Abstract
Introduction
Gestational age is estimated by ultrasound using fetal size as a proxy for age, although variance in early growth affects reliability. The aim of this study was to identify characteristics associated with discrepancies between last menstrual period‐based (EDD‐LMP) and ultrasound‐based (EDD‐US) estimated delivery dates.
Material and methods
We identified all singleton births (n = 1 201 679) recorded in the Swedish Medical Birth Register in 1995–2010, to assess the association between maternal/fetal characteristics and large negative and large positive discrepancies (EDD‐LMP earlier than EDD‐US and 10th percentile in the discrepancy distribution vs. EDD‐LMP later than EDD‐US and 90th percentile). Analyses were adjusted for age, parity, height, body mass index, smoking, and employment status.
Results
Women with a body mass index >40 kg/m2 had the highest odds for large negative discrepancies (−9 to −20 days) [odds ratio (OR) 2.16, 95% CI 2.01–2.33]. Other factors associated with large negative discrepancies were: diabetes, young maternal age, multiparity, body mass index between 30 and 39.9 kg/m2 or <18.5 kg/m2, a history of gestational diabetes, female fetus, shorter stature (<−1 SD), a history of preeclampsia, smoking or snuff use, and unemployment. Large positive discrepancies (+4 to +20 days) were associated with male fetus (OR 1.80, 95% CI 1.77–1.83), age ≥30 years, multiparity, not living with a partner, taller stature (>+1 SD), and unemployment.
Conclusions
Several maternal and fetal characteristics were associated with discrepancies between dating methods. Systematic associations of discrepancies with maternal height, fetal sex, and partly obesity, may reflect an influence on the precision of the ultrasound estimate due to variance in early growth.
Keywords: Pregnancy dating, pregnancy, gestational age, ultrasonography, prenatal, menstruation, female, humans
Abbreviations
- BMI
body mass index
- CI
confidence interval
- EDD
estimated delivery date
- GA
gestational age
- LMP
last menstrual period
- OR
odds ratio
- US
ultrasound
Key Message.
Variance in early fetal growth can bias ultrasound‐based pregnancy dating. Maternal obesity, height, and fetal sex may partly explain large discrepancies between pregnancy‐dating methods.
Introduction
The method of estimating gestational age (GA) by ultrasound (US), using fetal size as a proxy for age, is usually more precise than the method based on the last menstrual period (LMP). However, there is variance in early growth, which may affect the reliability of the US‐based estimate that is used for clinical decision‐making 1, 2. For example, early discrepancies between expected and actual fetal size can be detected in assisted reproductive technology pregnancies and are associated with maternal obesity and the birth of small‐for‐gestational‐age infants 1. In addition, fetal sex, by association with size, affects the accuracy of US pregnancy dating. Male fetuses have on average a 1‐mm larger biparietal diameter early in the second trimester, corresponding to about one day in growth 2.
Average differences between LMP‐ and US‐based estimated delivery dates (EDD) are small, with LMP estimates of one to three days more advanced pregnancy length than US estimates 3. Nevertheless, the choice of method affects the rates of preterm and post‐term deliveries, as well as neonatal outcomes, which is probably related to correction of EDD in pregnancies with large discrepancies between dating methods 4, 5.
Maternal and fetal characteristics associated with discrepancies between EDD‐LMP and EDD‐US estimates include maternal age, parity, smoking, obesity, diabetes, maternal height, educational level, fetal sex, malformations, and small‐for‐gestational‐age infants 1, 6, 7, 8, 9. Large discrepancies between methods have clinical implications through association with adverse outcomes 6, 10.
Because mean differences in early fetal growth are small, and adverse outcomes are thought to occur predominantly in pregnancies with a large deviation from average growth, the aim of this study was to identify characteristics, present at the time of pregnancy dating, associated with large discrepancies between EDD‐LMP and EDD‐US in a population‐based sample of Swedish pregnancies.
Material and methods
Data were obtained from the Swedish Medical Birth Register and the Swedish Patient Register. We included all singleton births in Sweden from 1995 to 2010, with valid documentation of EDD based on both LMP and US. To avoid data entry errors, and larger discrepancies because of reasons other than variation in early growth, pregnancies with discrepancies >20 days were excluded. The Medical Birth Register contains information on more than 99% of all births in Sweden since 1973, including maternal sociodemographic characteristics and prospectively collected information during pregnancy, delivery, and the neonatal period 11. The register has been found reliable for research purposes, with good internal validity 12. Since 1990, US scanning has been offered to all pregnant women with more than 95% acceptance. Since 1995, nearly all clinics have used EDD‐US 11.
Variables from the Medical Birth Register included maternal age, parity, height, weight, smoking and snuff (smokeless tobacco) use at first antenatal visit, family situation, occupational status, LMP, EDD‐LMP, EDD‐US, and infant sex (Table 1).
Table 1.
