to be reprogrammed. In the following section, we will discuss several strategies used to improve nuclear transfer efficiencies.
Serum starvation of donor cells
Serum starvation was used in the creation of Dolly and was believed essential to the success of nuclear transfer [1]. Serum starvation induces quiescence of cultured cells, and arrests them at the cell cycle stage of G0. Most laboratories that have succeeded with nuclear transfer have utilized a serum starvation treatment. However, there is a debate as to whether inducing quiescence is required for successful nuclear transfer. Cibelli et al. [52] proposed that G0 was unnecessary and that calves could be produced from cycling cells. In his study, actively dividing bovine fibroblasts were used for nuclear transfer and four calves were born from 28 embryos transferred to 11 recipients. Because 56% of cycling cells in that study were in G1 stage, it is likely that all cloned animals produced in this study were from donor cells at G1 stage. Cells at G2, S or M would not be expected to generate cloned animals in this study because they are incompatible with the recipient oocytes used. This study demonstrated that cells at G1 stage can produce live cloned animals and G0 induction is not essential.
Since the report of Cibelli and colleagues, many laboratories have compared nuclear transfer using donor cells with and without serum starvation. In our study, we used cells from a 17-year old male Japanese Black beef bull and found that serum starvation was not required for successful cloning because cloned embryos and animals were produced from cells not subjected to serum starvation (Table 1) [16]. Furthermore, serum starvation did not have a beneficial effect on the blastocyst development of cloned embryos.
Table 1.
Development of embryos cloned from donor cells from a 17-year old bull with and without serum starvation treatment
| Serum starvation | No. oocytes | No (%) fused | No (%) cleaved | No (%) blastocysts |
| Yes | 288 | 114 (40) | 75 (66) | 24 (21)a |
| No | 282 | 102 (36) | 79 (78) | 28 (28)a |
Values with the same superscripts are not significantly different (P > 0.05).
In other studies in which serum starvation vs. no starvation were directly compared, evidence was found that both quiescent and proliferating somatic donor cells can be fully reprogrammed after nuclear transfer and result in viable offspring [25,26,29,53,54]. However, it is still debatable which cell cycle stage, G0 or G1, result in the best cloning efficiency. Interestingly, Zechkerchenko et al. [53] observed a positive effect of serum starvation on the efficiency of nuclear transfer using bovine fetal fibroblasts. Although Cho et al. [55] did not observe an improvement in blastocyst rate from any of four different cell types (cumulus, fibroblast, uterine and oviduct epithelial cells). Similar observations were noted by Hills et al. [29] who reported that serum starvation of adult donor cells did not improve development rates of cloned embryos to blastocyst, but when fetal cells were serum-starved, there was a significant increase in their blastocyst development. Conversely, Rho et al. [54] found that fetal transgenic lines were not different in blastocyst development with or without serum starvation or confluency.
Recently, Kasinathan et al. [25] evaluated methods for generating G0 and G1 cell populations and compared their development following cloning. They found that a high degree of confluence was more effective than serum starvation for arresting cells in G0, and G1 cells could be obtained using a "shake-off" procedure. In this study, no differences in in vitro development were observed between embryos derived from the high-confluence cells (G0) or from the "shaken-off" cells (G1). Nevertheless, when embryos from each treatment were transferred into 50 recipients, five calves (10% of embryos transferred) were obtained from embryos derived from the "shake-off" cells, whereas no embryos from the confluent cells survived beyond 180 days of gestation. Kasinathan et al. [25] concluded that nuclear transfer donor cell cycle stage is important, particularly effecting late fetal development, and that actively dividing G1 cells support higher development rates than cells in G0. Despite the fact that Kasinathan's study did not produce live clones from G0 cells, a high nuclear transfer success rate was obtained by Cho et al. [55] who subjected donor cells to serum starvation and found no improvement in blastocyst development from adult donor cells, but resulted in a 27.3% calving rate.
To further complicate the matter, Wells et al. [26] compared two different types of non-transfected bovine fetal fibroblasts (BFFs) that were synchronized in G0, G1 or different phases within G1. They showed that serum starvation into G0 resulted in a significantly higher percentage of viable calves at term than did synchronization in early G1 or late G1. For transgenic fibroblasts, however, cells selected in G1 showed significantly higher development to term of calves and higher post-natal survival to weaning, than cells in G0. They suggest that it may be necessary to coordinate donor cell type and cell cycle stage to maximize overall cloning efficiency.
