Summary
Concerns have been raised about the effects on cognition of anaesthesia for surgery, especially in elderly people. We recorded cognitive decline in a cohort of 394 people (198 women) with median (IQR) age at recruitment of 72.6 (66.6–77.8) years, of whom 109 had moderate or major surgery during a median (IQR) follow‐up of 4.1 (2.0–7.6) years. Cognitive decline was more rapid in people who on recruitment were: older, p = 0.0003; male, p = 0.027; had worse cognition, p < 0.0001; or carried the ε4 allele of apoliprotein E (APOEε4), p = 0.008; and after an operation if cognitive impairment was already diagnosed, p = 0.0001. Cognitive decline appears to accelerate after surgery in elderly patients diagnosed with cognitive impairment, but not other elderly patients.
Keywords: anaesthesia, cognitive decline, surgery
Short abstract
☛ CPD available at http://www.learnataagbi.org
Introduction
A recent editorial in International Psychogeriatrics concluded that ‘anaesthesia and surgery induce cognitive dysfunction in susceptible individuals’ 1. Susceptible people are thought to include the elderly 2. If true, this is a serious problem. It was recently estimated that there are annually over 230 million procedures with general anaesthesia worldwide 3. There are over 880 million people > 60 years old in the world today 4. The latter figure is predicted to grow rapidly as life expectancy increases, particularly in developing countries.
Cell and animal studies have gone some way in showing some potentially damaging effects of certain anaesthetics, for example, inhalational anaesthetics such as isoflurane and sevoflurane 5, 6, 7, 8, 9, 10. In contrast, the intravenous anaesthetic, propofol, may have largely protective effects 6, 11, 12, 13, mainly shown in animal and cellular models, although it has also been suggested to have some negative actions 14.
However, it has been harder to establish the effects of general anaesthetics on long‐term cognitive decline in people. Short‐term postoperative cognitive dysfunction is well known. But there have been rather few longitudinal studies of longer term (> 3 months) cognitive decline 15, 16, 17, 18. There have been more studies of the risk of dementia, or specifically of Alzheimer's disease, following surgery with general anaesthesia. But those studies had until recently produced mixed results 19. However, two very large recent Taiwanese studies 20, 21 both found an increased risk of dementia following surgery with various types of anaesthesia.
The consensus statement of the First International Workshop on Anesthetics and Alzheimer's Disease 22 concluded that ‘there is sufficient evidence at multiple levels to warrant further and more definitive investigations of the onset and progression of Alzheimer's disease and neurodegeneration after anesthesia and surgery’. We therefore undertook a longitudinal cohort study of cognitive function in patients and controls from the Oxford Project to Investigate Memory and Ageing (OPTIMA), who were followed with frequent cognitive assessments and records of episodes of anaesthesia for surgery. Our aim was to assess factors influencing the effects of anaesthesia and surgery on the trajectory of cognitive decline with increasing age. We included the effects of age and gender, and of the apolipoprotein E ε4 variant (APOEε4) on this relationship.
Methods
We interrogated the database of the Oxford Project to Investigate Memory and Ageing (OPTIMA) in order to examine whether the time‐course of cognitive decline with age is related to episodes of anaesthesia and surgery. We identified patients with cognitive impairment and normal elderly people recruited by OPTIMA from 1988 until 2008; the follow‐up included in this analysis extends from 1988 until 2012. Participants were seen at least annually, at many of which visits a Cambridge Cognition Examination (CAMCOG) score (variable termed ‘C’ below) was obtained 23 and details of episodes of surgery since the preceding assessment recorded. Patients were genotyped with regard to the APOEε4 allele, which is known to be associated with a greater risk of cognitive decline 24, 25 and diagnosis of Alzheimer's dementia 26. We sought participants with at least two measures of CAMCOG who had joined the study as controls, or with mild cognitive impairment 27, who had participated in at least 3 years of follow‐up. We identified episodes of surgery that could be regarded as moderate or major in severity, and therefore highly likely to be associated with general or regional anaesthesia, or both. Minor surgical procedures, such as carpal tunnel release, cystoscopy and cataract surgery, were not included. We took account of clinical diagnoses: the presence of mild cognitive impairment possible Alzheimer's dementia or probable Alzheimer's dementia 28 was recorded from diagnosis onwards as a binary variable ‘D’, and the time of first diagnosis of any one of the three was defined to be TD.
We used mixed‐effects modelling with open‐source computer software ‘R’ to model the relationship between CAMCOG score and multiple explanatory variables on which it might be dependent. Although the CAMCOG scores are integers with a maximum of 107, it is not appropriate to model them with a binomial distribution because the answers to the questions which go to make up a CAMCOG score are not independent; a binomial generalised linear model therefore cannot be expected to provide a valid fit, and diagnostics carried out on a mixed‐effects binomial generalised linear model indeed confirmed this to be the case. With data concentrated largely towards one end of a scale of fixed length, a folded transformation usually provides acceptable normality. In practice, it turned out that fitting a linear mixed‐effects model with a folded transformation (z) of the CAMCOG score (C) of the form
| (1) |
produced normally distributed homoscedastic residuals, thereby satisfying the model's diagnostic requirements (Fig. 2).
Figure 2.
