Abstract
The first Ir-catalyzed enantioselective allylation of trisubstituted allylic electrophiles has been developed. Through modification of the leaving group of allylic electrophiles, we found that trisubstituted allylic phosphates are suitable electrophiles for asymmetric allylation. The reaction of allylic phosphates with enol silanes derived from dioxinones gave allylated products in good yields with high enantioselectivities.
Keywords: Ir-catalyzed asymmetric allylic substitution; trisubstituted allylic electrophiles; allylic phosphates; 1,1’-substituted olefin; dioxinone
Graphical Abstract
The first Ir-catalyzed enantioselective allylic substitution of trisubstituted allylic electrophiles has been developed. By employing allylic phosphates as electrophiles, asymmetric allylic substitution of enol silanes derived from dioxinones gave allylated products in good yields with high enantioselectivities.
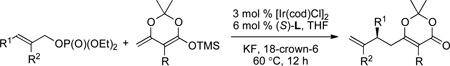
Iridium-catalyzed, enantioselective allylic substitution has become a powerful method to access highly enantioenriched molecules.1,2 This class of reaction began to occur in high yield and ee when a metalacyclic catalyst derived from a phosphoramidite ligand was developed. Over the past decade, research with these catalysts has focused on assessing and increasing the scope of nucleophile. In this body of work, cinnamyl carbonates and, in some cases, aliphatic allylic carbonates have been used as electrophiles. With few exceptions,3 these carbonates contain one aryl, alkyl or vinyl substituent and one leaving group to generate products in which the terminal alkene unit contains a single substituent (Figure 1).4–7
Figure 1.
Ir-catalyzed Enantioselective Allylic Substitution of Trisubstituted Electrophiles 1 with Enol Silane 2.
The inability to use allylic electrophiles containing more than one substituent, besides the leaving group, in asymmetric allylic substitutions is a major limitation on the eventual application of such reactions to the syntheses of complex molecules. In the current work, we have sought to develop systems that allow asymmetric allylic substitutions to occur with allylic electrophiles that are more substituted than those used previously, while maintaining the high regioselectivity for formation of branched products that characterizes iridium-catalyzed allylic substitution. In particular, we have sought to develop systems for asymmetric reactions of allylic esters containing 1,2-disubstituted alkene units to form products containing 1,1’-disubstituted olefins. Under the standard conditions comprising a carbonate leaving group and cyclometalated catalyst derived from a phosphoramidite, such reactions do not occur. This lack of reactivity is consistent with the absence of such reactions in the prior publications and highlights the need to find combinations of catalyst, leaving group, and nucleophile that will enable such processes.