Correlates of large discrepancies between pregnancy dating methods. Odds ratios (OR) with 95% confidence intervals (95% CI) for large negative discrepancy (below the 10th percentile in the discrepancy distribution; −9 to −20 days) and large positive discrepancy (above the 90th percentile in the discrepancy distribution; +4 to +20 days) between EDD‐LMP and EDD‐USa in association with maternal characteristics at the first antenatal visit, fetal sex, and maternal medical history variables
| Large negative discrepancies | Large positive discrepancies | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Neg (%)b | Ref (%)c | cOR | 95% CI | aORe | 95% CI | n | Pos (%)d | Ref (%)c | cOR | 95% CI | aORe | 95% CI | |
| Age at delivery (years) | ||||||||||||||
| <20 | 18 662 | 5 | 3 | 1.84 | 1.78–1.90 | 1.79 | 1.71–1.86 | 15 712 | 3 | 3 | 1.14 | 1.09–1.19 | 1.05 | 1.00–1.11 |
| 20–24 | 109 111 | 24 | 16 | 1.42 | 1.40–1.44 | 1.40 | 1.37–1.43 | 96 840 | 14 | 16 | 1.00 | 0.98–1.03 | 1.00 | 0.97–1.02 |
| 25–29 | 226 304 | 38 | 35 | 1.00 | – | 1.00 | – | 217 512 | 32 | 35 | 1.00 | – | 1.00 | – |
| 30–34 | 198 848 | 25 | 33 | 0.71 | 0.69–0.72 | 0.68 | 0.67–0.70 | 206 725 | 33 | 33 | 1.11 | 1.09–1.13 | 1.12 | 1.10–1.14 |
| 35–39 | 73 707 | 7 | 13 | 0.54 | 0.53–0.56 | 0.49 | 0.48–0.50 | 81 893 | 15 | 13 | 1.30 | 1.28–1.33 | 1.29 | 1.26–1.32 |
| >40 | 10 300 | 1 | 2 | 0.46 | 0.43–0.49 | 0.38 | 0.35–0.40 | 12 211 | 3 | 2 | 1.60 | 1.53–1.67 | 1.57 | 1.50–1.65 |
| Total | 636 932 | 100 | 100 | 630 893 | 100 | 100 | ||||||||
| Height (cm) | ||||||||||||||
| <160 | 119 842 | 22 | 18 | 1.22 | 1.21–1.24 | 1.17 | 1.15–1.20 | 113 261 | 17 | 18 | 0.98 | 0.96–1.00 | 0.96 | 0.94–0.98 |
| 160–172f | 412 279 | 64 | 65 | 1.00 | – | 1.00 | – | 407 312 | 63 | 65 | 1.00 | – | 1.00 | – |
| >172 | 104 811 | 14 | 17 | 0.82 | 0.81–0.84 | 0.86 | 0.84–0.88 | 110 320 | 20 | 17 | 1.17 | 1.15–1.19 | 1.19 | 1.17–1.21 |
| Total | 636 932 | 100 | 100 | 630 893 | 100 | 100 | ||||||||
| BMI classes according to WHO classification | ||||||||||||||
| Underweight | 15 836 | 3 | 2 | 1.36 | 1.31–1.42 | 1.18 | 1.13–1.24 | 15 049 | 3 | 2 | 1.03 | 0.98–1.07 | 1.03 | 0.98–1.09 |
| Normal range | 402 285 | 60 | 64 | 1.00 | – | 1.00 | – | 404 877 | 65 | 64 | 1.00 | – | 1.00 | – |
| Pre‐obese | 153 378 | 24 | 24 | 1.08 | 1.06–1.09 | 1.07 | 1.05–1.08 | 151 804 | 24 | 24 | 0.98 | 0.97–1.00 | 0.97 | 0.95–0.98 |
| Obese class I | 47 165 | 9 | 7 | 1.39 | 1.36–1.42 | 1.34 | 1.30–1.37 | 43 689 | 7 | 7 | 0.91 | 0.89–0.94 | 0.90 | 0.87–0.92 |
| Obese class II | 13 860 | 3 | 2 | 1.74 | 1.68–1.81 | 1.70 | 1.63–1.78 | 11 950 | 2 | 2 | 0.83 | 0.79–0.87 | 0.80 | 0.76–0.85 |
| Obese class III | 4408 | 1 | 1 | 2.18 | 2.05–2.33 | 2.16 | 2.01–2.33 | 3524 | 1 | 1 | 0.78 | 0.71–0.86 | 0.74 | 0.66–0.82 |
| Total | 636 932 | 100 | 100 | 630 893 | 100 | 100 | ||||||||
| Living with partner | ||||||||||||||
| Yes | 599 095 | 93 | 94 | 1.00 | – | 1.00 | – | 593 478 | 93 | 94 | 1.00 | – | 1.00 | – |
| No | 30 259 | 6 | 5 | 1.23 | 1.20–1.27 | 0.99 | 0.95–1.02 | 30 030 | 6 | 5 | 1.25 | 1.22–1.29 | 1.21 | 1.17–1.25 |
| Missing | 7578 | 1 | 1 | 7385 | 1 | 1 | ||||||||
| Total | 636 932 | 100 | 100 | 630 893 | 100 | 100 | ||||||||
| Employed | ||||||||||||||
| Yes | 461 462 | 68 | 74 | 1.00 | – | 1.00 | – | 461 500 | 71 | 74 | 1.00 | – | 1.00 | – |
| No | 113 304 | 21 | 17 | 1.32 | 1.30–1.34 | 1.09 | 1.07–1.11 | 109 636 | 19 | 17 | 1.12 | 1.10–1.14 | 1.16 | 1.14–1.19 |
| Missing | 62 166 | 12 | 9 | 59 757 | 10 | 9 | ||||||||
| Total | 636 932 | 100 | 100 | 630 893 | 100 | 100 | ||||||||
| Smoking at first antenatal visit | ||||||||||||||
| No | 568 365 | 87 | 90 | 1.00 | – | 1.00 | – | 565 880 | 89 | 90 | 1.00 | – | 1.00 | – |
| Yes | 59 502 | 12 | 9 | 1.35 | 1.33–1.38 | 1.13 | 1.11–1.16 | 56 077 | 9 | 9 | 1.04 | 1.02–1.07 | 1.04 | 1.02–1.07 |
| Missing | 9065 | 2 | 1 | 8936 | 1 | 1 | ||||||||
| Total | 636 932 | 100 | 100 | 630 893 | 100 | 100 | ||||||||
| Snuff use at first antenatal visitg | ||||||||||||||
| No | 483 073 | 73 | 77 | 1.00 | – | 1.00 | – | 481 672 | 75 | 77 | 1.00 | – | 1.00 | – |