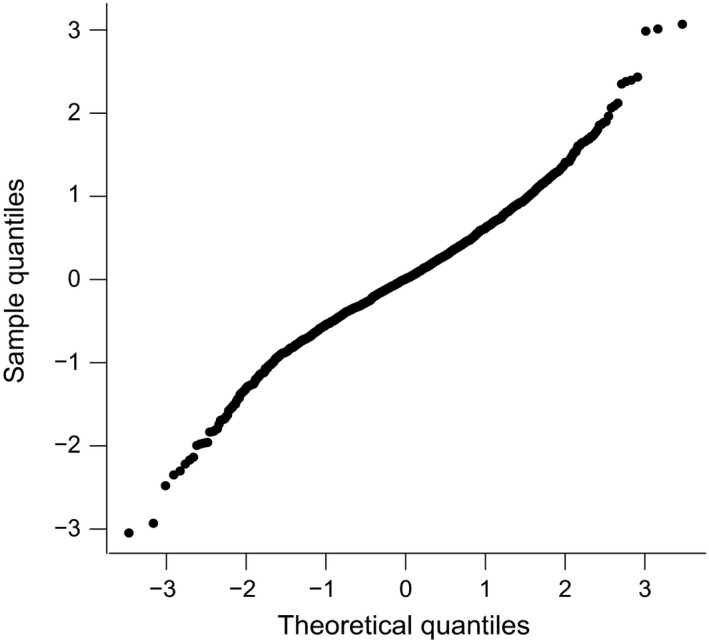
The commonly termed ‘QQ‐Plot’ assessing the normality assumptions of the statistical model. Residuals of the measured values of the folded transformation variable z representing the Cambridge Cognition Examination (CAMCOG) score (sample quantiles) are plotted against the standardised theoretical quantiles taken from the model expressed in Eqn. (3). The degree to which the assumption of Normality of the distribution of residuals is appropriate can be judged by the linearity of this plot, which in this case is entirely satisfactory.
We modelled z as a linear function of the following variables and all relevant interactions between the variables: baseline age on recruitment (BA); initial, first, CAMCOG score on recruitment (IC); male gender (G); presence of APOEε4 heterozygosity or homozygosity (AP); time after recruitment (t); first surgery having occurred (S1); second surgery having occurred (S2); time at first surgery (T1); time at second surgery (T2); history of a clinical diagnosis of mild cognitive impairment or possible or probable Alzheimer's disease (D); time at first diagnosis (TD); whether diagnosis was made before or after surgery (A). The initial CAMCOG score was incorporated as an independent variable to allow for a practice effect that could be expected to result from first exposure to the test 29.
Data were fitted to a model in which continuity of C was assumed at episodes of surgery and onset of diagnosis, at first with all of the possible contributing factors allowed, both singly and as interactions. The model was then refined by excluding the least significant factor until parsimony was achieved with all remaining factors showing significance at the level of p < 0.05. This procedure was first undertaken excluding the clinical diagnoses, so as to concentrate on the fully objective indices: BA, IC, G, AP, S1, S2, T1 and T2. This then resulted in estimates of the coefficients (β0–β9) that define the linear model:
| (2) |
The procedure was then undertaken including the clinical diagnoses, D. This resulted in estimates of the coefficients (β0–β13) that define the linear model:
| (3) |
A final analysis was conducted introducing the binary factor A expressing whether diagnosis was made before or after surgery to examine the possibility of surgery leading to a diagnosis. This explored the possible addition of a variable β14A.D.S1.(t − TD) into Eqn. (3) above.
We took the value of p < 0.05 to indicate significance throughout.
Results
From 982 participants recruited by OPTIMA at the start of this study, we identified 394 (198 women, 196 men) satisfying the criteria of having at least two measures of CAMCOG score who had joined the study as controls, or with mild cognitive impairment, and who had participated in at least 3 years of follow‐up. The median age at recruitment was 72.6 years (IQR 66.6–77.8 years). One‐thousand nine‐hundred and twenty‐two observations of CAMCOG were made during a median follow‐up time of 4.1 years (IQR 2.0–7.6 years) up to a maximum of 23 years. APOEε4 allele heterozygosity was present in 117 and homozygosity in 14 participants. A first episode of surgery occurred in 109 participants and this was at a median (IQR) time of 3.0 (2.0–6.1) years from recruitment. A second episode of surgery occurred in 37 of these and this was at a median (IQR) time of 5.1 (2.6–7.1) years from recruitment. Third and subsequent episodes of surgery were too few to warrant specific identification in the analysis. Initial CAMCOG score on recruitment had a median (IQR) value of 98.0 (93.0–101.0). Mild cognitive impairment was diagnosed in 79 participants at some stage of their follow‐up. In many of these, the clinical diagnosis progressed to possible or probable Alzheimer's disease. A total of 150 patients were diagnosed with one or more of these three categories. Of these 36 had surgery, 16 of them receiving their diagnosis before (usually shortly before) their first surgery. A few other clinical diagnoses were recorded on patients, which were not separately included in the analysis.
Figure 1 provides an overview of the dataset, showing the time‐course of mean values of CAMCOG over the first 10 years from recruitment for participants. Data have been collected into bins of 1‐year periods for this visual summary. Ten participants received their surgery after the 10‐year period depicted in Fig. 1 and are therefore not included in this graph. It is important to note that these data cannot be regarded as trajectories for individual participants; they are included to provide an overview of the ranges of CAMCOG and follow‐up times. In relation to the range of CAMCOG seen here, it may be helpful to note that a cut‐off score of 79 is traditionally used to indicate dementia.
Figure 1.
