Abstract
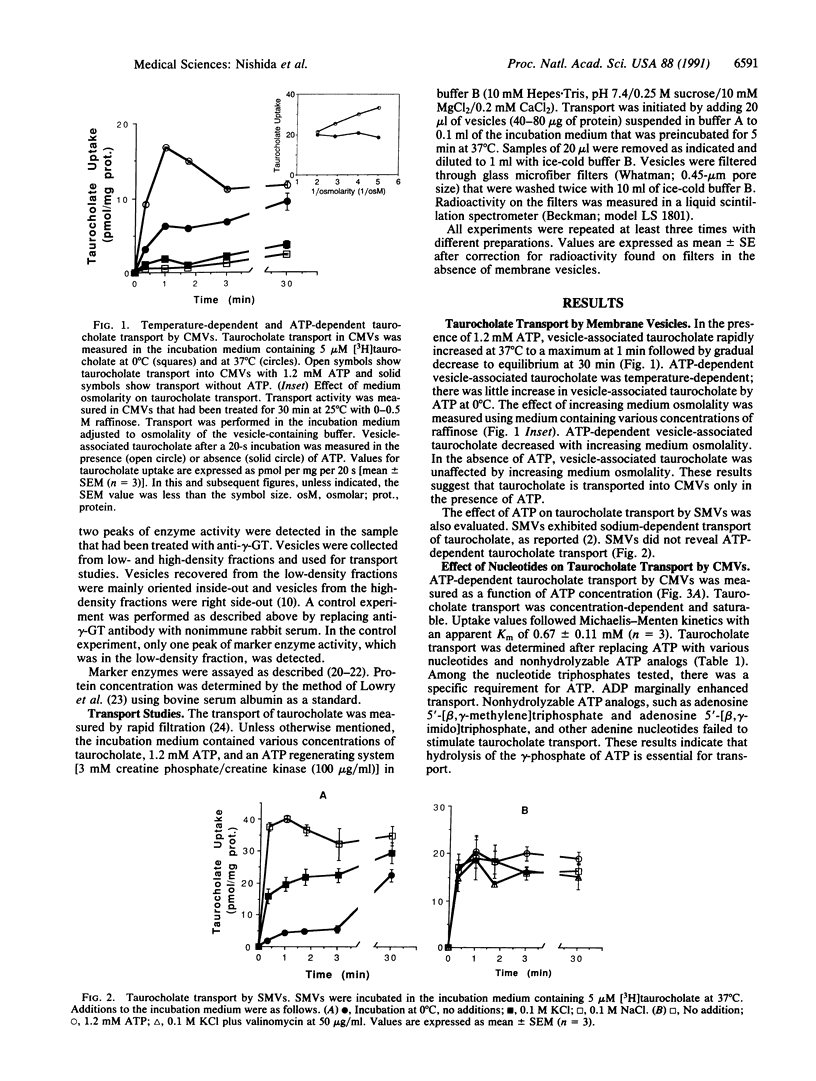
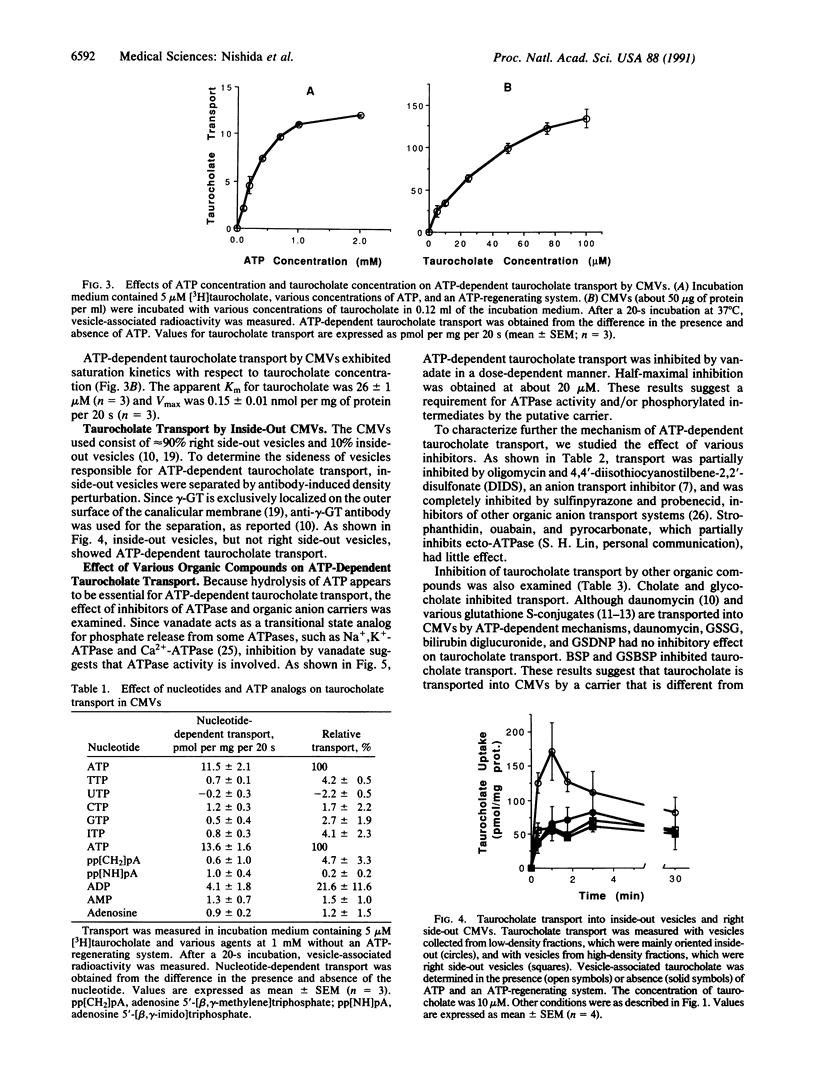
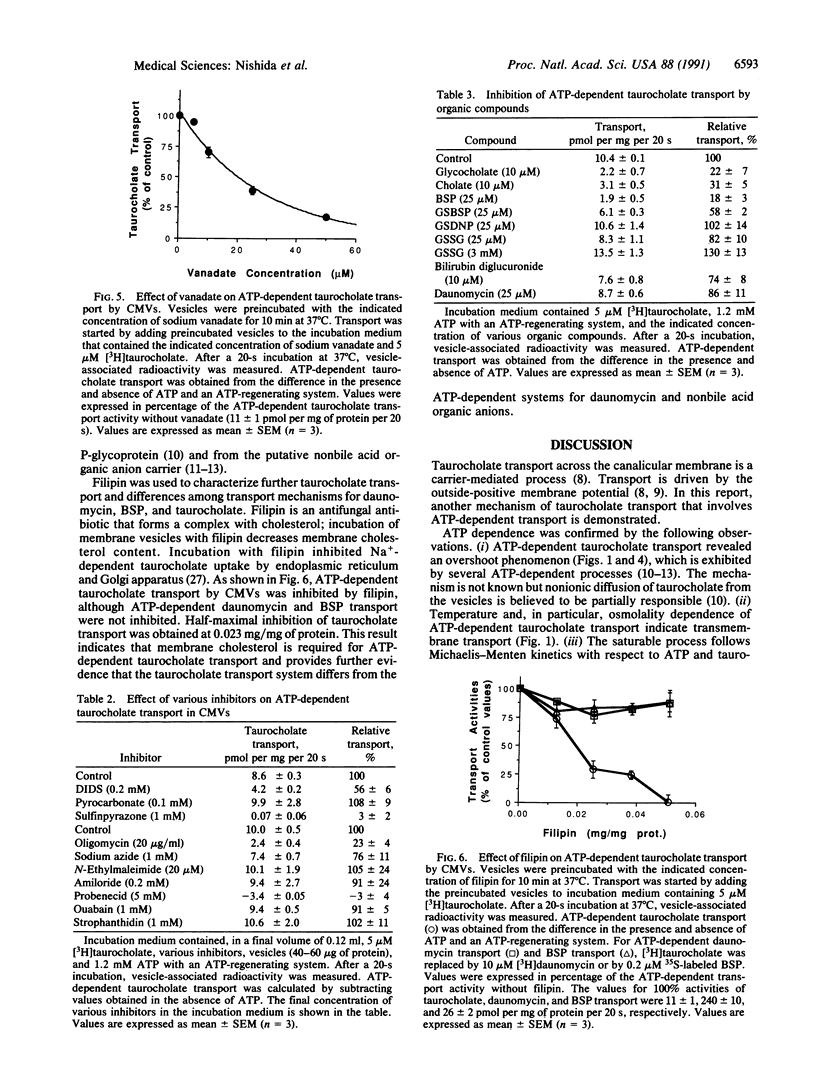
The secretion of bile by the liver is primarily determined by the ability of the hepatocyte to transport bile acids into the bile canaliculus. A carrier-mediated process for the transport of taurocholate, the major bile acid in humans and rats, was previously demonstrated in canalicular membrane vesicles from rat liver. This process is driven by an outside-positive membrane potential that is, however, insufficient to explain the large bile acid concentration gradient between the hepatocyte and bile. In this study, we describe an ATP-dependent transport system for taurocholate in inside-out canalicular membrane vesicles from rat liver. The transport system is saturable, temperature-dependent, osmotically sensitive, specifically requires ATP, and does not function in sinusoidal membrane vesicles and right side-out canalicular membrane vesicles. Transport was inhibited by other bile acids but not by substrates for the previously demonstrated ATP-dependent canalicular transport systems for organic cations or nonbile acid organic anions. Defects in ATP-dependent canalicular transport of bile acids may contribute to reduced bile secretion (cholestasis) in various developmental, inheritable, and acquired disorders.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anwer M. S., Hegner D. Effect of Na on bile acid uptake by isolated rat hepatocytes. Evidence for a heterogeneous system. Hoppe Seylers Z Physiol Chem. 1978 Feb;359(2):181–192. [PubMed] [Google Scholar]
- Booth J., Boyland E., Sims P. An enzyme from rat liver catalysing conjugations with glutathione. Biochem J. 1961 Jun;79(3):516–524. doi: 10.1042/bj0790516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantley L. C., Jr, Cantley L. G., Josephson L. A characterization of vanadate interactions with the (Na,K)-ATPase. Mechanistic and regulatory implications. J Biol Chem. 1978 Oct 25;253(20):7361–7368. [PubMed] [Google Scholar]
- Coleman R. Biochemistry of bile secretion. Biochem J. 1987 Jun 1;244(2):249–261. doi: 10.1042/bj2440249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford J. M., Berken C. A., Gollan J. L. Role of the hepatocyte microtubular system in the excretion of bile salts and biliary lipid: implications for intracellular vesicular transport. J Lipid Res. 1988 Feb;29(2):144–156. [PubMed] [Google Scholar]