| Yes | 5844 | 1 | 1 | 1.16 | 1.09–1.24 | 1.12 | 1.05–1.20 | 5670 | 1 | 1 | 1.01 | 0.94–1.08 | 1.01 | 0.94–1.09 |
| Missing | 148 015 | 26 | 23 | 143 551 | 24 | 23 | ||||||||
| Total | 636 932 | 100 | 100 | 630 893 | 100 | 100 | ||||||||
| Parity | ||||||||||||||
| 0‐parous | 280 971 | 45 | 44 | 1.00 | – | 1.00 | – | 276 029 | 43 | 44 | 1.00 | – | 1.00 | – |
| 1‐parous | 236 861 | 36 | 38 | 0.92 | 0.91–0.93 | 1.05 | 1.03–1.07 | 233 995 | 35 | 38 | 0.94 | 0.93–0.96 | 0.91 | 0.89–0.92 |
| 2‐parous | 85 121 | 13 | 13 | 0.94 | 0.92–0.95 | 1.20 | 1.17–1.24 | 86 219 | 15 | 13 | 1.10 | 1.08–1.12 | 1.02 | 0.99–1.04 |
| 3‐parous | 22 807 | 4 | 4 | 1.04 | 1.00–1.07 | 1.40 | 1.34–1.46 | 23 246 | 4 | 4 | 1.25 | 1.21–1.29 | 1.13 | 1.08–1.17 |
| 4‐parous or more | 11 172 | 2 | 2 | 1.24 | 1.19–1.30 | 1.77 | 1.67–1.87 | 11 404 | 3 | 2 | 1.49 | 1.43–1.56 | 1.29 | 1.23–1.37 |
| Total | 636 932 | 100 | 100 | 630 893 | 100 | 100 | ||||||||
| Fetal sex | ||||||||||||||
| Male | 319 057 | 44 | 52 | 1.00 | – | 1.00 | – | 340 140 | 65 | 52 | 1.76 | 1.74–1.78 | 1.80 | 1.77–1.83 |
| Female | 317 875 | 56 | 49 | 1.34 | 1.33–1.36 | 1.36 | 1.34–1.38 | 290 753 | 35 | 49 | 1.00 | – | 1.00 | – |
| Total | 636 932 | 100 | 100 | 630 893 | 100 | 100 | ||||||||
| Medical history variablesh | ||||||||||||||
| Diabetes mellitus | ||||||||||||||
| No | 635 613 | 100 | 100 | 1.00 | – | 1.00 | – | 629 860 | 100 | 100 | 1.00 | – | 1.00 | – |
| Yes | 1319 | 0 | 0 | 2.15 | 1.91–2.41 | 1.95 | 1.69–2.25 | 1033 | 0 | 0 | 0.78 | 0.65–0.92 | 0.86 | 0.70–1.04 |
| Total | 636 932 | 100 | 100 | 630 893 | 100 | 100 | ||||||||
| Gestational diabetes in a former pregnancyi | ||||||||||||||
| No | 353 236 | 99 | 99 | 1.00 | – | 1.00 | – | 353 236 | 99 | 100 | 1.00 | – | 1.00 | – |
| Yes | 2725 | 1 | 1 | 1.61 | 1.48–1.75 | 1.47 | 1.38–1.63 | 2725 | 1 | 0 | 0.75 | 0.67–0.85 | 0.74 | 0.65–0.84 |
| Total | 355 961 | 100 | 100 | 355 961 | 100 | 100 | ||||||||
| Hypertensive disorders | ||||||||||||||
| No | 636 565 | 100 | 100 | 1.00 | – | 1.00 | – | 630 560 | 100 | 100 | 1.00 | – | 1.00 | – |
| Yes | 367 | 0 | 0 | 1.37 | 1.08–1.74 | 1.26 | 0.94–1.68 | 333 | 0 | 0 | 0.88 | 0.66–1.18 | 0.76 | 0.54–1.07 |
| Total | 636 932 | 100 | 100 | 630 893 | 100 | 100 | ||||||||
| Preeclampsia in a former pregnancyi | ||||||||||||||
| No | 345 235 | 96 | 97 | 1.00 | – | 1.00 | – | 345 235 | 98 | 97 | 1.00 | – | 1.00 | – |
| Yes | 10 726 | 4 | 3 | 1.22 | 1.17–1.28 | 1.16 | 1.09–1.23 | 10 726 | 2 | 3 | 0.86 | 0.82–0.91 | 0.92 | 0.86–0.98 |
| Total | 355 961 | 100 | 100 | 355 961 | 100 | 100 | ||||||||
| Hypothyroidism | ||||||||||||||
| No | 635 635 | 100 | 100 | 1.00 | – | 1.00 | – | 629 595 | 100 | 100 | 1.00 | – | 1.00 | – |
| Yes | 1297 | 0 | 0 | 0.91 | 0.78–1.05 | 0.97 | 0.82–1.14 | 1298 | 0 | 0 | 0.96 | 0.83–1.11 | 0.85 | 0.72–1.01 |
| Total | 636 932 | 100 | 100 | 630 893 | 100 | 100 | ||||||||
| Systematic lupus erythematosus | ||||||||||||||
| No | 636 720 | 100 | 100 | 1.00 | – | 1.00 | – | 630 695 | 100 | 100 | 1.00 | – | 1.00 | – |
| Yes | 212 | 0 | 0 | 1.17 | 0.84–1.63 | 1.24 | 0.83–1.84 | 198 | 0 | 0 | 0.85 | 0.58–1.25 | 0.79 | 0.50–1.24 |
| Total | 636 932 | 100 | 100 | 630 893 | 100 | 100 | ||||||||
| Ulcerative colitis | ||||||||||||||
| No | 636 163 | 100 | 100 | 1.00 | – | 1.00 | – | 630 101 | 100 | 100 | 1.00 | – | 1.00 | – |
| Yes | 769 | 0 | 0 | 0.76 | 0.62–0.92 | 0.82 | 0.64–1.04 | 792 | 0 | 0 | 0.96 | 0.80–1.15 | 1.09 | 0.89–1.34 |
| Total | 636 932 | 100 | 100 | 630 893 | 100 | 100 | ||||||||
| Crohn's disease | ||||||||||||||
| No | 636 174 | 100 | 100 | 1.00 | – | 1.00 | – | 630 124 | 100 | 100 | 1.00 | – | 1.00 | – |
| Yes | 758 | 0 | 0 | 0.92 | 0.77–1.11 | 0.92 | 0.73–1.14 | 769 | 0 | 0 | 1.05 | 0.88–1.26 | 1.17 | 0.96–1.44 |
| Total | 636 932 | 100 | 100 | 630 893 | 100 | 100 | ||||||||
| Depressive disorders | ||||||||||||||
| No | 634 329 | 100 | 100 | 1.00 | – | 1.00 | – | 628 468 | 100 | 100 | 1.00 | – | 1.00 | – |
| Yes | 2603 | 1 | 0 | 1.26 | 1.15–1.38 | 1.09 | 0.98–1.21 | 2425 | 0 | 0 | 0.92 | 0.83–1.03 | 0.91 | 0.81–1.03 |
| Total | 636 932 | 100 | 100 | 630 893 | 100 | 100 | ||||||||