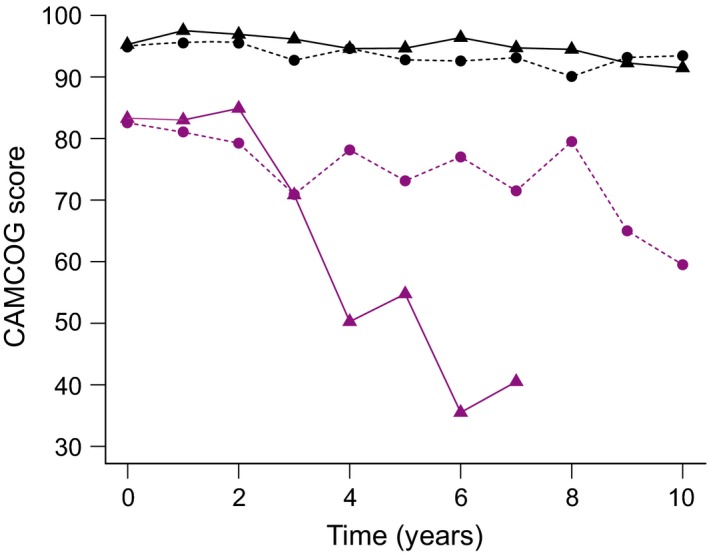
Summary overview of Cambridge Cognition Examination (CAMCOG) scores for the participants in the study, showing the time‐course of mean values of CAMCOG over the first 10 years from recruitment for participants divided into four groups. Black lines depict participants with a CAMCOG of 86 or higher at recruitment; purple lines depict those with a CAMCOG of 85 or lower at recruitment. Dashed lines show data for participants who received no surgery within the 10‐year period; solid lines show data for those who receive an episode of surgery within the 10‐year period. Ten of 394 participants received their surgery after the 10‐year period depicted in this figure and are therefore not included in this graph.
The results of the modelling associated with Eqn. (2) are shown in Table 1. Recall that the coefficients and p values here are obtained without reference to clinical diagnoses. The low p values for the negative coefficients β1, β3 and β4, respectively for BA, G and AP, indicate that the absolute level of CAMCOG score is significantly lowered by increasing age at recruitment, male gender and the presence of the APOEε4 allele. The significance of the negative co‐efficient β5 shows the anticipated decrease in CAMCOG score with increasing age during the follow‐up. The significance of the co‐efficient β6 for the interaction term G.IC shows that there is a positive component to the CAMCOG score, throughout the follow‐up period, that depends upon IC but is enhanced by the male gender; this will tend to offset the deleterious influence of male gender alone expressed in the negative co‐efficient β3, but the degree of offset is dependent upon IC. The significance of the co‐efficient β7 for the interaction term G.t shows a greater rate of decline in CAMCOG with time in men. The effect of gender is therefore complex, acting via three coefficients in the model, two of which are interactive terms.
Table 1.
Co‐efficients obtained by mixed‐effects modelling associated with Eqn. (2). Central to the question addressed by this paper is the finding that the co‐efficient β8 is significant, suggesting that surgery with anaesthesia increases the rate of cognitive decline in this cohort as a whole
| Variable | Co‐efficient | Value | 95% CI | Std. Error | p value |
|---|---|---|---|---|---|
| Intercept | β0 | 1.143 | 0.635–1.650 | 0.259 | 0.0000 |
| BA, baseline age | β1 | −0.010 | −0.016 to −0.004 | 0.003 | 0.0006 |
| IC, initial CAMCOG | β2 | 0.890 | 0.786–0.994 | 0.053 | 0.0000 |
| G, male gender | β3 | −0.106 | −0.198 to −0.014 | 0.047 | 0.0253 |
| AP, APOEε4 a | β4 | −0.637 | −1.003 to −0.241 | 0.202 | 0.0017 |
| t, time | β5 | −0.038 | −0.058 to −0.018 | 0.010 | 0.0001 |
| G.IC | β6 | 0.289 | 0.116–0.461 | 0.088 | 0.0011 |
| G.t | β7 | −0.068 | −0.099 to −0.037 | 0.016 | 0.0000 |
| S1.(t − T1) | β8 | −0.043 | −0.080 to −0.006 | 0.019 | 0.0251 |
| S2.(t − T2) | β9 | 0.059 | 0.000–0.118 | 0.030 | 0.0498 |
Heterozygous or homozygous for gene allele. S1 and S2 refer to first and second surgery having occurred, respectively, at times T1 and T2.
The significance of the negative co‐efficient β8 for the interaction term S1.(t − T1) indicates that first surgery significantly increases the rate of decline in CAMCOG (p = 0.025). Interestingly, the borderline significance of the positive co‐efficient β9 for the interaction term S2.(t − T2) indicates that second surgery may slow the rate of decline in CAMCOG score to a similar extent to which it had been worsened by first surgery (p = 0.05). A further possibility is that patients receiving second surgery were selected in part because they had shown less cognitive decline following first surgery, and cannot therefore be regarded as randomly selected from those having first surgery.
