Abstract
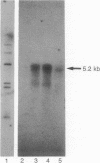
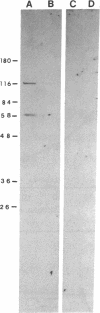
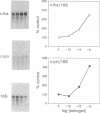
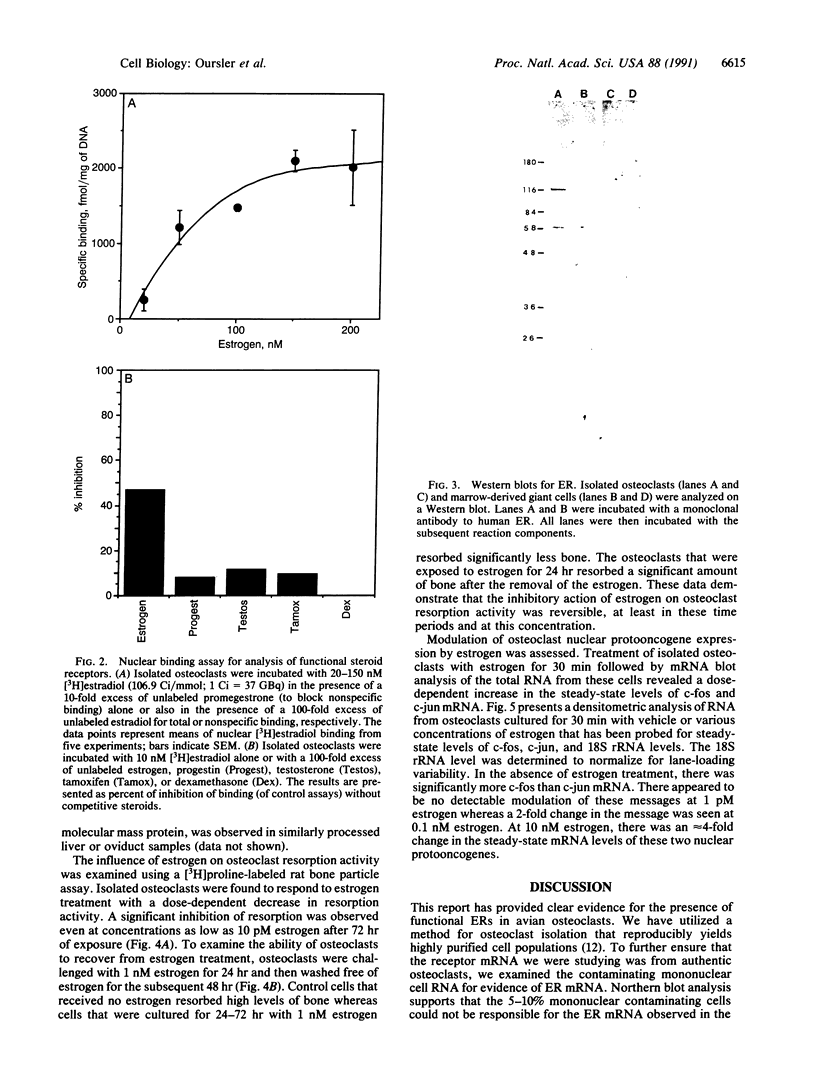
Although in vivo effects of 17 beta-estradiol (estrogen) on bone turnover have been shown to occur mainly through influences on osteoclast-mediated bone resorption, the mechanism by which estrogen reduces bone resorption is unclear. To approach this question, we have examined authentic osteoclasts for evidence of a direct osteoclast response to estrogen in vitro. Highly purified (greater than (90%) viable avian osteoclasts from birds maintained on a low calcium diet were obtained using an osteoclast-specific monoclonal antibody coupled to magnetic beads. Isolated cells were either analyzed directly for estrogen receptor (ER) levels or cultured to assess the biological effects of estrogen. Northern blot analysis revealed a 5.2-kilobase mRNA that hybridized with a cDNA to human ER mRNA in the osteoclasts. An anti-human ER antibody recognized proteins of 66 kDa and 140 kDa in osteoclast extracts by Western blot analysis. The 66-kDa size is in close agreement with the reported size of the human ER. Nuclear binding of estrogen to intact viable osteoclasts was steroid-specific and saturable, with 5662 +/- 1420 molecules bound per nucleus (mean +/- SEM). In vitro estrogen responses in osteoclasts included a dose-dependent decrease in resorption as well as an increase in nuclear protooncogene mRNA levels. These observations indicate that osteoclasts are capable of directly responding to estrogen in vivo.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair H. C., Kahn A. J., Crouch E. C., Jeffrey J. J., Teitelbaum S. L. Isolated osteoclasts resorb the organic and inorganic components of bone. J Cell Biol. 1986 Apr;102(4):1164–1172. doi: 10.1083/jcb.102.4.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake M. S., Johnston K. H., Russell-Jones G. J., Gotschlich E. C. A rapid, sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on Western blots. Anal Biochem. 1984 Jan;136(1):175–179. doi: 10.1016/0003-2697(84)90320-8. [DOI] [PubMed] [Google Scholar]
- Bohmann D., Tjian R. Biochemical analysis of transcriptional activation by Jun: differential activity of c- and v-Jun. Cell. 1989 Nov 17;59(4):709–717. doi: 10.1016/0092-8674(89)90017-2. [DOI] [PubMed] [Google Scholar]
- Caputo C. B., Meadows D., Raisz L. G. Failure of estrogens and androgens to inhibit bone resorption in tissue culture. Endocrinology. 1976 Apr;98(4):1065–1068. doi: 10.1210/endo-98-4-1065. [DOI] [PubMed] [Google Scholar]
- Chen T. L., Feldman D. Distinction between alpha-fetoprotein and intracellular estrogen receptors: evidence against the presence of estradiol receptors in rat bone. Endocrinology. 1978 Jan;102(1):236–244. doi: 10.1210/endo-102-1-236. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Eriksen E. F., Colvard D. S., Berg N. J., Graham M. L., Mann K. G., Spelsberg T. C., Riggs B. L. Evidence of estrogen receptors in normal human osteoblast-like cells. Science. 1988 Jul 1;241(4861):84–86. doi: 10.1126/science.3388021. [DOI] [PubMed] [Google Scholar]