| Anxiety disorders and phobias | ||||||||||||||
| No | 635 326 | 100 | 100 | 1.00 | – | 1.00 | – | 629 406 | 100 | 100 | 1.00 | – | 1.00 | – |
| Yes | 1606 | 0 | 0 | 1.30 | 1.16–1.47 | 1.14 | 1.00–1.30 | 1487 | 0 | 0 | 0.93 | 0.81–1.07 | 0.92 | 0.79–1.07 |
| Total | 636 932 | 100 | 100 | 630 893 | 100 | 100 | ||||||||
Estimated due date based on the date of the last menstrual period (EDD‐LMP) and estimated due date based ultrasound biometry (EDD‐US).
Pregnancies with negative discrepancy below the 10th percentile (−9 to −20 days), n = 119 275 births.
Reference category is median discrepancy ±2 days (−1 to +3 days), n = 517 657 births.
Pregnancies with positive discrepancy above the 90th percentile (+4 to +20 days), n = 113 236 births.
Adjusted, as appropriate, for age, parity, height, BMI, smoking at first antenatal visit, and employment status.
Mean ± 1 SD.
Variable available for analyses for births in years 2000–2010.
Inpatient diagnoses registered at least once from 5 years to 9 months before registered birth.
Analyses excluding 0‐parous women.
[Correction added on 23 December 2016 after online publication: In Table 1, the values of Male and Female Fetal Sex under Large positive discrepancies have been corrected.]
Maternal height was defined as shorter stature if height was below 1 standard deviation (SD) from the mean, and taller stature was defined as above 1 SD from the mean. Maternal body mass index (BMI) was calculated based on height and weight, and categorized according to the World Health Organization classification as underweight <18.5 kg/m2, normal weight 18.5–24.9 kg/m2, pre‐obesity 25.0–29.9 kg/m2, obesity class I (30.0–34.9 kg/m2), obesity class II (35.0–39.9 kg/m2), and obesity class III (>40 kg/m2) 13.
Information on maternal diagnoses, from five years before until nine months (EDD‐US minus 280 days) before delivery, was collected from the Swedish Patient Register. In the inpatient setting, diagnoses were included if they appeared once. In the outpatient setting, diagnoses would have to be registered twice to avoid any preliminary diagnoses. Inpatient diagnoses were retrieved for the whole study period, and outpatient diagnoses were retrieved from 2001. Diagnoses that theoretically could affect early fetal growth were chosen, and included diabetes, gestational diabetes in a former pregnancy, hypertensive disorders, preeclampsia in a former pregnancy, hypothyroidism, systemic lupus erythematosus, inflammatory bowel disease (Crohn's disease and ulcerative colitis), depressive‐affective disorders, and phobias and anxiety disorders.
Discrepancy was defined as EDD‐LMP minus EDD‐US, corresponding to the difference in days between the two pregnancy‐dating methods as recorded in the Medical Birth Register. Negative discrepancy corresponds to EDD‐LMP earlier than EDD‐US, reflecting a more advanced GA by LMP estimation than by US estimation, i.e. the fetus was smaller than expected when dated by US and EDD was postponed. Positive discrepancy was defined as EDD‐LMP later than EDD‐US, corresponding to a less advanced GA by LMP‐estimation than the GA estimated by US‐examination, i.e. the fetus was larger than expected when dated by US and EDD was moved to an earlier date.
Discrepancies of interest were defined as those below the 10th percentile (large negative discrepancy) and above the 90th percentile (large positive discrepancy) in the discrepancy distribution, and the reference category was defined as discrepancies within 2 days of the median. Remaining pregnancies, not corresponding to the large discrepancy categories or the reference category, were excluded from further analyses. Analyses were conducted with the purpose of assessing to what extent maternal characteristics, disease, and fetal sex were associated with large discrepancies in days between EDD‐LMP and EDD‐US. Multiple logistic regression analyses were used to calculate odds ratios (OR) and 95% confidence intervals (95% CI) for large positive or negative discrepancy, in association with maternal characteristics, disease, and fetal sex.