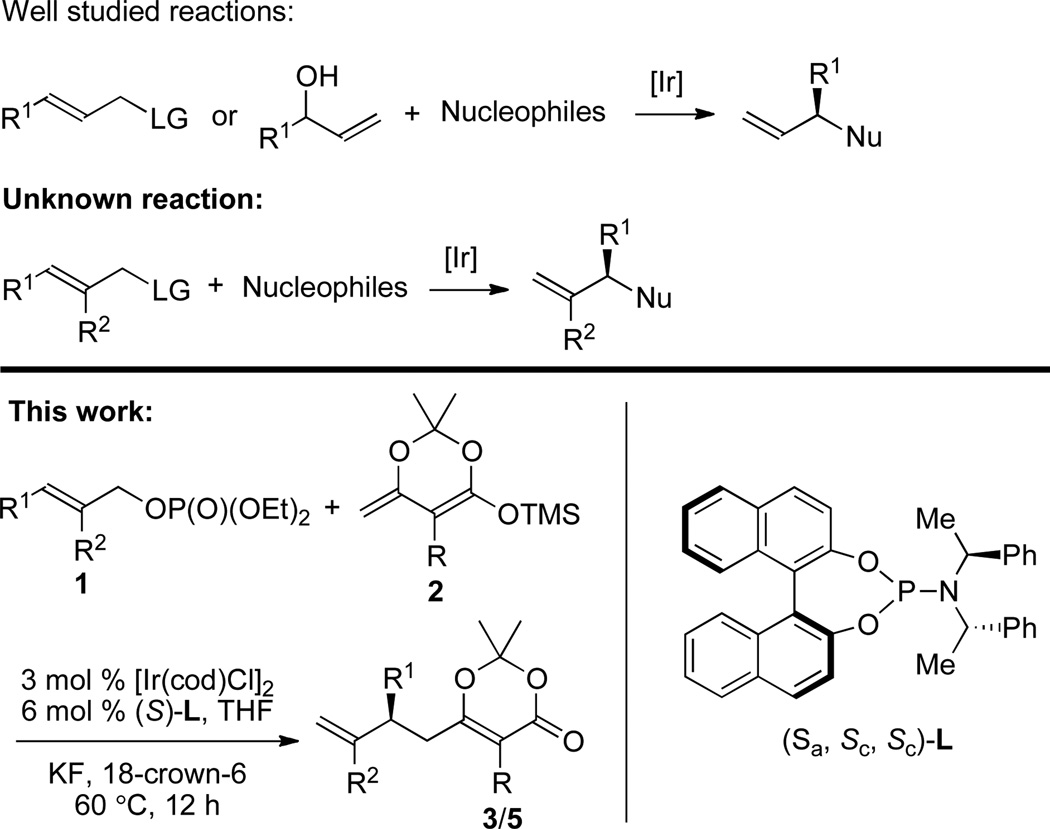
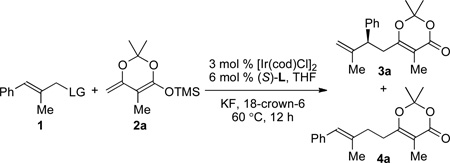
Here, we report the first Ir-catalyzed, enantioselective allylic substitution of a trisubstituted allylic electrophile. By using a phosphate as the leaving group, and enol silanes derived from dioxinones8 as nucleophiles, the established cyclometalated iridium complexes catalyze allylic substitution with trisubstituted allylic phosphates to form products containing a 1,1’-disubstituted alkenes in good yields with high enantioselectivities and branched-to-linear ratios (Figure 1). The 1,1’-disubstituted olefin unit and the dioxinone moiety can be transformed to a variety of functional groups by established methods.9
We began our studies to achieve allylic substitution of trisubstituted allylic esters by examining the reactions of a series of nucleophiles with methyl carbonate derived from 2-methyl cinnamyl alcohol in the presence of [Ir(cod)Cl]2 (cod = cyclooctadiene) and the phosphoramidite ligand (Sa,Sc,Sc)-L. We found that the reaction of methyl carbonate with enol silane 2a derived from dioxanone we reported recently to undergo Ir-catalyzed substitution reactions to form products from formal allylation at the less acidic position of a dicarbonyl compound6e gave product 3a in 23% yield. Reactions with enol silanes derived from ketones and α,β-unsaturated ketones did not form any detectable amount of product.
To improve the yield of product 3a formed from the reaction, next we surveyed reactions of enol silane 2a with several allylic esters. As shown in Table 1, treatment of cinnamyl trifluoroacetate (1 equiv) and enol silane 2a (2 equiv) in the presence of 3 mol % [Ir(cod)Cl]2 and 6 mol % (S)-L with KF and 18-crown-6 as additives at 60 °C for 12 hours did not form a detectable amount of product 3a (entry 1, Table 1). The reaction of allyl methyl carbonate with silane 2a under the same conditions gave a 4:1 ratio of branched product 3a to the linear adduct 4a in 23% yield (entry 2, Table 1). The reaction of cinnamyl trichloroethyl carbonate gave a lower 3:1 branched-to-linear (b/l) ratio in 31% yield (entry 3, Table 1), and the reaction of the analogous t-Bu-carbonate gave no product (entry 4, Table 1). However, allylic substitution of allyl ethyl phosphate with 2a provided branched product 3a in 77% yield, 98% ee, and a 20:1 b/l ratio (entry 5, Table 1).
Table 1.
Evaluation of the Reaction Conditions for the Ir-catalyzed Asymmetric Allylic Substitution of Esters 1 with Dienolate 2a.a
 | ||||
|---|---|---|---|---|
| entry | LG | b:lb | Yield (3a)c | % eed |
| 1 | OCOCF3 | N.D. | N.R. | N.D. |
| 2 | OCO2Me | 4:1 | 23% | N.D. |
| 3 | OCO2CH2CCl3 | 3:1 | 31% | N.D. |
| 4 | OCO2t-Bu | N.D. | N.R. | N.D. |
| 5 | OP(O)(OEt)2 | 20:1 | 77% | 98% |
Reaction conditions: allylic ester 1 (0.1 mmol, 1.0 equiv), dinenolate 2a (0.2 mmol, 2.0 equiv), [Ir(cod)Cl]2 (3 mol %), (Sa,Sc,Sc)-L (6 mol %), KF (1.0 equiv), 18-crown-6 (1.0 equiv), THF (0.2 mL), 60 °C, 12 h.
