Abstract
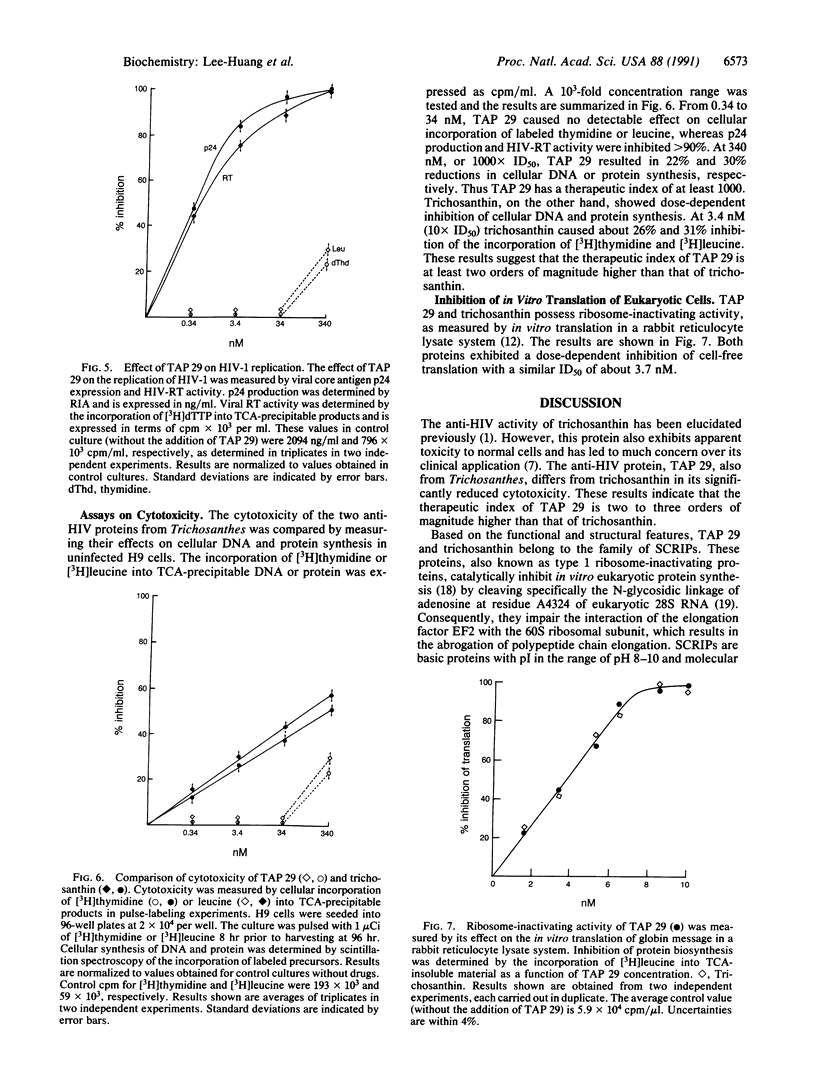
An anti-human immunodeficiency virus (anti-HIV) protein capable of inhibiting HIV-1 infection and replication has been isolated and purified to homogeneity from Trichosanthes kirilowii. This protein, TAP 29 (Trichosanthes anti-HIV protein, 29 kDa), is distinct from trichosanthin [also known as GLQ 223 (26 kDa)] in size, N-terminal amino acid sequence, and cytotoxicity. In addition to three conservative substitutions--namely, Arg-29 to Lys, Ile-37 to Val, and Pro-42 to Ser--a total difference of residues 12-16 was found. TAP 29 yielded -Lys-Lys-Lys-Val-Tyr-, whereas trichosanthin has -Ser-Ser-Tyr-Gly-Val-. Although the two proteins exhibit similar anti-HIV activity, as measured by syncytium formation, p24 expression, and HIV reverse transcriptase activity, they differ significantly in cytotoxicity, as measured by their effects on cellular DNA and protein syntheses. At the dose level of the bioassays, 0.34-340 nM, trichosanthin demonstrates a dose-dependent toxic effect on host cells. TAP 29 displays no toxic effect, even at 100 X ID50, whereas trichosanthin demonstrates 38% and 44% inhibition on cellular DNA and protein synthesis, respectively. These results indicate that the therapeutic index of TAP 29 is at least two orders of magnitude higher than that of trichosanthin. Thus TAP 29 may offer a broader safe dose range in the treatment of AIDS.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbieri L., Aron G. M., Irvin J. D., Stirpe F. Purification and partial characterization of another form of the antiviral protein from the seeds of Phytolacca americana L. (pokeweed). Biochem J. 1982 Apr 1;203(1):55–59. doi: 10.1042/bj2030055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbieri L., Zamboni M., Lorenzoni E., Montanaro L., Sperti S., Stirpe F. Inhibition of protein synthesis in vitro by proteins from the seeds of Momordica charantia (bitter pear melon). Biochem J. 1980 Feb 15;186(2):443–452. doi: 10.1042/bj1860443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan W. Y., Tam P. P., Yeung H. W. The termination of early pregnancy in the mouse by beta-momorcharin. Contraception. 1984 Jan;29(1):91–100. doi: 10.1016/0010-7824(84)90062-3. [DOI] [PubMed] [Google Scholar]
- Chow T. P., Feldman R. A., Lovett M., Piatak M. Isolation and DNA sequence of a gene encoding alpha-trichosanthin, a type I ribosome-inactivating protein. J Biol Chem. 1990 May 25;265(15):8670–8674. [PubMed] [Google Scholar]
- Collins E. J., Robertus J. D., LoPresti M., Stone K. L., Williams K. R., Wu P., Hwang K., Piatak M. Primary amino acid sequence of alpha-trichosanthin and molecular models for abrin A-chain and alpha-trichosanthin. J Biol Chem. 1990 May 25;265(15):8665–8669. [PubMed] [Google Scholar]
- Endo Y., Tsurugi K. RNA N-glycosidase activity of ricin A-chain. Mechanism of action of the toxic lectin ricin on eukaryotic ribosomes. J Biol Chem. 1987 Jun 15;262(17):8128–8130. [PubMed] [Google Scholar]
- Hoffman A. D., Banapour B., Levy J. A. Characterization of the AIDS-associated retrovirus reverse transcriptase and optimal conditions for its detection in virions. Virology. 1985 Dec;147(2):326–335. doi: 10.1016/0042-6822(85)90135-7. [DOI] [PubMed] [Google Scholar]