Analyses were restricted to births with maternal height 140–189 cm and maternal weight 35–139 kg, to reduce data entry errors. Analyses of diagnoses in former pregnancies were restricted to parous women. All models were adjusted for age, parity, height, BMI, smoking in early pregnancy, and employment status, as appropriate. Analyses were further adjusted for maternal comorbidity, which was a composite variable defined as the woman having one of the selected diagnoses recorded at least once. Statistical analyses were conducted using the statistical software R version 3.1.3, R Core Team (2015).
The Regional Ethical Review Board in Uppsala, Sweden, approved the study protocol reference number 2012/412, 19 December 2012.
Results
The total number of births in the study population was 1 201 679. Mean maternal age was 30 years (SD = 5 years), mean height was 166 cm (SD = 6 cm), and mean BMI was 24 kg/m2 (SD = 4 kg/m2). Most commonly, there was no (16%) or only a few days' discrepancy between EDD‐LMP and EDD‐US (Figure 1). Mean discrepancy was +2 days, and the median discrepancy was +1 day. A large negative discrepancy, below the 10th percentile, corresponded to −9 to −20 days, whereas a large positive discrepancy, above the 90th percentile, corresponded to +4 to +20 days. Information on maternal height or weight was missing in 101 630 births (8.5%). A total of 1 100 049 births had valid documentation for maternal height and weight: large negative discrepancies (n = 119 275), reference category (discrepancy −1 to +3 days, n = 517 657), and large positive discrepancies (n = 113 236).
Figure 1.
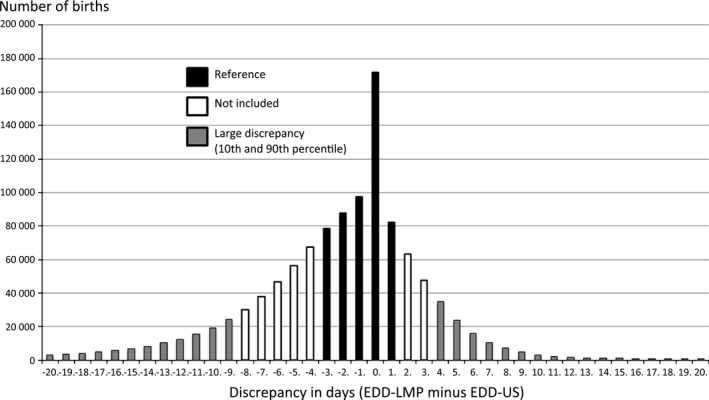
Number of births and discrepancy in days between ultrasound (US)‐based and last menstrual period (LMP)‐based estimated delivery date (EDD).
For large negative discrepancies, the strongest association was observed for obesity class III (OR 2.16, 95% CI 2.01–2.33). Other factors associated with large negative discrepancies were: diabetes (OR 1.95, 95% CI 1.69–2.25), maternal age <20 years (OR 1.79, 95% CI 1.71–1.86), being four‐parous or more (OR 1.77, 95% CI 1.67–1.87), and obesity class II, a history of gestational diabetes, age 20–24 years, three‐parous, female fetus, obesity class I, underweight, shorter stature, a history of preeclampsia, smoking, snuff use, unemployment, pre‐obesity, or primiparity (Table 1). Women who had a taller stature or were aged 30 years or above were less likely to have large negative discrepancies.
For large positive discrepancies, the strongest association was observed for pregnancies with a male fetus (OR 1.80, 95% CI 1.77–1.83). Other factors associated with large positive discrepancies were: age 40 years or above (OR 1.57, 95% CI 1.50–1.65), 35–39 years (OR 1.29, 95% CI 1.26–1.32), being four‐parous or more (OR 1.29, 95% CI 1.23–1.37), not living with a partner, taller stature, unemployed, being three‐parous, 30–34 years, or smoking (Table 1). Women who were overweight, obese, had gestational diabetes or preeclampsia, were primiparous, or had a shorter stature were less likely to have large positive discrepancies.
When outpatient diagnoses were included in the adjusted analyses, possible for the years 2006–2010, there were higher odds for large negative discrepancies in association with depressive disorder OR 1.11 (95% CI 1.02–1.21) and higher odds for large positive discrepancies with Crohn's disease (OR 1.36, 95% CI 1.10–1.67). There were lower odds for large positive discrepancies with diabetes (OR 0.79, 95% CI 0.66–0.94), hypothyroid disease (OR 0.85, 95% CI 0.73–0.99), and depressive disorders (OR 0.85, 95% CI 0.78–0.94). Adjusting for maternal co‐morbidity only marginally affected the estimates.
Discussion
This study confirmed previously reported associations of discrepancies between pregnancy‐dating methods and characteristics such as obesity, maternal age, parity, fetal sex, maternal height, smoking, and diabetes 6, 8, 9. Our study population was partly overlapping with the populations in two of the studies 8, 9. However, we included a more recent and longer time period and also assessed other possible risk factors for discrepancies in dating methods, such as snuff use, unemployment, living without a partner, and preeclampsia in a former pregnancy.
Discrepancies could be the result of variation in menstrual cycles or difficulties recalling the LMP, as well as differences in early fetal growth and technical issues in US biometry 1, 2. In most situations, it may be difficult to determine which of the estimates is biased. However, the systematic effect of both fetal sex, attributable to the smaller size of female fetuses, and maternal height, because of genetic influence on fetal growth, is not likely to be explained by unreliable LMP.