Ratios of branched (3a) to linear product (4a) were determined by 1H NMR analysis of the crude reaction mixtures.
Yield of isolated product.
Ee % of 3a was determined by HPLC analysis using a chiral stationary phase.
N.D. = not determined. N.R. = no reaction.
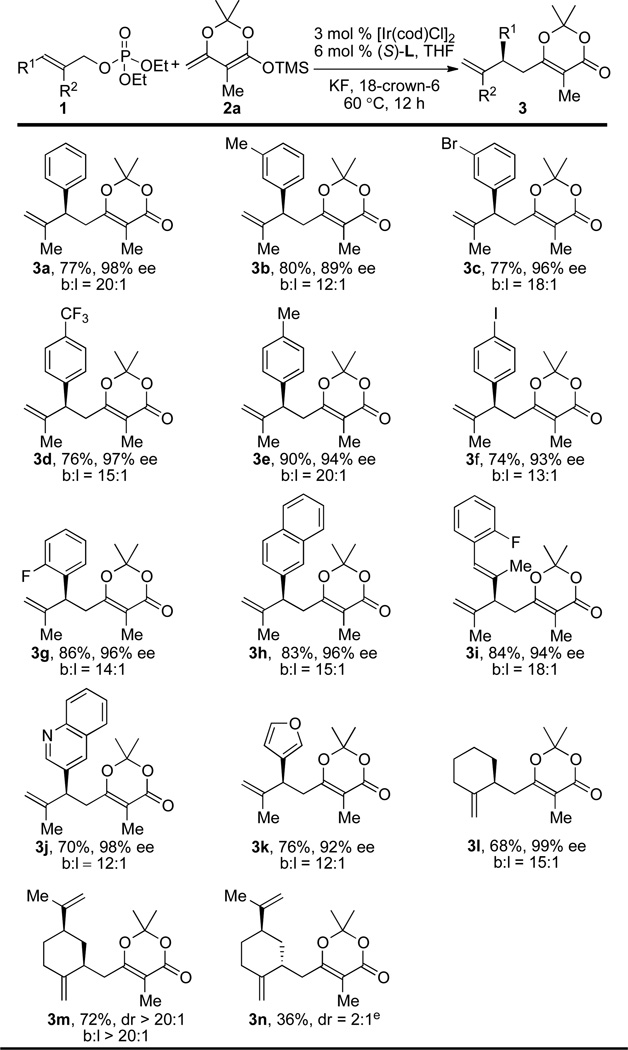
The scope of the allylic phosphate that underwent the Ir-catalyzed enantioselective allylic substitution with enol silane 2a is summarized in Table 2. Reactions with a variety of trisubstituted allylic phosphates containing a series of substitution patterns on the arene were examined. Asymmetric allylation of substrates bearing substituents at the meta-position of the arene gave products 3b–c in 77–80% yield with 89–96% ee and between 12:1 and 18:1 b/l ratios (entries 2–3, Table 2). Allylic phosphates bearing para-substituents also reacted to afford products 3d–f in 74–90% yield with 93–97% ee and 13:1 to 20:1 b/l ratios (entries 4–6, Table 2). Allylic substitution of ortho-fluoro substituted allylic phosphate provided product 3g in 86% yield with 96% ee and a 14:1 b/l ratio (entry 7, Table 2). Similar results were obtained with a naphthyl-substituted allylic phosphate (entry 8, Table 2). A dienyl phosphate reacted to give branched product 3i selectively (18:1 b/l ratio) in 84% yield with 94% ee. Heterocycles are tolerated under the developed reaction conditions. Reactions with allylic phosphates containing quinolinyl and furyl groups provided products 3j–k in 70–76% yield with 92–98% ee and a 12:1 branched to linear ratio (entries 10–11, Table 2).
Table 2.