- Irvin J. D., Kelly T., Robertus J. D. Purification and properties of a second antiviral protein from Phytolacca americana which inactivates eukaryotic ribosomes. Arch Biochem Biophys. 1980 Apr 1;200(2):418–425. doi: 10.1016/0003-9861(80)90372-0. [DOI] [PubMed] [Google Scholar]
- Irvin J. D. Purification and partial characterization of the antiviral protein from Phytolacca americana which inhibits eukaryotic protein synthesis. Arch Biochem Biophys. 1975 Aug;169(2):522–528. doi: 10.1016/0003-9861(75)90195-2. [DOI] [PubMed] [Google Scholar]
- Jiménez A., Vázquez D. Plant and fungal protein and glycoprotein toxins inhibiting eukaryote protein synthesis. Annu Rev Microbiol. 1985;39:649–672. doi: 10.1146/annurev.mi.39.100185.003245. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee-Huang S., Huang P. L., Nara P. L., Chen H. C., Kung H. F., Huang P., Huang H. I., Huang P. L. MAP 30: a new inhibitor of HIV-1 infection and replication. FEBS Lett. 1990 Oct 15;272(1-2):12–18. doi: 10.1016/0014-5793(90)80438-o. [DOI] [PubMed] [Google Scholar]
- Maraganore J. M., Joseph M., Bailey M. C. Purification and characterization of trichosanthin. Homology to the ricin A chain and implications as to mechanism of abortifacient activity. J Biol Chem. 1987 Aug 25;262(24):11628–11633. [PubMed] [Google Scholar]
- McGrath M. S., Hwang K. M., Caldwell S. E., Gaston I., Luk K. C., Wu P., Ng V. L., Crowe S., Daniels J., Marsh J. GLQ223: an inhibitor of human immunodeficiency virus replication in acutely and chronically infected cells of lymphocyte and mononuclear phagocyte lineage. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2844–2848. doi: 10.1073/pnas.86.8.2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nara P. L., Fischinger P. J. Quantitative infectivity assay for HIV-1 and-2. Nature. 1988 Mar 31;332(6163):469–470. doi: 10.1038/332469a0. [DOI] [PubMed] [Google Scholar]
- Nara P. L., Hatch W. C., Dunlop N. M., Robey W. G., Arthur L. O., Gonda M. A., Fischinger P. J. Simple, rapid, quantitative, syncytium-forming microassay for the detection of human immunodeficiency virus neutralizing antibody. AIDS Res Hum Retroviruses. 1987 Fall;3(3):283–302. doi: 10.1089/aid.1987.3.283. [DOI] [PubMed] [Google Scholar]
- Palca J. Trials and tribulations of AIDS drug testing. Science. 1990 Mar 23;247(4949 Pt 1):1406–1406. doi: 10.1126/science.247.4949.1406. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Stirpe F., Barbieri L. Ribosome-inactivating proteins up to date. FEBS Lett. 1986 Jan 20;195(1-2):1–8. doi: 10.1016/0014-5793(86)80118-1. [DOI] [PubMed] [Google Scholar]
- Stirpe F., Williams D. G., Onyon L. J., Legg R. F., Stevens W. A. Dianthins, ribosome-damaging proteins with anti-viral properties from Dianthus caryophyllus L. (carnation). Biochem J. 1981 May 1;195(2):399–405. doi: 10.1042/bj1950399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarling J. M., Moran P. A., Haffar O., Sias J., Richman D. D., Spina C. A., Myers D. E., Kuebelbeck V., Ledbetter J. A., Uckun F. M. Inhibition of HIV replication by pokeweed antiviral protein targeted to CD4+ cells by monoclonal antibodies. Nature. 1990 Sep 6;347(6288):92–95. doi: 10.1038/347092a0. [DOI] [PubMed] [Google Scholar]
- Zhang X. J., Wang J. H. Homology of trichosanthin and ricin A chain. 1986 May 29-Jun 4Nature. 321(6069):477–478. doi: 10.1038/321477b0. [DOI] [PubMed] [Google Scholar]