Only small average discrepancies between LMP‐based and US‐based methods are expected because both methods predict EDD relatively well; US is the preferred method, being more precise in the general population 14. However, as average differences may not be clinically meaningful, we compared pregnancies where a substantial difference was observed to those with minimal differences. In this study, discrepancies exceeded one week in more than one of six pregnancies (14% with negative and 4% with positive discrepancies). In addition, large negative discrepancies were associated with obesity and diabetes. These conditions, as well as large negative discrepancies between pregnancy‐dating methods, have been associated with adverse outcomes 1, 6, 10, 15, 16. The reason for the association between large negative discrepancies between the dating methods and adverse outcomes may be misclassifications of GA, resulting for example in delayed post‐term inductions 6, 10. Another reason is that a smaller‐than‐expected fetus at the time of US pregnancy dating may be growth‐restricted 1, 10.
Maternal age >30 years was associated with larger fetuses than expected at the US scan, and a similar effect of maternal age has been reported earlier 6. This is consistent with another study relating maternal age to increasing embryonic growth trajectories 17. However, the follicular phase can be one day shorter in women from 35 years of age, which could increase the probability of earlier conception and a large‐than‐expected fetus at the US scan 18. Tunón et al. found that maternal age affected the precision of EDD‐US estimates but not of EDD‐LMP estimates, indicating an effect on fetal growth 19. Age below 25 years was associated with higher odds for both negative and positive large discrepancies, hypothetically because of greater LMP uncertainty.
Maternal height systematically affected the odds for large discrepancies; taller mothers were more likely to have larger fetuses and shorter mothers to have smaller fetuses than expected according to EDD‐LMP. The effect of height was expected, based on genetic influence rather than menstrual cycle differences 5, 20.
Obesity class III had the largest effect on large negative discrepancies. Every increase in BMI class above the normal range increased the odds for large negative discrepancies, and decreased the odds for large positive discrepancies. It could be hypothesized that obese women would have larger fetuses than expected at the US scan because of associated macrosomia. The association between high maternal BMI and negative discrepancies is, however, in accordance with results of earlier studies 6, 9. In particular, in another study based on data from the Swedish Medical Birth Register between 1992 and 2005, EDD was more often postponed among overweight and obese women 9. One explanation could be prolonged menstruation cycles associated with obesity 21, 22. Other reasons might be due to the accuracy of the US measurements of fetal size when examining obese women, or a possible repressive influence of obesity on early fetal growth. According to a study on assisted reproductive technology pregnancies, there is a risk of underestimating the GA when it is based on US examination in obese women 1. In Sweden, 13% of pregnant women in 2010 were obese 6, 10, 23. Both maternal obesity and negative discrepancies are known to be associated with adverse pregnancy outcomes 6, 10. Underweight women were also at higher odds for large negative discrepancies, as expected based on a higher risk for small‐for‐gestational‐age infants and delayed ovulations 22, 24.
Of the socioeconomic factors that were assessed, living without a partner and being unemployed increased odds for both negative and positive discrepancies. However, the association between living without a partner and a negative discrepancy was not significant after adjustment. Even though Sweden offers free antenatal care for all pregnant women, and living without a partner is a socially accepted family structure, this group differs from the rest of the population in terms of risk for discrepancies for mostly unknown reasons.
Smoking and snuff tobacco use were associated with higher odds for large negative discrepancies. There is a well‐known repressive effect on fetal growth caused by smoking, with linear dose–response curves for increased smoking in relation to shorter biparietal diameter and femur length in the third trimester 25. Our results suggested an effect of smoking also on early fetal growth, which was in line with another study, where fetal crown rump length was found to be shorter in women who smoked 10 or more cigarettes per day as compared with non‐smokers 26.
Advanced parity increased the odds for large discrepancies. These results are in line with a previous Swedish study with data partly overlapping those included in this study 8. The effect estimates in the current study remained significant for large negative discrepancies after adjustments; however, the associations with large positive discrepancies were weaker after adjustments, strengthening the notion of a more complex association.
Fetal sex had a systematic effect on discrepancies. The odds for large negative discrepancies were higher for female fetuses, whereas the odds for large positive discrepancies were higher for male fetuses. This could be attributed to the smaller average size of female fetuses in early pregnancy, and confirms results from earlier studies 2, 8, 27. Although most fetuses are mildly misclassified, a number of fetuses are misclassified by more than a week because they are considerably larger (more often males) or smaller (more often females) than the mean. This is reflected in adversely affected odds for postmaturity‐related morbidity and mortality in post‐term female infants in a Swedish register study after introduction of – predominantly second trimester – US pregnancy dating, and also reflected in a successively increasing male‐to‐female birth ratio in gestational weeks 41, 42, and 43 28, 29. According to a Norwegian study, this increased post‐term male–female ratio is normalized when estimates are based on LMP or first trimester instead of second trimester US 27.
The association of maternal diabetes with negative discrepancies confirms the results of other studies 6, 30. In a Norwegian study, women with type 1 diabetes had negative discrepancies of seven or more days in 25% of pregnancies, and 14 or more days in 10% of pregnancies, compared with a certain LMP date, with a risk of going beyond the optimal pregnancy length because of systematic postponement of EDD‐US 30.
Odds of negative discrepancies for those with a history of hypertensive disease were not significant after adjustments, which could indicate that the effect on fetal growth occurs later in pregnancy, although hypertension has also been associated with shorter crown rump length measurements 7. A history of preeclampsia in former pregnancy was associated with higher odds for negative discrepancies and lower odds for positive discrepancies. This is in accordance with the higher risk among these women, also in the currently studied pregnancy, for preeclampsia and intrauterine growth retardation, which can be of early onset 31.
A history of anxiety disorders was associated with higher odds for negative discrepancies. However, there were no statistically significant associations with large negative or large positive discrepancies when having a history of hypothyroidism, systemic lupus erythematosus, ulcerative colitis, Crohn's disease or depressive disorders, which could be the result of a later effect on growth.