Scope of the Ir-catalyzed Asymmetric Allylic Substitution of Allylic Phosphates 1 with Dinenolate 2a.a–d
Reaction conditions: allylic phosphate 1 (0.2 mmol, 1.0 equiv), dinenolate 2a (0.4 mmol, 2.0 equiv), [Ir(cod)Cl]2 (3 mol %), (Sa,Sc,Sc)-L (6 mol %), KF (1.0 equiv), 18-crown-6 (1.0 equiv), THF (0.4 mL), 60 °C, 12 h.
Branched to linear ratios were determined by 1H NMR analysis of the crude reaction mixtures.
Yields of isolated products are listed (the average of at least two runs).
Enantioselectivities were determined by HPLC analysis using a chiral stationary phase.
(Ra,Rc,Rc)-L was used.
Aliphatic allylic phosphates also reacted to give the substitution products in good yields, stereoselectivity and regioselectivity. In particular, the reaction occurred with cyclic allylic phosphates derived from allylic alcohols. The aliphatic allylic phosphate from cyclohexenyl methanol reacted to give 3l with high branched selectivity and enantioselectivity (68% yield, 99% ee, b/l = 15:1; entry 12, Table 2).
The reactions of a related chiral, non-racemic aliphatic allylic phosphate were also studied. The phosphate derived from (R)-perillyl alcohol converted under the standard reaction conditions (with (Sa,Sc,Sc)-L as the ligand) to the allylated product 3m in 72% yield with a >20:1 branched selectivity and a >20:1 diastereoselectivity. The reaction with the antipode of the phosphoramidite ligand, (Ra,Rc,Rc)-L, under otherwise identical reaction conditions formed a mixture of products 3n and 3m in a combined 36% yield and low diastereoselectivity (2:1), indicating that the reactions of these trisubstituted allylic esters occur with a matched and mismatched pairing of the catalyst with the enantioenriched allylic phosphate.
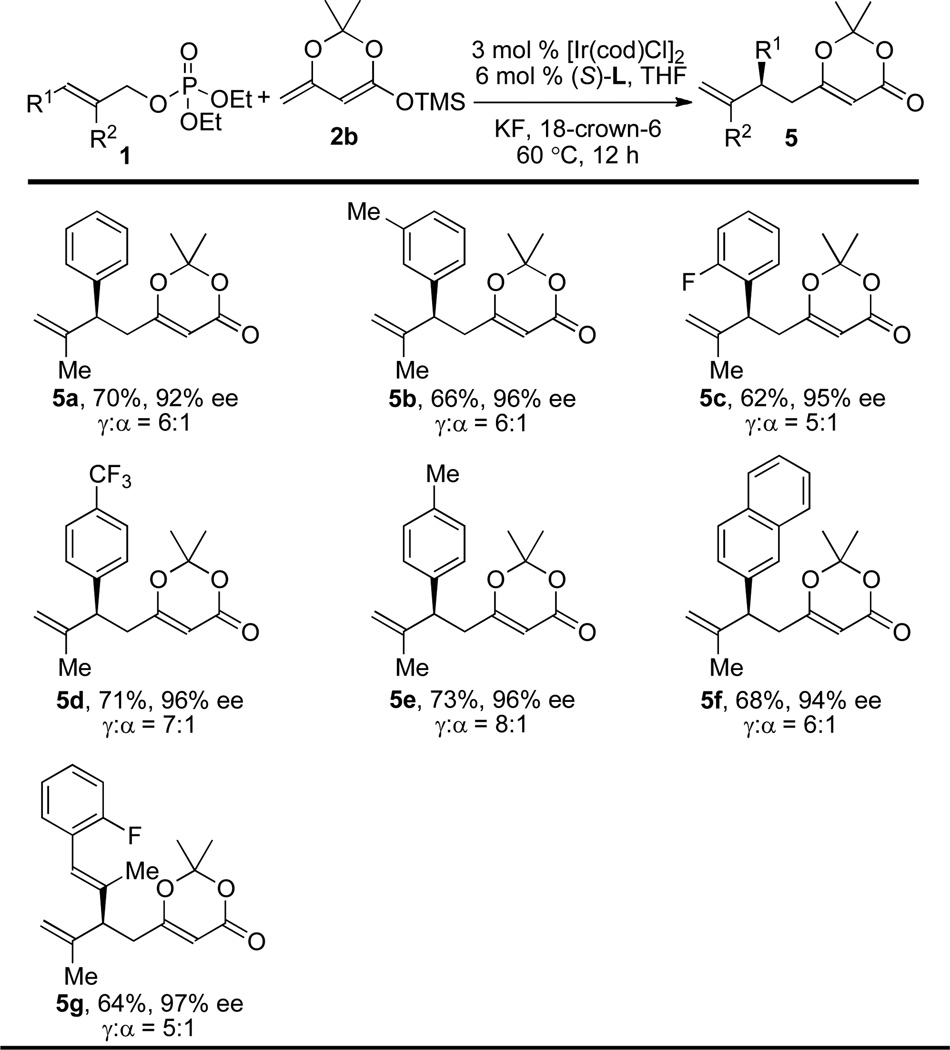
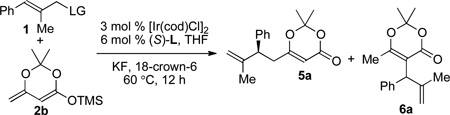
In contrast to nucleophilic additions of enol silane 2a, nucleophilic additions of enol silane 2b, which lacks a substituent at the α-position, can occur at both the α- and γ-positions. It is challenging to achieve high regioselectivity for the reaction of nucleophile 2b at the γ-position.10 To explore whether the allylation could occur regioselectively at the γ-position with enol silane 2b, allylic substitutions of several allylic electrophiles were examined (Table 3). The reaction of allylic methyl carbonate formed a 3:1 