In summary, it is clear that quiescence is not necessary for the success of nuclear transfer because cells not subjected to serum starvation can also produce live clones. Even so, it remains unclear which cell cycle stage, G0 or G1, imparts a higher nuclear transfer efficiency. This question will continue to be debated until large-scale nuclear transfer studies can be conducted.
Cloning competence of various somatic cell types
Many somatic cell types, including mammary epithelial cells, ovarian cumulus cells, fibroblast cells from skin and internal organs, various internal organ cells, Sertoli cells [38,56], macrophage [56] and blood leukocytes [34,35] have been successfully utilized for nuclear transfer. A clear consensus, however, has not yet been reached as to the superior somatic cell type for nuclear transfer. This is due in part to the fact that different laboratories employ diverse procedures; and cell culture, nuclear transfer, and micromanipulation all require critical technical skills. In order to make these comparisons valid, the procedures and techniques used, as well as the skill of lab personnel, must be identical for each donor animal and cell type. To compare the competence of different cell types for reprogramming by cloning, we avoided animal variation by looking at the cloning competence of three cell types: ovarian cumulus, mammary epithelial and skin fibroblast cells, all from the same donor animal, a 13-year-old elite diary cow.
The ability of donor cells to be reprogrammed was assessed by the development of cloned embryos in vitro and by the birth of cloned calves following embryo transfer. As shown in Tables 2 and 3, although no differences were detected in the cleavage rates of embryos from three different cell types, cumulus cells produced the highest rate of blastocyst development in this study and resulted in 6 full-term cloned calves. Furthermore, four out of the six calves derived from cumulus cells survived and were still healthy at nearly 4 years of age (Table 3). In contrast, the poorest in vitro development, and no full-term survival, was obtained with mammary epithelial cells. Skin fibroblast cells resulted in an intermediate rate of in vitro development and gave rise to 4 full-term cloned calves.
Table 2.
Summary of in vitro development of cloned embryos from different cell types
| Cell types | No. reconstructed embryos | Embryo development (%) | |
| Cleavage | Blastocyst | ||
| Cumulus | 92 | 65a | 57a |
| Fibroblast | 110 | 63a | 34b |
| Epithelium | 96 | 66a | 23c |
Numbers with different superscripts within columns are significantly different (P < 0.05).
Table 3.
Summary of embryo transfer and calving of cloned embryos from different cell types
| Cell type | No. embryo Transferred | No. recipients | No. (%) calves born | Alive to adulthood | |
| Total | Pregnant* | ||||
| Cumulus | 109 | 58 | 10 | 6 (5.5)** | 4 |
| Fibroblast | 57 | 29 | 8 | 4 (7.0)** | 0 |
| Epithelium | 34 | 24 | 1 | 0 | 0 |
*: Pregnancy determined by ultrasound examination at 60 days of gestation. **: A set of twins included.
Our results showed that the donor cell type can significantly affect embryo development in vitro as well as in vivo. Cumulus cells proved to be the most effective cell type for somatic cloning according to both the in vitro development test as well as full-term survival. These results suggest that DNA from cumulus cells is more effectively reprogrammed following nuclear transfer. Our results agreed with those obtained in mice [57] where they compared the nuclear transfer efficiency of neuronal, Sertoli and cumulus cells, and obtained the best live birth rate from cumulus cell-derived cloned embryos. Furthermore, it was reported that cumulus cell-derived cloned mice do not have widespread dysregulation of imprinting [23]. Kato et al. [15,36] compared cells from the liver, testis, skin, ear, along with cumulus and oviductal cells and concluded that cumulus and oviduct epithelial cells are the most suitable for nuclear donors. Evidence supporting the superiority of cumulus cells for nuclear transfer also comes from the study of Forsberg et al. [58] who conducted large numbers of embryo transfer in cattle. It was shown that cumulus cells gave an overall 15.2% calving rate, while fetal genital ridge cells, and fibroblast cells produced a 9% calving rate. Adult fibroblast cells, in this study, gave the lowest calving rate of only 5%.
In summary, among the somatic cell types tested, the consensus from numerous laboratories is that cumulus cells give the highest cloning efficiency and result in the least number of abnormalities in cloned animals.
Effect of donor age
By using a design similar to the donor cell type comparison, we studied the cloning efficiency of fibroblast cells from donors of different ages. We found that cells from fetuses and newborn animals were more efficient in nuclear transfer. However, when cells from adult animals were used, little changes were observed in the cloning efficiency of cells from cattle varying in age from 2 to16-years-old (Table 4).
Table 4.