- Elferink R. P., Ottenhoff R., Liefting W., de Haan J., Jansen P. L. Hepatobiliary transport of glutathione and glutathione conjugate in rats with hereditary hyperbilirubinemia. J Clin Invest. 1989 Aug;84(2):476–483. doi: 10.1172/JCI114189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evers J., Murer H., Kinne R. Phenylalanine uptake in isolated renal brush border vesicles. Biochim Biophys Acta. 1976 Apr 5;426(4):598–615. doi: 10.1016/0005-2736(76)90124-3. [DOI] [PubMed] [Google Scholar]
- Frimmer M., Ziegler K. The transport of bile acids in liver cells. Biochim Biophys Acta. 1988 Feb 24;947(1):75–99. doi: 10.1016/0304-4157(88)90020-2. [DOI] [PubMed] [Google Scholar]
- Heidrich H. G., Kinne R., Kinne-Saffran E., Hannig K. The polarity of the proximal tubule cell in rat kidney. Different surface charges for the brush-border microvilli and plasma membranes from the basal infoldings. J Cell Biol. 1972 Aug;54(2):232–245. doi: 10.1083/jcb.54.2.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M., Kinne R., Tran T., Arias I. M. Taurocholate transport by rat liver canalicular membrane vesicles. Evidence for the presence of an Na+-independent transport system. J Clin Invest. 1984 Mar;73(3):659–663. doi: 10.1172/JCI111257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M., Kinne R., Tran T., Arias I. M. Taurocholate transport by rat liver sinusoidal membrane vesicles: evidence of sodium cotransport. Hepatology. 1982 Sep-Oct;2(5):572–579. doi: 10.1002/hep.1840020510. [DOI] [PubMed] [Google Scholar]
- Inoue M., Kinne R., Tran T., Biempica L., Arias I. M. Rat liver canalicular membrane vesicles. Isolation and topological characterization. J Biol Chem. 1983 Apr 25;258(8):5183–5188. [PubMed] [Google Scholar]
- Ishikawa T., Müller M., Klünemann C., Schaub T., Keppler D. ATP-dependent primary active transport of cysteinyl leukotrienes across liver canalicular membrane. Role of the ATP-dependent transport system for glutathione S-conjugates. J Biol Chem. 1990 Nov 5;265(31):19279–19286. [PubMed] [Google Scholar]