The results of the modelling associated with Eqn. (3) are shown in Table 2. Here we note similar co‐efficients β1–β7, together with similar associated p values, expressing similar dependence of CAMCOG score on BA, IC, G, AP, t and the interactive terms G.IC and G.t. The model resolves the effect of surgery, however, by examining the influence of diagnosis, D. Co‐efficient β10 indicates that a diagnosis is followed by a significantly more rapid rate of decline in CAMCOG score. Co‐efficients β11 and β12 indicate that this rate of decline is lessened by having a higher IC but worsened by being of male gender. The highly significant co‐efficient β13 associated with variable D.S1(t − T1) suggests that it is those patients with a diagnosis who are susceptible to the effects of first surgery; for the cohort as a whole, the non‐significant co‐efficient β8 for the variable S1.(t − T1) indicates that individuals without a diagnosis may be exempted from the risk of surgery. The effects of second surgery remain poorly defined in the second model, as in the first.
Table 2.
Co‐efficients obtained by mixed‐effects modelling associated with Eqn. (3). Central to the question addressed by this paper is the finding that the co‐efficient β8 is here insignificant, while the co‐efficient β13 is highly significant, suggesting that the patients who are susceptible to experiencing an increased rate of cognitive decline after first surgery with anaesthesia are those who have had a clinical diagnosis of mild cognitive impairment, or possible or probable Alzheimer's disease
| Variable | Co‐efficient | Value | 95% CI | Std. Error | p value |
|---|---|---|---|---|---|
| Intercept | β0 | 1.164 | 0.658–1.670 | 0.258 | 0.0000 |
| BA, baseline age | β1 | −0.010 | −0.016 to −0.004 | 0.003 | 0.0003 |
| IC, initial CAMCOG | β2 | 0.890 | 0.786–0.994 | 0.053 | 0.0000 |
| G, male gender | β3 | −0.105 | −0.197 to −0.013 | 0.047 | 0.0267 |
| AP, APOEε4 a | β4 | −0.538 | −0.934 to −0.142 | 0.202 | 0.0079 |
| t, time | β5 | −0.032 | −0.050 to −0.014 | 0.009 | 0.0003 |
| G.IC | β6 | 0.227 | 0.054–0.399 | 0.088 | 0.0101 |
| G.t | β7 | −0.038 | −0.067 to −0.009 | 0.015 | 0.0133 |
| S1.(t − T1) | β8 | −0.029 | −0.064 to 0.006 | 0.018 | 0.1151 |
| S2.(t − T2) | β9 | 0.055 | 0.000–0.110 | 0.028 | 0.0500 |
| D.(t − TD) | β10 | −0.129 | −0.223 to −0.035 | 0.048 | 0.0076 |
| D.IC.(t − TD) | β11 | 0.055 | 0.006–0.104 | 0.025 | 0.0264 |
| D.G.(t − TD) | β12 | −0.085 | −0.130 to −0.040 | 0.023 | 0.0002 |
| D.S1.(t − T1) | β13 | −0.050 | −0.073 to −0.026 | 0.012 | 0.0001 |
Heterozygous or homozygous for gene allele. S1 and S2 refer to first and second surgery having occurred, respectively, at times T1 and T2. D refers to the presence of a clinical diagnosis of cognitive impairment having been made at time TD.
We finally examined the model expressed in Eqn. (3) by introducing a further factor A expressing the timing of diagnosis with regard to the timing of S1. Co‐efficient β14 associated with the variable β14A.D.S1.(t − TD) received the value −0.009 with p value 0.743, indicating that the surgery appeared not to be related to the subsequent making of a diagnosis. All other co‐efficients were largely unaffected by this additional term in the model.
The validity of the modelling requirement of normality in the response can be assessed by plotting the residuals of measured values of z (sample quantiles) against the standardised theoretical quantiles (units of standard deviation) taken from the model in Eqn. (3). This plot is shown in Fig. 2, and its approximate linearity confirms that there is no reason to reject the normality assumption. A measure of the success of the model in representing the data is R2, the proportion of the variance that is explained by the model. This was a remarkable 73% for the model of Eqn. (3).
We present sample predictions from the model to illustrate the time course of CAMCOG in hypothetical individuals with characteristics that are typical for our data set. Figure 3 shows the model predictions from Eqn. (2) for women and men, without diagnoses, incorporating four different scenarios for each of two starting values of CAMCOG score: high and low. The scenarios are as follows: no APOEε4 allele gene, black lines; APOEε4 hetero‐ or homozygosity, blue lines; no surgery is indicated by short dashes; a single episode of surgery at 3 years, is indicated by solid lines. Initial CAMCOG scores are 98 and 84 for women; the (lower) male initial CAMCOG values are those for all variables being identical other than gender.
Figure 3.