Cloning competence of cells from donor animals of different ages
| Donor age | No. Oocytes used | (%) Development | |
| Cleavage | Blastocyst | ||
| Fetus (D57) | 630 | 82 | 48a |
| New born | 302 | 76 | 51a |
| 2 years | 158 | 79 | 38b |
| 10–12 years | 424 | 73 | 35b |
| 16 years | 269 | 63 | 37b |
Numbers with different superscripts within columns are significantly different (P < 0.05).
Similarly, Renard et al. [31], Hills et al. [29] and Wakayama and Yanagimachi [56] also reported that development rates of somatic cloned embryo remained similar regardless of donor age. However, Kato et al. [36] noted that clones derived from adult cells frequently aborted in the later stages of pregnancy, and calves developing to term showed a higher number of abnormalities than did those derived from newborn or fetal cells. Forsberg et al. [58] transferred a large number of cloned embryos in cattle. They also concluded that, in general, embryos cloned from fetal cells produced higher pregnancy and calving rates than those from adult cells.
In conclusion, it appears that cells from fetuses, as well as aged adults, can lead to comparable blastocyst development of cloned embryos. Nevertheless, fetal cells may be better than adult cells in producing healthy live births. This might be due to the fact that the somatic cells of adult animals have accumulated more genetic mutations/are more terminally differentiated than fetal cells, and are thus more likely to fail at full term development.
Effect of cell culture duration (passage numbers)
Our group was the first to directly compare passage effect of donor cells on the outcome of nuclear transfer [16]. In our study, we found that cells of later passages (up to 15) could also support clone development to full term (Table 5).
Table 5.
Cloning efficiency of cells at different passages
| No. Passage | No. NT | No. (%) fused | No (%) cleaved | No. (%) blastocyst |
| 5 | 288 | 114 (40) | 75 (66) | 24 (21)a |
| 10 | 269 | 115 (43) | 72 (63) | 43 (37)b |
| 15 | 264 | 109 (41) | 81 (74) | 36 (33)b |
Numbers with different superscripts within columns are significantly different (P < 0.05).
Comparable to our findings were those of Arat et al. [40] who established a primary cell line from granulosa cells and transfected them with the green fluorescence protein (GFP) gene. Non-transfected cells were used for cloning between passage 10 and 15 as either serum-starved or serum-fed donor cells. There were no differences in development to the blastocyst stage for nuclear transfer embryos from transfected or non-transfected or from serum-starved or serum-fed cells. Blastocyst development rates of embryos produced from donor cells at passage 15, however, were significantly higher than those produced with cells at passage 10, 11, and 13. Developmental competence of later passages, up to 16 [54] and as high as 36, from fibroblast from a cloned fetus [41], have also been reported.
The demonstration that later passages can support clone development is essential for utilizing somatic cloning for gene-knockout studies, in which single cells must be clonally expanded to generate sufficient cells for nuclear transfer [7]. These afore-mentioned studies suggest that cells of higher passages were receptive to nuclear reprogramming. Additional support for this hypothesis comes from a recent study by Enright et al. [59] who showed that cells of later passages contain less epigenetic modifications, i.e., their histones are more acetylated than in earlier passages. This observation agrees with an earlier notion that in vitro culture of cells can induce expression of genes that were not expressed before culture [60,61]. Furthermore, Hills et al. [62] reported that a greater proportion of late passage cells (passage 18), vs. earlier passage cells (passage 2), were found to be in G0/G1 whether or not they were in serum-starved culture conditions.
Effect of modification of pre-existing epigenetic marks in donor cells
Histone acetylation and DNA methylation are heritable modifications of the chromatin that do not involve changes in gene sequences (epigenetic signals). These epigenetic modifications are believed responsible for the derivation of various cell types with the same genetic makeup. In natural reproduction, relatively low levels of DNA methylation exist in the gametes, which are further de-methylated during early embryo development [63,64]. With nuclear transplantation, the somatic donor nucleus carries the specific epigenetic modifications of its tissue type, which must be erased during nuclear reprogramming. Therefore, the levels of epigenetic modification existing in donor cells may affect their reprogrammability following nuclear transfer. As discussed earlier, a discrepancy in the donor cell's susceptibility to reprogramming has been observed between different cell types, resulting in differences in vitro and in vivo development of cloned embryos. Therefore, treating donor cells with pharmacological agents to remove some epigenetic marks prior to nuclear transfer may improve the ability of the donor cells to be fully reprogrammed by the recipient karyoplast.