- Greene G. L., Nolan C., Engler J. P., Jensen E. V. Monoclonal antibodies to human estrogen receptor. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5115–5119. doi: 10.1073/pnas.77.9.5115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsman A., Gallagher J. C., Simpson M., Nordin B. E. Prospective trial of oestrogen and calcium in postmenopausal women. Br Med J. 1977 Sep 24;2(6090):789–792. doi: 10.1136/bmj.2.6090.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King W. J., Greene G. L. Monoclonal antibodies localize oestrogen receptor in the nuclei of target cells. Nature. 1984 Feb 23;307(5953):745–747. doi: 10.1038/307745a0. [DOI] [PubMed] [Google Scholar]
- Komm B. S., Terpening C. M., Benz D. J., Graeme K. A., Gallegos A., Korc M., Greene G. L., O'Malley B. W., Haussler M. R. Estrogen binding, receptor mRNA, and biologic response in osteoblast-like osteosarcoma cells. Science. 1988 Jul 1;241(4861):81–84. doi: 10.1126/science.3164526. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McSheehy P. M., Chambers T. J. Osteoblast-like cells in the presence of parathyroid hormone release soluble factor that stimulates osteoclastic bone resorption. Endocrinology. 1986 Oct;119(4):1654–1659. doi: 10.1210/endo-119-4-1654. [DOI] [PubMed] [Google Scholar]
- Oursler M. J., Collin-Osdoby P., Anderson F., Li L., Webber D., Osdoby P. Isolation of avian osteoclasts: improved techniques to preferentially purify viable cells. J Bone Miner Res. 1991 Apr;6(4):375–385. doi: 10.1002/jbmr.5650060409. [DOI] [PubMed] [Google Scholar]
- Oursler M. J., Osdoby P. Osteoclast development in marrow cultured in calvaria-conditioned media. Dev Biol. 1988 May;127(1):170–178. doi: 10.1016/0012-1606(88)90198-4. [DOI] [PubMed] [Google Scholar]
- Pensler J. M., Radosevich J. A., Higbee R., Langman C. B. Osteoclasts isolated from membranous bone in children exhibit nuclear estrogen and progesterone receptors. J Bone Miner Res. 1990 Aug;5(8):797–802. doi: 10.1002/jbmr.5650050802. [DOI] [PubMed] [Google Scholar]
- Ransone L. J., Verma I. M. Nuclear proto-oncogenes fos and jun. Annu Rev Cell Biol. 1990;6:539–557. doi: 10.1146/annurev.cb.06.110190.002543. [DOI] [PubMed] [Google Scholar]
- Rauscher F. J., 3rd, Sambucetti L. C., Curran T., Distel R. J., Spiegelman B. M. Common DNA binding site for Fos protein complexes and transcription factor AP-1. Cell. 1988 Feb 12;52(3):471–480. doi: 10.1016/s0092-8674(88)80039-4. [DOI] [PubMed] [Google Scholar]
- Riggs B. L., Melton L. J., 3rd Involutional osteoporosis. N Engl J Med. 1986 Jun 26;314(26):1676–1686. doi: 10.1056/NEJM198606263142605. [DOI] [PubMed] [Google Scholar]
- Spelsberg T. C., Graham M. L., 2nd, Berg N. J., Umehara T., Riehl E., Coulam C. B., Ingle J. N. A nuclear binding assay to assess the biological activity of steroid receptors in isolated animal and human tissues. Endocrinology. 1987 Aug;121(2):631–644. doi: 10.1210/endo-121-2-631. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka T., Sato B., Matsumoto K., Ono K. Steroid receptors in osteoblasts. Clin Orthop Relat Res. 1980 May;(148):297–303. [PubMed] [Google Scholar]
- van Paassen H. C., Poortman J., Borgart-Creutzburg I. H., Thijssen J. H., Duursma S. A. Oestrogen binding proteins in bone cell cytosol. Calcif Tissue Res. 1978 Aug 18;25(3):249–254. doi: 10.1007/BF02010778. [DOI] [PubMed] [Google Scholar]