- Kamimoto Y., Gatmaitan Z., Hsu J., Arias I. M. The function of Gp170, the multidrug resistance gene product, in rat liver canalicular membrane vesicles. J Biol Chem. 1989 Jul 15;264(20):11693–11698. [PubMed] [Google Scholar]
- Kitamura T., Gatmaitan Z., Arias I. M. Serial quantitative image analysis and confocal microscopy of hepatic uptake, intracellular distribution and biliary secretion of a fluorescent bile acid analog in rat hepatocyte doublets. Hepatology. 1990 Dec;12(6):1358–1364. doi: 10.1002/hep.1840120617. [DOI] [PubMed] [Google Scholar]
- Kitamura T., Jansen P., Hardenbrook C., Kamimoto Y., Gatmaitan Z., Arias I. M. Defective ATP-dependent bile canalicular transport of organic anions in mutant (TR-) rats with conjugated hyperbilirubinemia. Proc Natl Acad Sci U S A. 1990 May;87(9):3557–3561. doi: 10.1073/pnas.87.9.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K., Sogame Y., Hara H., Hayashi K. Mechanism of glutathione S-conjugate transport in canalicular and basolateral rat liver plasma membranes. J Biol Chem. 1990 May 15;265(14):7737–7741. [PubMed] [Google Scholar]
- Kurisu H., Nilprabhassorn P., Wolkoff A. W. Preparation of [35S]sulfobromophthalein of high specific activity. Anal Biochem. 1989 May 15;179(1):72–74. doi: 10.1016/0003-2697(89)90202-9. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lamri Y., Roda A., Dumont M., Feldmann G., Erlinger S. Immunoperoxidase localization of bile salts in rat liver cells. Evidence for a role of the Golgi apparatus in bile salt transport. J Clin Invest. 1988 Oct;82(4):1173–1182. doi: 10.1172/JCI113714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipman B. J., Silverstein S. C., Steinberg T. H. Organic anion transport in macrophage membrane vesicles. J Biol Chem. 1990 Feb 5;265(4):2142–2147. [PubMed] [Google Scholar]
- Meier P. J., Meier-Abt A. S., Boyer J. L. Properties of the canalicular bile acid transport system in rat liver. Biochem J. 1987 Mar 1;242(2):465–469. doi: 10.1042/bj2420465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ORLOWSKI M., MEISTER A. GAMMA-GLUTAMYL-P-NITROANILIDE: A NEW CONVENIENT SUBSTRATE FOR DETERMINATION AND STUDY OF L- AND D-GAMMA-GLUTAMYLTRANSPEPTIDASE ACTIVITIES. Biochim Biophys Acta. 1963 Aug 6;73:679–681. doi: 10.1016/0006-3002(63)90348-2. [DOI] [PubMed] [Google Scholar]