Two reagents have been widely used for the alteration of the levels of epigenetic modification of somatic cells. Trichostatin A (TSA) and 5-aza-deoxy-cytadine (5-aza-dC) have been found to increase histone acetylation and decrease DNA methylation, respectively. These changes have been associated with increases of gene expression. Recently, we conducted studies in which the pre-existing epigenetic marks in donor cells were reduced by these drugs [49]. We found that global epigenetic marks in donor cells can be modified by treatment with TSA or 5-aza-dC. Unfortunately, treating donor cells with 5-aza-dC reduced blastocyst formation of cloned embryos. Previously, Jones et al. [50] and Zhou et al. [51] treated bovine fetal fibroblast cells and mouse stem cells with much higher doses of 5-aza-C (1 or 5 μm) and also found that blastocyst development of cloned embryos were reduced. The consensus from these studies [49-51] suggests that lowering the levels of DNA methylation in donor cells does not always improve development of cloned embryos. At high concentrations, 5-aza-dC may have been cytotoxic to the donor cells. Additionally, prolonged treatment at a lower concentration, as was the case in our study, may have caused severe hypo-methylation, and resulted in disrupted expression of essential genes important for embryo development. Therefore, further experiments are required to test the effects of lower concentrations and shorter durations of 5-aza-dC treatment on donor cells.
Treating donor cells with TSA, by contrast, significantly improved development of cloned embryos. Previous reports indicated that treatment of mouse stem cells with TSA reduced development of cloned embryos [51]. The differences between these findings may be due to the variation in the concentrations of TSA used. Prior to nuclear transfer, we treated donor cells with a wide range of TSA concentrations and identified the lowest concentration capable of inducing histone hyperacetylation (1.25 μM). The lowest concentration tested (0.08 μM), did not cause hyperacetylation, but resulted in observable changes in cell morphology, similar to those described previously [65]. It was this lower concentration of TSA (0.08 μM) that improved development of cloned embryos in our study, while the higher concentration (1.25 μM) inhibited embryo development. The detrimental effect of a higher dose of TSA on embryo development may be explained by the fact that treatment of cells with high concentrations of TSA causes chromatin breaks and apoptosis [66].
Conclusion
Somatic cell cloning by nuclear transfer is a relatively new technology with many potential applications. However, at the current stage of development, the reprogramming of epigenetic inheritance by nuclear transfer is still incomplete. Further efforts and new paradigms are needed to perfect this technology and extend it to its fullest potential.
Acknowledgments
Acknowledgement
The authors would like to thank Marina Julian for careful reading and editing this manuscript.
Contributor Information
X Cindy Tian, Email: xtian@canr.uconn.edu.
Chikara Kubota, Email: CYR01275@nifty.ne.jp.
Brian Enright, Email: benright@canr.uconn.edu.
Xiangzhong Yang, Email: jyang@canr.uconn.edu.
References
- Wilmut I, Schnieke AE, McWhir J, Kind AJ, Campbell KHS. Viable offspring derived from fetal and adult mammalian cells. Nature. 1997;385:810–813. doi: 10.1038/385810a0. [DOI] [PubMed] [Google Scholar]
- Anderson GB, Seidel GE. Cloning for profit. Science. 1998;280:1400–1401. doi: 10.1126/science.280.5368.1400. [DOI] [PubMed] [Google Scholar]
- Polejaeva IA, Campbell KHS. New advances in somatic cell nuclear transfer: Application in transgenesis. Theriogenology. 2000;53:117–126. doi: 10.1016/S0093-691X(99)00245-9. [DOI] [PubMed] [Google Scholar]