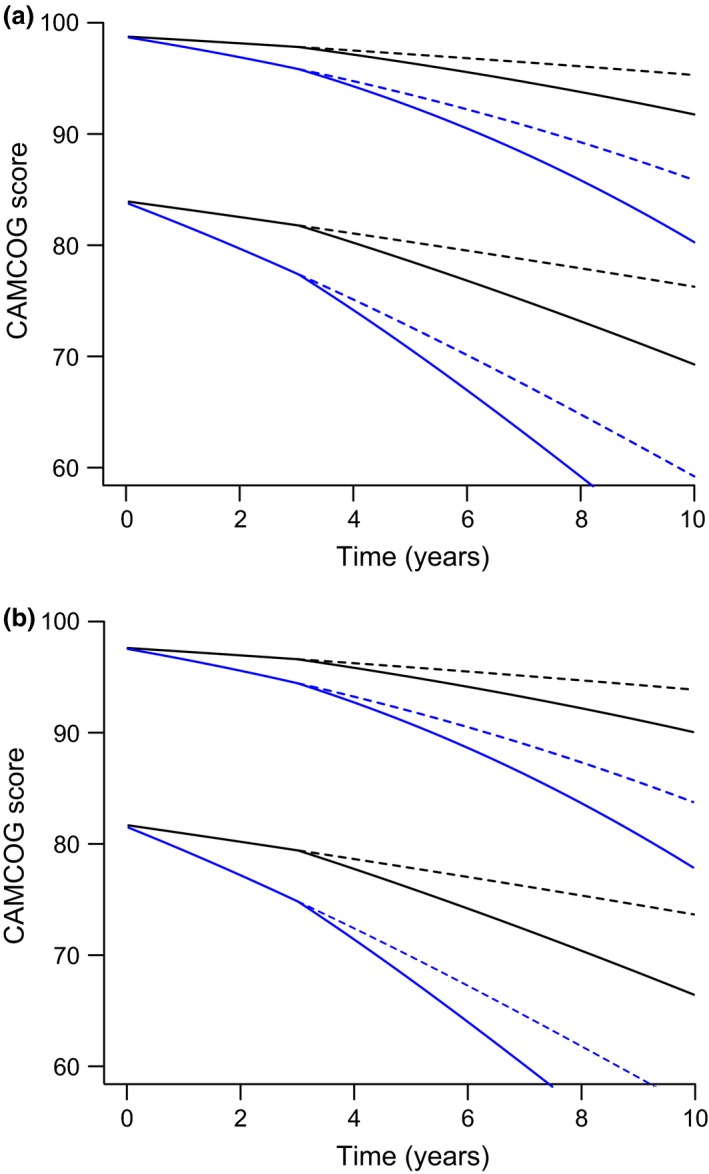
Sample predictions from the model of Eqn. (2) and Table 1 to illustrate the trajectory of Cambridge Cognition Examination (CAMCOG) in hypothetical individuals with characteristics that are typical for the data set used in the analysis. (a) women; (b) men, both without diagnoses. Four different scenarios are depicted for each of two starting values of CAMCOG score: high and low. The scenarios are as follows: no APOEε4 allele, black lines; APOEε4 hetero‐ or homozygosity, blue lines; no surgery is indicated by dashes; a single episode of surgery at 3 years is indicated by solid lines. Initial CAMCOG scores are 98 and 84 for women; the (lower) male initial CAMCOG values are those for all variables being identical other than gender.
Figure 4 examines the effect of diagnoses. It shows the model predictions for women and men, without APOEε4 alleles, incorporating four different scenarios for each of two starting values of CAMCOG score: high and low. The scenarios are as follows: no diagnoses, black lines; diagnoses made at 2.5 years, red lines; no surgery is indicated by short dashes; a single episode of surgery at 3 years, is indicated by solid lines. Initial CAMCOG scores are 98 and 85 for women; the (lower) male initial CAMCOG values are those for all variables being identical other than gender. In Fig. 4, the solid red lines illustrate the substantial significant increase in the rate of decline in CAMCOG score occurring at first surgery in patients with a diagnostic category.
Figure 4.
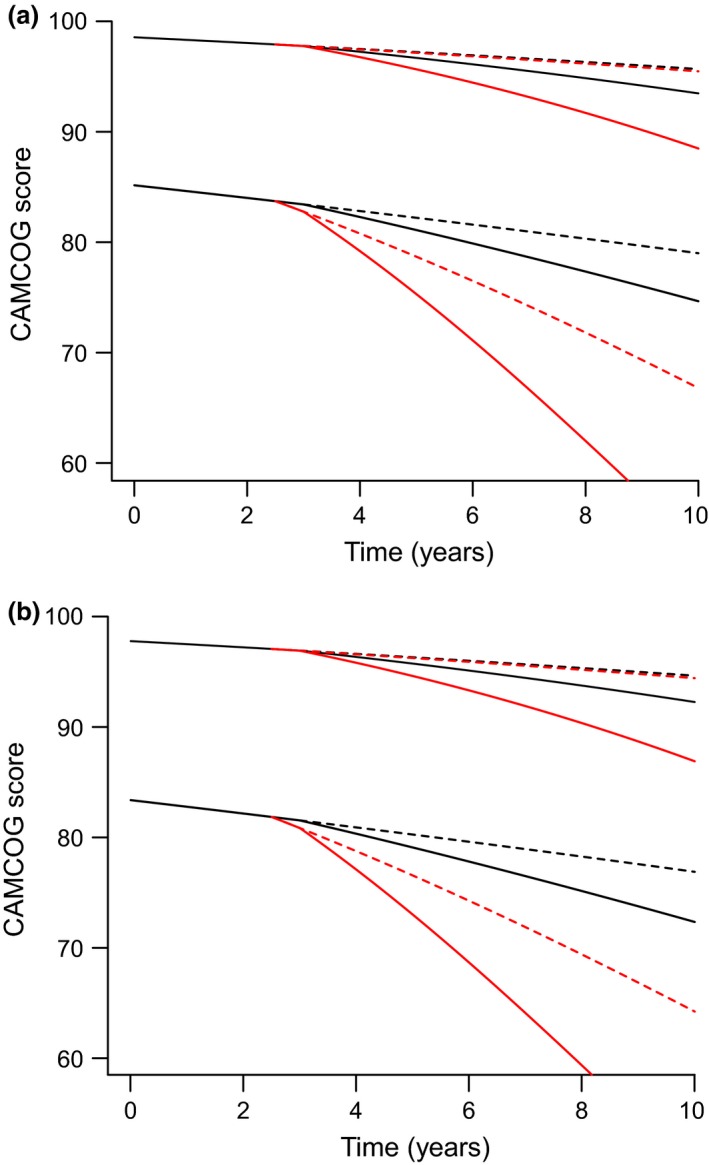
Sample predictions from the model of Eqn. (3) and Table 2 to illustrate the trajectory of Cambridge Cognition Examination (CAMCOG) in hypothetical individuals with characteristics that are typical for the data set used in the analysis. (a) women; (b) men, both without APOEε4 allele. Four different scenarios are depicted for each of two starting values of CAMCOG score: high and low. The scenarios are as follows: no diagnoses (mild cognitive impairment or possible or probable Alzheimer's dementia), black lines; diagnoses made at 2.5 years, red lines; no surgery is indicated by dashes; a single episode of surgery at 3 years is indicated by solid lines. Initial CAMCOG scores are 98 and 85 for womens; the (lower) male initial CAMCOG values are those for all variables being identical other than gender.