The present large register‐based cohort, which could be considered population‐based as more than 99% of all births were registered, allowed for high external validity compared with an earlier hospital‐based study 6. In comparison with two earlier Swedish register studies, the current study includes a larger sample, addresses a more recent time period and adds several other characteristics, such as maternal medical history variables (for example preeclampsia and diabetes in an former pregnancy), as well as snuff use, unemployment, and living without a partner 8, 9. Detailed information was prospectively recorded, minimizing recall bias. A limitation of the study was that no information of regularity on menstrual cycles was included in the register. There was also some missing information on maternal weight, possibly introducing a degree of inclusion bias.
Large discrepancies between the LMP and US methods are not rare, and clinicians should be aware that one or both methods may be unreliable. This is particularly important in the extremes of pregnancy length, when GA influences clinical decision‐making. Early pregnancy dating is preferred – due to increasing growth variance with advancing GA – and is optimally performed at 10 weeks + 0 days to 13 weeks + 6 days according to the recommendations of the International Society of Ultrasound in Obstetrics and Gynecology 32.
Increased awareness of maternal and fetal characteristics that can bias GA estimates can be helpful in clinical situations when large discrepancies between methods are noted. A large discrepancy combined with characteristics affecting early growth should lead to critical evaluation of the GA estimate based on US, and additional information, such as the date of the LMP or an assumed date of conception, should be considered before decision‐making. As large negative discrepancies are associated with adverse outcomes, these pregnancies may benefit from closer monitoring in order to detect intrauterine growth retardation and to avoid post‐term pregnancies 14, 33.
Conclusions
Maternal and fetal characteristics affect discrepancies between pregnancy‐dating methods based on LMP and US. The US estimate is often regarded as the most reliable method and is used for clinical decision‐making. This study indicates that the precision of the US estimate is systematically affected by characteristics such as maternal height and fetal sex, and possibly partly by obesity. In clinical settings, large discrepancies between GA estimates, in combination with certain maternal or fetal characteristics, can help clinicians identify pregnancies with high risk for uncertain dating estimates in order to make appropriate management decisions.
Funding
This study was supported by Uppsala University Hospital, Center for Clinical Research, Västerås, Uppsala University, Karolinska Institutet and the County Council of Västmanland, Sweden.
Acknowledgments
Bengt Haglund contributed to the study design and gave valuable advice on the analyses, interpretation, and presentation of data. Per Wikman contributed to the statistical analyses.
Kullinger M, Wesström J, Kieler H, Skalkidou A. Maternal and fetal characteristics affect discrepancies between pregnancy‐dating methods: a population‐based cross‐sectional register study. Acta Obstet Gynecol Scand 2017; 96:86–95.
Conflict of interest
The authors have stated explicitly that there are no conflicts of interest in connection with this article.
References
- 1. Källén B, Finnström O, Nygren K‐G, Olausson PO. Maternal and fetal factors which affect fetometry: use of in vitro fertilization and birth register data. Eur J Obstet Gynecol Reprod Biol. 2013;170:372–6. [DOI] [PubMed] [Google Scholar]
- 2. Moore WM, Ward BS, Jones VP, Bamford FN. Sex difference in fetal head growth. Br J Obstet Gynaecol. 1988;95:238–42. [DOI] [PubMed] [Google Scholar]
- 3. Hoffman CS, Messer LC, Mendola P, Savitz DA, Herring AH, Hartmann KE. Comparison of gestational age at birth based on last menstrual period and ultrasound during the first trimester. Paediatr Perinat Epidemiol. 2008;22:587–96. [DOI] [PubMed] [Google Scholar]
- 4. Bennett KA, Crane JMG, O'Shea P, Lacelle J, Hutchens D, Copel JA. First trimester ultrasound screening is effective in reducing postterm labor induction rates: a randomized controlled trial. Am J Obstet Gynecol. 2004;190:1077–81. [DOI] [PubMed] [Google Scholar]
- 5. Kramer MS. Determinants of low birth weight: methodological assessment and meta‐analysis. Bull World Health Organ. 1987;65:663–737. [PMC free article] [PubMed] [Google Scholar]
- 6. Morin I, Morin L, Zhang X, Platt RW, Blondel B, Bréart G, et al. Determinants and consequences of discrepancies in menstrual and ultrasonographic gestational age estimates. BJOG. 2005;112:145–52. [DOI] [PubMed] [Google Scholar]
- 7. Mook‐Kanamori DO, Steegers EAP, Eilers PH, Raat H, Hofman A, Jaddoe VWV. Risk factors and outcomes associated with first‐trimester fetal growth restriction. JAMA. 2010;303:527–34. [DOI] [PubMed] [Google Scholar]
- 8. Källén K. Mid‐trimester ultrasound prediction of gestational age: advantages and systematic errors. Ultrasound Obstet Gynecol. 2002;20:558–63. [DOI] [PubMed] [Google Scholar]
- 9. Simic M, Wåhlin IA, Marsal K, Källén K. Maternal obesity is a potential source of error in mid‐trimester ultrasound estimation of gestational age. Ultrasound Obstet Gynecol. 2010;35:48–53. [DOI] [PubMed] [Google Scholar]
- 10. Källén K. Increased risk of perinatal/neonatal death in infants who were smaller than expected at ultrasound fetometry in early pregnancy. Ultrasound Obstet Gynecol. 2004;24:30–4. [DOI] [PubMed] [Google Scholar]
- 11. Cnattingius S, Ericson A, Gunnarskog J, Källén B. A quality study of a medical birth registry. Scand J Soc Med. 1990;18:143–8. [DOI] [PubMed] [Google Scholar]
- 12. Petersson K, Persson M, Lindkvist M, Hammarström M, Nilses C, Haglund I, et al. Internal validity of the Swedish Maternal Health Care Register. BMC Health Serv Res. 2014;14:364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. WHO BMI classification. Available online at: http://www.euro.who.int/en/health-topics/disease-prevention/nutrition/a-healthy-lifestyle/body-mass-index-bmi (accessed April 8, 2016).