- Robl J. Development and application of technology for large scale cloning of cattle. Theriogenology. 1999;51:499–508. doi: 10.1016/S0093-691X(98)00243-X. [DOI] [PubMed] [Google Scholar]
- Stice SL, Robl JM, Ponce de Leon FA, Jerry J, Golueke PG, Cibelli JB, Kane JJ. Cloning: new breakthroughs leading to commercial opportunities. Theriogenology. 1998;49:129–138. doi: 10.1016/S0093-691X(97)00407-X. [DOI] [PubMed] [Google Scholar]
- Lanza RP, Cibelli JB, West MD. Human therapeutic cloning. Nat Med. 1999;5:975–977. doi: 10.1038/12404. [DOI] [PubMed] [Google Scholar]
- Capecchi MR. How close are we to implementing gene targeting in animals other than the mouse? Proc Natl Acad Sci USA. 2000;97:956–957. doi: 10.1073/pnas.97.3.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solter D. Imprinting. Int J Dev Biol. 1998;42:951–954. [PubMed] [Google Scholar]
- De Sousa PA, Winger Q, Hill JR, Jones K, Watson AJ, Westhusin ME. Reprogramming of fibroblast nuclei after transfer into bovine oocytes. Cloning. 1999;1:63–69. doi: 10.1089/15204559950020102. [DOI] [PubMed] [Google Scholar]
- Munsie MJ, Michalska AE, O'Brien CM, Trounson AO, Pera MF, Mountford PS. Isolation of pluripotent embryonic stem cells from reprogrammed adult mouse somatic nuclei. Curr Biol. 2000;10:989–992. doi: 10.1016/S0960-9822(00)00648-5. [DOI] [PubMed] [Google Scholar]
- Surani MA. Reprogramming of genome function through epigenetic inheritance. Nature. 2001;414:122–128. doi: 10.1038/35102186. [DOI] [PubMed] [Google Scholar]
- Winger QA, Hill JR, Shin T, Watson AJ, Kraemer DC, Westhusin ME. Genetic reprogramming of lactate dehydrogenase, citrate synthase, and phosphofructokinase mRNA in bovine nuclear transfer embryos produced using bovine fibroblast cell nuclei. Mol Reprod Dev. 2000;56:458–464. doi: 10.1002/1098-2795(200008)56:4<458::AID-MRD3>3.3.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Garry FB, Adams R, McCann JP, Odde KG. Postnatal characteristics of calves produced by nuclear transfer cloning. Theriogenology. 1996;45:141–152. doi: 10.1016/0093-691X(95)00363-D. [DOI] [Google Scholar]
- Hill JR, Roussel AJ, Cibelli JB, Edwards JF, Hooper NL, Miller MW, Thompson JA, Looney CR, Westhusin ME, Robl JM, Stice SL. Clinical and pathologic features of cloned transgenic calves and fetuses (13 case studies) Theriogenology. 1999;51:1451–1465. doi: 10.1016/S0093-691X(99)00089-8. [DOI] [PubMed] [Google Scholar]
- Kato Y, Tani T, Sotomaru Y, Kurokawa K, Kato J, Doguchi H, Yasue H, Tsunoda Y. Eight calves cloned from somatic cells of a single adult. Science. 1998;282:2095–2098. doi: 10.1126/science.282.5396.2095. [DOI] [PubMed] [Google Scholar]
- Kubota C, Yamakuchi H, Todoroki J, Mizoshita K, Tabara N, Barber M, Yang X. Six cloned calves produced from adult fibroblast cells after long-term culture. Proc Natl Acad Sci USA. 2000;97:990–995. doi: 10.1073/pnas.97.3.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renard J-P, Chastnat S, Chesne P, Richard C, Marchal J, Cordonnier N, Chavatte P, Vignon X. Lymphoid hypoplasia and somatic cloning. Lancet. 1999;353:1489–1491. doi: 10.1016/S0140-6736(98)12173-6. [DOI] [PubMed] [Google Scholar]
- Walker SK, Hartwich KM, Seamark RF. The production of unusually large offspring following embryo manipulation: concepts and changes. Theriogenology. 1996;45:111–120. doi: 10.1016/0093-691X(95)00360-K. [DOI] [Google Scholar]
- Young LE, Sinclear KD, Wilmut I. Large offspring syndrome in cattle and sheep. Rev Reprod. 1998;3:155–163. doi: 10.1530/revreprod/3.3.155. [DOI] [PubMed] [Google Scholar]
- Bourc'his D, Le Bourhis D, Patin D, Niveleau A, Comizzoli P, Renard J-P, Viegas-Pe'quignot E. Delayed and incomplete reprogramming of chromosome methylation patterns in bovine cloned embryos. Curr Biol. 2001;11:1542–1546. doi: 10.1016/S0960-9822(01)00480-8. [DOI] [PubMed] [Google Scholar]