Discussion
We found that elderly people were at risk of more rapid cognitive decline after an episode of moderate or major surgery with general or regional anaesthesia (Fig. 3). On further analysis, we discovered that in our cohort this effect was limited to those who had already suffered cognitive impairment (Fig. 4), that is, either mild cognitive impairment or possible or probable Alzheimer's disease. A second episode of such surgery reversed that decline at borderline significance (Tables 1 and 2). We found no difference between men and women. We found no independent influence of the APOEε4 allele on the effects of surgery.
A few longitudinal studies have examined cognitive 15, 16, 17, 18 or functional decline 16, 30 after surgery. An early study 15, with a wide age‐range (24–86 years), found no association of cognitive performance with a history of general anaesthesia, nor any interaction with age. However, two recent studies 16, 18 have found effects of surgery on cognitive decline in the elderly. One of those studies 18, of 1731 cognitively normal participants, with a mean age of 79 years at recruitment, found no association of surgery after the age of 40 years with incident mild cognitive impairment during a median follow‐up of 4.8 years, but did find a risk of mild cognitive impairment associated with surgery after the age of 60 years or during the 10 years or 20 years before diagnosis of mild cognitive impairment. They did not find any increased risk associated with multiple (≥ 4) episodes of surgery, however. Our findings, including the apparently beneficial effect of a second surgery, were consistent with those of their results relating to the elderly. Some reports have suggested 17, as we have shown above, that pre‐existing cognitive impairment may contribute to greater postoperative cognitive dysfunction. One study 31 replicated, in elderly people with mild cognitive impairment, the pre‐clinical findings 6, 13 of relatively benign actions of propofol, compared with inhalational anaesthetics.
Schenning et al. 16 reported an interaction between surgery and APOEε4 contributing to cognitive decline. But neither we nor Sprung et al. 18 found any such effect. Further, a meta‐analysis of 1063 APOEε4 carriers and 2983 non‐carriers 32 found no association of APOEε4 with increased cognitive decline at 1 year after surgery (OR 1.15, 95% CI 0.71–1.86). However, APOEε4 has been shown to accelerate cognitive decline in elderly people, whether non‐demented 25 or with Alzheimer's disease 24, regardless of surgery. Thus, elderly cognitively impaired, APOEε4 carriers who experience moderate or major surgery may be in danger of suffering particularly rapid cognitive decline.
More studies have investigated associations of surgery or anaesthesia with either dementia 20, 21, 33, 34, 35, 36 or Alzheimer's disease 33, 37, 38, 39, 40. Several 20, 21, 38, 40 have reported increased risks for these outcomes, but several have not 33, 34, 35, 36, 39. Two very large Taiwanese studies 20, 21, both based on medical records, found an increased risk of dementia following surgery with anaesthesia, of various types 20. A large US study 40 found an increased risk of Alzheimer's disease for coronary artery bypass graft surgery compared with percutaneous transluminal coronary angioplasty. In contrast, a recent large US study 33 reported a reduced risk of dementia or of Alzheimer's disease associated with previous surgery with general anaesthesia. But their method, based on the elderly participants’ reports of lifetime exposures, is very likely to be subject to recall bias, as the authors accept. However, a meta‐analysis of 15 case–control, mostly general epidemiological, studies 19 found no evidence of an association between general anaesthesia and risk of Alzheimer's disease: pooled OR 1.05 (0.93–1.19). But the authors commented on the few high‐quality studies and found no valid cohort studies. Also, some large studies, for example, the two Taiwanese studies 20, 21, have been reported since that meta‐analysis. Generally, it appears unreliable to base primary data on the recollections of elderly participants, especially those shortly to be diagnosed with mild cognitive impairment or dementia. This is likely to bias the result against a positive association with such risk.
To improve the definition(s) of the vulnerable, population(s) will require the study of elements such as age, sex and genetic and environmental factors. Although we found no effects of age or sex, we studied a rather narrow age range (IQR 66.6–77.8 years). A younger cohort would be unlikely to show any effect, since very few would already be cognitively impaired pre‐surgery. But the study of a sufficiently large cohort, including cognitively impaired people, with a broad interquartile range extending beyond the age of 80 years, could be valuable. We note that several of the studies that failed to find a relevant association 15, 33, 35, 39, but not all 34, 36, had a substantial proportion of participants who had surgery when < 70 years old. As the definition of the vulnerable population(s) is refined, some of the apparent contradictions above may be explained.