- 14. Salomon LJ. Early fetal growth: concepts and pitfalls. Ultrasound Obstet Gynecol. 2010;35:385–9. [DOI] [PubMed] [Google Scholar]
- 15. Marchi J, Berg M, Dencker A, Olander EK, Begley C. Risks associated with obesity in pregnancy, for the mother and baby: a systematic review of reviews. Obes Rev. 2015;16:621–38. [DOI] [PubMed] [Google Scholar]
- 16. Yang J, Cummings EA, O'connell C, Jangaard K. Fetal and neonatal outcomes of diabetic pregnancies. Obstet Gynecol. 2006;1:644–50. [DOI] [PubMed] [Google Scholar]
- 17. van Uitert EM, Exalto N, Burton GJ, Willemsen SP, Koning AHJ, Eilers PHC, et al. Human embryonic growth trajectories and associations with fetal growth and birthweight. Hum Reprod. 2013;28:1753–61. [DOI] [PubMed] [Google Scholar]
- 18. Liu J, Shi Y, Dong J‐Y, Zheng T, Li J‐Y, Lu L‐L, et al. Clinical characteristics, diagnosis and management of respiratory distress syndrome in full‐term neonates. Chin Med J. 2010;123:2640–4. [PubMed] [Google Scholar]
- 19. Tunón K, Eik‐Nes SH, Grøttum P. The impact of fetal, maternal and external factors on prediction of the day of delivery by the use of ultrasound. Ultrasound Obstet Gynecol. 1998;11:99–103. [DOI] [PubMed] [Google Scholar]
- 20. Han Z, Lutsiv O, Mulla S, McDonald SD. Maternal height and the risk of preterm birth and low birth weight: a systematic review and meta‐analyses. J Obstet Gynaecol Can. 2012;34:721–46. [DOI] [PubMed] [Google Scholar]
- 21. Wei S, Schmidt MD, Dwyer T, Norman RJ, Venn AJ. Obesity and menstrual irregularity: associations with SHBG, testosterone, and insulin. Obesity (Silver Spring). 2009;17:1070–6. [DOI] [PubMed] [Google Scholar]
- 22. Symons JP, Sowers MF, Harlow SD. Relationship of body composition measures and menstrual cycle length. Ann Hum Biol. 1997;24:107–16. [DOI] [PubMed] [Google Scholar]
- 23. socialstyrelsen.se. Available online at: http://www.socialstyrelsen.se (accessed April 12, 2015).
- 24. Kramer MS. The epidemiology of low birthweight. Nestle Nutr Inst Workshop Ser. 2013;74:1–10. [DOI] [PubMed] [Google Scholar]
- 25. Iñiguez C, Ballester F, Costa O, Murcia M, Souto A, Santa‐Marina L, et al. Maternal smoking during pregnancy and fetal biometry: the INMA Mother and Child Cohort Study. Am J Epidemiol. 2013;178:1067–75. [DOI] [PubMed] [Google Scholar]
- 26. van Uitert EM, van der Elst‐Otte N, Wilbers JJ, Exalto N, Willemsen SP, Eilers PHC, et al. Periconception maternal characteristics and embryonic growth trajectories: the Rotterdam Predict study. Hum Reprod. 2013;28:3188–96. [DOI] [PubMed] [Google Scholar]
- 27. Koch S, Lynggaard M, Jensen MS, Henriksen TB, Uldbjerg N. Sex bias in ultrasound measures of gestational age: assessment by sex ratio in post‐term births. Epidemiology. 2014;25:513–7. [DOI] [PubMed] [Google Scholar]
- 28. Skalkidou A, Kieler H, Stephansson O, Roos N, Cnattingius S, Haglund B. Ultrasound pregnancy dating leads to biased perinatal morbidity and neonatal mortality among post‐term‐born girls. Epidemiology. 2010;21:791–6. [DOI] [PubMed] [Google Scholar]
- 29. Björkman K, Wesström J. Risk for girls can be adversely affected post‐term due to underestimation of gestational age by ultrasound in the second trimester. Acta Obstet Gynecol Scand. 2015;94:1373–9. [DOI] [PubMed] [Google Scholar]
- 30. Eidem I, Vangen S, Henriksen T, Vollset SE, Hanssen KF, Joner G, et al. Discrepancy in term calculation from second trimester ultrasound scan versus last menstrual period in women with type 1 diabetes. Acta Obstet Gynecol Scand. 2014;93:809–16. [DOI] [PubMed] [Google Scholar]
- 31. Mifsud W, Sebire NJ. Placental pathology in early‐onset and late‐onset fetal growth restriction. Fetal Diagn Ther. 2014;36:117–28. [DOI] [PubMed] [Google Scholar]
- 32. Salomon LJ, Alfirevic Z, Berghella V, Bilardo C, Hernandez‐Andrade E, Johnsen SL, et al. Practice guidelines for performance of the routine mid‐trimester fetal ultrasound scan. Ultrasound Obstet Gynecol. 2011;37:116–26. [DOI] [PubMed] [Google Scholar]
- 33. DeVore GR. The importance of the cerebroplacental ratio in the evaluation of fetal well‐being in SGA and AGA fetuses. Am J Obstet Gynecol. 2015;213:5–15. [DOI] [PubMed] [Google Scholar]