- Dean W, Santos F, Stojkovic M, Zakhartchenko V, Walter J, Wolf E, Reik W. Conservation of methylation reprogramming in mammalian development: aberrant reprogramming in cloned embryos. Proc Natl Acad Sci USA. 2001;98:13734–13738. doi: 10.1073/pnas.241522698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Y-K, Koo D-B, Park J-S, Choi Y-H, Lee K-K, Han Y-M. Aberrant methylation of donor genome in cloned bovine embryos. Nat Genet. 2001;28:173–177. doi: 10.1038/88903. [DOI] [PubMed] [Google Scholar]
- Rideout WM, III, Eggan K, Jaenisch R. Nuclear cloning and epigenetic reprogramming of the genome. Science. 2001;293:1093–1098. doi: 10.1126/science.1063206. [DOI] [PubMed] [Google Scholar]
- Gibbons J, Arat S, Rzucidlo J, Miyoshi K, Waltenburg R, Respess D, Venable A, Stice S. Enhanced survivability of cloned calves derived from roscovitine-treated adult somatic cells. Biol Reprod. 2002;66:895–900. doi: 10.1095/biolreprod66.4.895. [DOI] [PubMed] [Google Scholar]
- Kasinathan P, Knott JG, Wang Z, Jerry DJ, Robl JM. Production of calves from G1 fibroblasts. Nat Biotechnol. 2001;19:1176–1178. doi: 10.1038/nbt1201-1176. [DOI] [PubMed] [Google Scholar]
- Wells DN, Laible G, Tucker FC, Miller AL, Oliver JE, Xiang T, Forsyth JT, Berg MC, Cockrem K, L'Huillier PJ, Tervit HR, Oback B. Coordination between donor cell type and cell cycle stage improves nuclear cloning efficiency in cattle. Theriogenology. 2003;59:45–59. doi: 10.1016/S0093-691X(02)01273-6. [DOI] [PubMed] [Google Scholar]
- Campbell KH, Loi P, Cappai P, Wilmut I. Improved development to blastocyst of ovine nuclear transfer embryos reconstructed during the presumptive S-phase of enucleated activated oocytes. Biol Reprod. 1994;50:1385–1393. doi: 10.1095/biolreprod50.6.1385. [DOI] [PubMed] [Google Scholar]
- Du F, Sung L-Y, Tian XC, Yang X. Differential Cytoplast Requirement for Embryonic and Somatic Cell Nuclear Transfer in Cattle. Mol Reprod Dev. 2002;63:183–191. doi: 10.1002/mrd.10172. [DOI] [PubMed] [Google Scholar]
- Hill JR, Winger QA, Long CR, Looney CR, Thompson JA, Westhusin ME. Development rates of male bovine nuclear transfer embryos derived from adult and fetal cells. Biol Reprod. 2000;62:1135–1140. doi: 10.1095/biolreprod62.5.1135. [DOI] [PubMed] [Google Scholar]
- Kasinathan P, Knott JG, Moreira PN, Burnside AS, Jerry DJ, Robl JM. Effect of fibroblast donor cell age and cell cycle on development of bovine nuclear transfer embryos in vitro. Biol Reprod. 2001;64:1487–1493. doi: 10.1095/biolreprod64.5.1487. [DOI] [PubMed] [Google Scholar]
- Renard JP. In Proceedings of Transgenic Animals in Research Conference Proceedings of Transgenic Animal Research Conference: Aug, 1999. Tahoe City, CA; 1999. Chromatin remodeling and potential for full term development of cloned embryos; p. 15. [Google Scholar]
- Tian XC, Xu J, Yang X. Normal telomere lengths found in cloned cattle. Nat Genet. 2000;26:272–273. doi: 10.1038/81559. [DOI] [PubMed] [Google Scholar]
- Xue F, Tian XC, Kubota C, Du F, Taneja M, Dinnyes A, Dai Y, Levine H, Pereira LV, Yang X. Aberrant X-Chromosome inactivation in deceased cattle derived from somatic cloning. Nat Genet. 2002;31:216–220. doi: 10.1038/ng900. [DOI] [PubMed] [Google Scholar]
- Galli C, Duchi R, Moor RM, Lazzari G. Mammalian leukocytes contain all the genetic information necessary for the development of a new individual. Cloning. 1999;1:161–170. doi: 10.1089/15204559950019924. [DOI] [PubMed] [Google Scholar]
- Hochedlinger K, Jaenisch R. Monoclonal mice generated by nuclear transfer from mature B and T donor cells. Nature. 2002;415:1035–1038. doi: 10.1038/nature718. [DOI] [PubMed] [Google Scholar]
- Kato Y, Tani T, Tsunoda Y. Cloning of calves from various somatic cell types of male and female adult, newborn and fetal cows. J Reprod Fertil. 2000;120:231–237. doi: 10.1530/reprod/120.2.231. [DOI] [PubMed] [Google Scholar]