The balance of current evidence suggests that anaesthesia and surgery may provoke long‐term cognitive damage in certain vulnerable elderly people, which may lead to dementia. These effects may be limited to those who are already cognitively impaired, even if only mildly. Carriage of the APOEε4 allele exacerbates cognitive decline, even if there is no direct independent interaction with the effects of surgery and anaesthesia. It also appears that certain anaesthetics, including some that are widely used, may be the harmful agents, while others may be relatively benign. Moreover, current options for preventing or even moderating these effects are somewhat limited. If this is so, then it becomes of considerable importance to define the susceptible population(s), to specify the harmful agents, to understand the damaging mechanisms and to discover appropriate solutions. That is because there will always be elderly people who will need major surgery, despite the risks to their cognitive powers.
Competing interests
No competing interests declared. Dr Dorrington is supported by the Dunhill Medical Trust (Grant R178/1110).
Acknowledgements
We thank Dr Grzegorz Agacinski for expert help with data retrieval from OPTIMA records.
☛ CPD available at http://www.learnataagbi.org
This article is accompanied by an editorial by Docherty and Shenkin, Anaesthesia 2016; 71: 1131–5.
You can respond to this article at http://www.anaesthesiacorrespondence.com
References
- 1. Scott DA, Silbert BS, Evered LA. Anesthesia and Alzheimer's disease: time to wake up! International Psychogeriatrics 2013; 25: 341–4. [DOI] [PubMed] [Google Scholar]
- 2. Jevtovic‐Todorovic V, Absalom AR, Blomgren K, et al. Anaesthetic neurotoxicity and neuroplasticity: an expert group report and statement based on the BJA Salzburg Seminar. British Journal of Anaesthesia 2013; 111: 143–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Xie Z, Xu Z. General anesthetics and b‐amyloid protein. Progress in Neuropsychopharmacological Biology and Psychiatry 2013; 47: 140–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. United Nations Department of Economic and Social Affairs PD . World Population Prospects: The 2015 Revision, Key Findings and Advance Tables. 2015.
- 5. Dong Y, Wu X, Xu Z, Zhang Y, Xie Z. Anesthetic isoflurane increases phosphorylated tau levels mediated by caspase activation and Aβ generation. PLoS ONE 2012; 7: e39386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Eckenhoff RG, Johansson JS, Wei H, et al. Inhaled anesthetic enhancement of amyloid‐beta oligomerization and cytotoxicity. Anesthesiology 2004; 101: 703–9. [DOI] [PubMed] [Google Scholar]
- 7. Ni C, Tan G, Luo A, et al. Melatonin premedication attenuates isoflurane anesthesia‐induced beta‐amyloid generation and cholinergic dysfunction in the hippocampus of aged rats. International Journal of Neuroscience 2013; 123: 213–20. [DOI] [PubMed] [Google Scholar]
- 8. Tian Y, Guo S, Guo Y, Jian L. Anesthetic propofol attenuates apoptosis, Aβ accumulation, and inflammation induced by sevoflurane through NF‐kB pathway in human neuroglioma cells. Cellular and Molecular Neurobiology 2015; 35: 891–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wei H, Kang B, Wei W, et al. Isoflurane and sevoflurane affect cell survival and BCL‐2/BAX ratio differently. Brain Research 2005; 1037: 139–47. [DOI] [PubMed] [Google Scholar]
- 10. Wei H, Xie Z. Anesthesia, calcium homeostasis and Alzheimer's disease. Current Alzheimer Research 2009; 6: 30–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Corcoran TB, Engel A, Sakamoto H, O'Shea A, O'Callaghan‐Enright S, Shorten GD. The effects of propofol on neutrophil function, lipid peroxidation and inflammatory response during elective coronary artery bypass grafting in patients with impaired ventricular function. British Journal of Anaesthesia 2006; 97: 825–31. [DOI] [PubMed] [Google Scholar]
- 12. Ye X, Lian Q, Eckenhoff MF, Eckenhoff RG, Pan JZ. Differential general anesthetic effects on microglial cytokine expression. PLoS ONE 2013; 8: e52887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhang Y, Zhen Y, Dong Y, et al. Anesthetic propofol attenuates the isoflurane‐induced caspase‐3 activation and Aβ oligomerization. PLoS ONE 2011; 6: e27019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Whittington RA, Virág L, Marcouiller F, et al. Propofol directly increases tau phosphorylation. PLoS ONE 2011; 6: e16648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dijkstra JB, Van Boxtel MP, Houx PJ, Jolles J. An operation under general anesthesia as a risk factor for age‐related cognitive decline: results from a large cross‐sectional population study. Journal of the American Geriatrics Society 1998; 46: 1258–65. [DOI] [PubMed] [Google Scholar]
- 16. Schenning KJ, Murchison CF, Mattek NC, Silbert LC, Kaye JA, Quinn JF. Surgery is associated with ventricular enlargement as well as cognitive and functional decline. Alzheimer‘s and Dementia 2016; 12: 590–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Silbert B, Evered L, Scott DA, et al. Preexisting cognitive impairment is associated with postoperative cognitive dysfunction after hip joint replacement surgery. Anesthesiology 2015; 122: 1224–34. [DOI] [PubMed] [Google Scholar]