- Ruetz S., Fricker G., Hugentobler G., Winterhalter K., Kurz G., Meier P. J. Isolation and characterization of the putative canalicular bile salt transport system of rat liver. J Biol Chem. 1987 Aug 15;262(23):11324–11330. [PubMed] [Google Scholar]
- Ruetz S., Hugentobler G., Meier P. J. Functional reconstitution of the canalicular bile salt transport system of rat liver. Proc Natl Acad Sci U S A. 1988 Aug;85(16):6147–6151. doi: 10.1073/pnas.85.16.6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharschmidt B. F., Keeffe E. B., Blankenship N. M., Ockner R. K. Validation of a recording spectrophotometric method for measurement of membrane-associated Mg- and NaK-ATPase activity. J Lab Clin Med. 1979 May;93(5):790–799. [PubMed] [Google Scholar]
- Scharschmidt B. F., Lake J. R. Hepatocellular bile acid transport and ursodeoxycholic acid hypercholeresis. Dig Dis Sci. 1989 Dec;34(12 Suppl):5S–15S. doi: 10.1007/BF01536656. [DOI] [PubMed] [Google Scholar]
- Simion F. A., Fleischer B., Fleischer S. Two distinct mechanisms for taurocholate uptake in subcellular fractions from rat liver. J Biol Chem. 1984 Sep 10;259(17):10814–10822. [PubMed] [Google Scholar]
- Sippel C. J., Ananthanarayanan M., Suchy F. J. Isolation and characterization of the canalicular membrane bile acid transport protein of rat liver. Am J Physiol. 1990 May;258(5 Pt 1):G728–G737. doi: 10.1152/ajpgi.1990.258.5.G728. [DOI] [PubMed] [Google Scholar]
- Spivak W., Yuey W. Application of a rapid and efficient h.p.l.c. method to measure bilirubin and its conjugates from native bile and in model bile systems. Potential use as a tool for kinetic reactions and as an aid in diagnosis of hepatobiliary disease. Biochem J. 1986 Feb 15;234(1):101–109. doi: 10.1042/bj2340101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinman S. A., Graf J., Boyer J. L. Voltage-driven, taurocholate-dependent secretion in isolated hepatocyte couplets. Am J Physiol. 1989 May;256(5 Pt 1):G826–G832. doi: 10.1152/ajpgi.1989.256.5.G826. [DOI] [PubMed] [Google Scholar]
- Whelan G., Hoch J., Combes B. A direct assessment of the importance of conjugation for biliary transport of sulfobromophthalein sodium. J Lab Clin Med. 1970 Apr;75(4):542–557. [PubMed] [Google Scholar]