- Miyashita N, Shiga K, Yonai M, Kaneyama K, Kobayashi S, Kojima T, Goto Y, Kishi M, Aso H, Suzuki T, Sakaguchi M, Nagai T. Remarkable differences in telomere lengths among cloned cattle derived from different cell types. Biol Reprod. 2002;66:1649–1655. doi: 10.1095/biolreprod66.6.1649. [DOI] [PubMed] [Google Scholar]
- Ogura A, Inoue K, Ogonuki N, Noguchi A, Takano K, Nagano R, Suzuki O, Lee J, Ishino F, Matsuda J. Production of male cloned mice from fresh, cultured, and cryopreserved immature Sertoli cells. Biol Reprod. 2000;62:1579–1584. doi: 10.1095/biolreprod62.6.1579. [DOI] [PubMed] [Google Scholar]
- Shiga K, Fujita T, Hirose K, Sasae Y, Nagai T. Production of calves by transfer of nuclei from cultured somatic cells obtained from Japanese black bulls. Theriogenology. 1999;52:527–535. doi: 10.1016/S0093-691X(99)00149-1. [DOI] [PubMed] [Google Scholar]
- Arat S, Rzucidlo SJ, Gibbons J, Miyoshi K, Stice SL. Production of transgenic bovine embryos by transfer of transfected granulosa cells into enucleated oocytes. Mol Reprod Dev. 2001;60:20–26. doi: 10.1002/mrd.1057. [DOI] [PubMed] [Google Scholar]
- Liu L, Shin T, Pryor JH, Kraemer D, Westhusin M. Regenerated bovine fetal fibroblasts support high blastocyst development following nuclear transfer. Cloning. 2001;3:51–58. doi: 10.1089/15204550152475554. [DOI] [PubMed] [Google Scholar]
- Zakhartchenko V, Alberio R, Stojkovic M, Prelle K, Schernthaner W, Stojkovic P, Wenigerkind H, Wanke R, Duchler M, Steinborn R, Mueller M, Brem GE. Adult cloning in cattle: potential of nuclei from a permanent cell line and from primary cultures. Mol Reprod Dev. 1999;54:264–272. doi: 10.1002/(SICI)1098-2795(199911)54:3<264::AID-MRD7>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Amano T, Kato Y, Tsunoda Y. Full-term development of enucleated mouse oocytes fused with embryonic stem cells from different cell lines. Reproduction. 2001;121:729–733. doi: 10.1530/reprod/121.5.729. [DOI] [PubMed] [Google Scholar]
- Eggan K, Akutsu H, Loring J, Jackson-Grusby L, Klemm M, Rideout WM, 3rd, Yanagimachi R, Jaenisch R. Hybrid vigor, fetal overgrowth, and viability of mice derived by nuclear cloning and tetraploid embryo complementation. Proc Natl Acad Sci USA. 2001;98:6209–6214. doi: 10.1073/pnas.101118898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humpherys D, Eggan K, Akutsu H, Hochedlinger K, Rideout WM, 3rd, Biniszkiewicz D, Yanagimachi R, Jaenisch R. Epigenetic instability in ES cells and cloned mice. Science. 2001;293:95–97. doi: 10.1126/science.1061402. [DOI] [PubMed] [Google Scholar]
- Kato Y, Rideout III W, Hilton K, Barton SC, Tsunoda Y, Surani MA. Developmental potential of mouse primordial germ cells. Development. 1999;126:1823–1832. doi: 10.1242/dev.126.9.1823. [DOI] [PubMed] [Google Scholar]
- Wakayama T, Rodriguez I, Perry ACF, Yanagimachi R, Mombaerts P. Mice cloned from embryonic stem cells. Proc Natl Acad Sci USA. 1999;96:14984–14989. doi: 10.1073/pnas.96.26.14984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Jouneau A, Brochard V, Adenot P, Renard JP. Developmental potential of mouse embryos reconstructed from metaphase embryonic stem cell nuclei. Biol Reprod. 2001;65:412–419. doi: 10.1093/biolreprod/65.2.412. [DOI] [PubMed] [Google Scholar]
- Enright BP, Kubota C, Yang X, Tian XC. Epigenetic characteristics and development of embryos cloned from donor cells treated by Trichostatin A or 5-aza-2'-deoxycytidine. Biol Reprod. 2003;69:896–903. doi: 10.1095/biolreprod.103.017954. [DOI] [PubMed] [Google Scholar]
- Jones KL, Hill J, Shin TY, Lui L, Westhusin M. DNA hypomethylation of karyoplasts for bovine nuclear transplantation. Mol Reprod Dev. 2001;60:208–213. doi: 10.1002/mrd.1079. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Baquir S, Brochard V, Smith LC, Renard JP. Donor nuclei are not well reprogrammed by nuclear transfer procedure. Biol Reprod. 2002;66:237–238. (s345) [Google Scholar]