- 18. Sprung J, Roberts RO, Knopman DS, et al. Association of mild cognitive impairment with exposure to general anaesthesia for surgical and nonsurgical procedures: a population‐based study. Mayo Clinic Proceedings 2016; 91: 208–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Seitz DP, Shah PS, Herrmann N, Beyene J, Siddiqui N. Exposure to general anesthesia and risk of Alzheimer's disease: a systematic review and meta‐analysis. BMC Geriatrics 2011; 11: 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen CW, Lin CC, Chen KB, Kuo YC, Li CY, Chung CJ. Increased risk of dementia in people with previous exposure to general anesthesia: a nationwide population‐based case‐control study. Alzheimer's and Dementia 2014; 10: 196–204. [DOI] [PubMed] [Google Scholar]
- 21. Chen PL, Yang CW, Tseng YK, et al. Risk of dementia after anaesthesia and surgery. British Journal of Psychiatry 2014; 204: 188–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Baranov D, Bickler PE, Crosby GJ, et al. Consensus statement: First International Workshop on Anesthetics and Alzheimer's disease. Anesthesia and Analgesia 2009; 108: 1627–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Huppert FA, Brayne C, Gill C, Paykel ES, Beardsall L. CAMCOG – a concise neuropsychological test to assist dementia diagnosis: socio‐demographic determinants in an elderly population sample. British Journal of Clinical Psychology 1995; 34(Pt 4): 529–41. [DOI] [PubMed] [Google Scholar]
- 24. Martins CA, Oulhaj A, de Jager CA, Williams JH. APOE alleles predict the rate of cognitive decline in Alzheimer disease: a nonlinear model. Neurology 2005; 65: 1888–93. [DOI] [PubMed] [Google Scholar]
- 25. Salmon DP, Ferris SH, Thomas RG, et al. Age and apolipoprotein E genotype influence rate of cognitive decline in nondemented elderly. Neuropsychology 2013; 27: 391–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Farrer LA, Cupples LA, Haines JL, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta‐analysis. APOE and Alzheimer Disease Meta Analysis Consortium. Journal of the American Medical Association 1997; 278: 1349–56. [PubMed] [Google Scholar]
- 27. Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Archives of Neurology 1999; 56: 303–8. [DOI] [PubMed] [Google Scholar]
- 28. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS‐ADRDA work group under the auspices of Department of Health and Human Services task force on Alzheimer's disease. Neurology 1984; 34: 939–44. [DOI] [PubMed] [Google Scholar]
- 29. Rabbitt P, Diggle P, Smith D, Holland F, Mc Innes L. Identifying and separating the effects of practice and of cognitive ageing during a large longitudinal study of elderly community residents. Neuropsychologia 2001; 39: 532–43. [DOI] [PubMed] [Google Scholar]
- 30. Steinmetz J, Christensen KB, Lund T, Lohse N, Rasmussen LS. Long‐term consequences of postoperative cognitive dysfunction. Anesthesiology 2009; 110: 548–55. [DOI] [PubMed] [Google Scholar]
- 31. Liu Y, Pan N, Ma Y, et al. Inhaled sevoflurane may promote progression of amnestic mild cognitive impairment: a prospective, randomized parallel‐group study. American Journal of Medical Sciences 2013; 345: 355–60. [DOI] [PubMed] [Google Scholar]
- 32. Cao L, Wang K, Gu T, Du B, Song J. Association between APOE epsilon 4 allele and postoperative cognitive dysfunction: a meta‐analysis. International Journal of Neuroscience 2014; 124: 478–85. [DOI] [PubMed] [Google Scholar]
- 33. Aiello Bowles EJ, Larson EB, Pong RP, et al. Anesthesia exposure and risk of dementia and Alzheimer's disease: a prospective study. Journal of the American Geriatrics Society 2016; 64: 602–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Knopman DS, Petersen RC, Cha RH, Edland SD, Rocca WA. Coronary artery bypass grafting is not a risk factor for dementia or Alzheimer disease. Neurology 2005; 65: 986–90. [DOI] [PubMed] [Google Scholar]
- 35. Sprung J, Jankowski CJ, Roberts RO, et al. Anesthesia and incident dementia: a population‐based, nested, case‐control study. Mayo Clinic Proceedings 2013; 88: 552–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yip AG, Brayne C, Matthews FE. Risk factors for incident dementia in England and Wales: the Medical Research Council Cognitive Function and Ageing Study. A population‐based nested case‐control study. Age and Ageing 2006; 35: 154–60. [DOI] [PubMed] [Google Scholar]
- 37. Bohnen NI, Warner MA, Kokmen E, Beard CM, Kurland LT. Alzheimer's disease and cumulative exposure to anesthesia: a case‐control study. Journal of the American Geriatrics Society 1994; 42: 198–201. [DOI] [PubMed] [Google Scholar]
- 38. Bufill E, Bartes A, Moral A, et al. Genetic and environmental factors that may influence in the senile form of Alzheimer's disease: nested case control studies. [Article in Spanish] Neurologia 2009; 24: 108–12. [PubMed] [Google Scholar]
- 39. Gasparini M, Vanacore N, Schiaffini C, et al. A case‐control study on Alzheimer's disease and exposure to anesthesia. Neurological Sciences 2002; 23: 11–4. [DOI] [PubMed] [Google Scholar]
- 40. Lee TA, Wolozin B, Weiss KB, Bednar MM. Assessment of the emergence of Alzheimer's disease following coronary artery bypass graft surgery or percutaneous transluminal coronary angioplasty. Journal of Alzheimer‘s Disease 2005; 7: 319–24. [DOI] [PubMed] [Google Scholar]