- Cibelli P, Stice SL, Golueke PJ, Kane JJ, Jerry J, Blackwell C, deLeon FAP, Robl JM. Cloned transgenic calves produced from non-quiescent fetal fibroblasts. Science. 1998;280:1256–1258. doi: 10.1126/science.280.5367.1256. [DOI] [PubMed] [Google Scholar]
- Zakhartchenko V, Durcova-Hills G, Stojkovic M, Schernthaner W, Prelle K, Steinborn R, Muller M, Brem G, Wolf E. Effects of serum starvation and re-cloning on the efficiency of nuclear transfer using bovine fetal fibroblasts. J Reprod Fertil. 1999;115:325–331. doi: 10.1530/jrf.0.1150325. [DOI] [PubMed] [Google Scholar]
- Roh S, Shim H, Hwang WS, Yoon JT. In vitro development of green fluorescent protein (GFP) transgenic bovine embryos after nuclear transfer using different cell cycles and passages of fetal fibroblasts. Reprod Fertil Dev. 2000;12:1–6. doi: 10.1071/RD00021. [DOI] [PubMed] [Google Scholar]
- Cho JK, Lee BC, Park JI, Lim JM, Shin SJ, Kim KY, Lee BD, Hwang WS. Development of bovine oocytes reconstructed with different donor somatic cells with or without serum starvation. Theriogenology. 2002;57:1819–1828. doi: 10.1016/S0093-691X(01)00699-9. [DOI] [PubMed] [Google Scholar]
- Wakayama T, Yanagimachi R. Mouse cloning with nucleus donor cells of different age and type. Mol Reprod Dev. 2001;58:376–383. doi: 10.1002/1098-2795(20010401)58:4<376::AID-MRD4>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Wakayama T, Perry AC, Zuccotti M, Johnson KR, Yanagimachi R. Full-term development of mice from enucleated oocytes injected with cumulus cell nuclei. Nature. 1998;394:369–374. doi: 10.1038/28615. [DOI] [PubMed] [Google Scholar]
- Forsberg EJ, Strelchenko NS, Augenstein ML, Betthauser JM, Childs LA, Eilertsen KJ, Enos JM, Forsythe TM, Golueke PJ, Koppang RW, Lange G, Lesmeister TL, Mallon KS, Mell GD, Misica PM, Pace MM, Pfister-Genskow M, Voelker GR, Watt SR, Bishop MD. Production of cloned cattle from in vitro systems. Biol Reprod. 2002;67:327–333. doi: 10.1095/biolreprod67.1.327. [DOI] [PubMed] [Google Scholar]
- Enright BP, Jeong BS, Yang X, Tian XC. Epigenetic Characteristics of Bovine Donor Cells for Nuclear Transfer: Levels of Histone Acetylation. Biol Reprod. 2003. [DOI] [PubMed]
- Hirayu H, Dere WH. Rapoport B. Initiation of normal thyroid cells in primary culture associated with enhanced c-myc messenger ribonucleic acid levels. Endocrinology. 1987;120:924–928. doi: 10.1210/endo-120-3-924. [DOI] [PubMed] [Google Scholar]
- Baker TK, Carfagna MA, Gao H, Dow ER, Li Q, Searfoss GH, Ryan TP. Temporal gene expression analysis of monolayer cultured rat hepatocytes. Chem Res Toxicol. 2001;14:1218–1231. doi: 10.1021/tx015518a. [DOI] [PubMed] [Google Scholar]
- Hill JR, Winger QA, Burghardt RC, Westhusin ME. Bovine nuclear transfer embryo development using cells derived from a cloned fetus. Anim Reprod Sci. 2001;67:17–26. doi: 10.1016/S0378-4320(01)00106-3. [DOI] [PubMed] [Google Scholar]
- Mayer W, Niveleau A, Walter J, Fundele R, Haaf T. Demethylation of the zygotic paternal genome. Nature. 2000;403:501–502. doi: 10.1038/35000656. [DOI] [PubMed] [Google Scholar]
- Oswald J, Engemann S, Lane N, Mayer W, Olek A, Fundele R, Dean W, Reik W, Walter J. Active demethylation of the paternal genome in the mouse zygote. Curr Biol. 2000;10:475–478. doi: 10.1016/S0960-9822(00)00448-6. [DOI] [PubMed] [Google Scholar]
- Hoshikawa Y, Kwon HJ, Yoshida M, Horinouchi S, Beppu T. Trichostatin A induces morphological changes and gelsolin expression by inhibiting histone deacetylase in human carcinoma cell lines. Exp Cell Res. 1994;214:189–197. doi: 10.1006/excr.1994.1248. [DOI] [PubMed] [Google Scholar]
- Nakajima H, Kim YB, Terano H, Yoshida M, Horinouchi S. FR90 a Potent Antitumor Antibiotic, Is a Novel Histone deacetylase inhibitor. Expt Cell Res. 1228;241:126–133. doi: 10.1006/excr.1998.4027. [DOI] [PubMed] [Google Scholar]