mixture of the product 5a from reaction at the γ-position of 2a to the product 6a from reaction at the α-position of 2a in a total yield of 17% (entry 1, Table 3). Reactions with allylic trifluoroacetate, t-Bu-carbonate and trichloroethyl carbonate failed to give product 5a in a substantial yield (entries 2–4, Table 3). However, the reaction of this nucleophile with the allylic ethyl phosphate as the electrophile occurred with a high ratio of products from addition at the γ-position to addition at the α-position, and the yield was significantly higher (entry 5, Table 3). The ratio of γ-substituted product 5a to α-substituted product 6a was 6:1, and the γ-adduct 5a was isolated in 70% yield with 92% ee (entry 5, Table 3). Allylic esters bearing dimethyl, di-2-ethylhexyl, or diisopropyl phosphates (entries 6–8, Table 3) reacted in the same or lower yield and γ– to α–selectivity. Therefore, diethylphosphates were used to evaluate the scope of the reaction of trisubstituted allylic esters with enol silane 2b.
Table 3.
Evaluation of the Reaction Conditions for the Ir-catalyzed Asymmetric Allylic Substitution of Allylic Esters 1 with Dienolate 2b.a
 | ||||
|---|---|---|---|---|
| entry | LG | γ:αb | Yield (5a)c | % eed |
| 1 | OCO2Me | 3:1 | 17% | N.D. |
| 2 | OCOCF3 | N.D. | N.R. | N.D. |
| 3 | OCO2t-Bu | N.D. | N.R. | N.D. |
| 4 | OCO2CH2CCl3 | N.D. | 11% | N.D. |
| 5 | OP(O)(OEt)2 | 6:1 | 70% | 92% |
| 6 | OP(O)(OMe)2 | 6:1 | 62% | 92% |
| 7 | OP(O)(Oi-Pr)2 | 3:1 | 49% | N.D. |
| 8 | OP(O)(OEH)2 | 3:1 | 41% | N.D. |
Reaction conditions: allylic ester 1 (0.1 mmol, 1.0 equiv), dinenolate 2b (0.2 mmol, 2.0 equiv), [Ir(cod)Cl]2 (3 mol %), (Sa,Sc,Sc)-L (6 mol %), KF (1.0 equiv), 18-crown-6 (1.0 equiv), THF (0.2 mL), 60 °C, 12 h.
Ratios of γ- to α-substitution were determined by 1H NMR analysis of the crude reaction mixtures.
Yield of isolated product (5a).
Ee % of 5a was determined by HPLC analysis using a chiral stationary phase.
N.D. = not determined. N.R. = no reaction. EH = 2-ethyl-hexyl.
The results of asymmetric allylic substitution of ethyl phosphates 1 with enol silane 2b are summarized in Table 4. Reactions of 2-methyl cinnamyl phosphates and alkenyl substituted allylic phosphates with silane 2b gave allylated products 5a–g in 62–73% yields with 92–97% ee and between 5:1 and 8:1 γ/α-selectivities (Table 4). In contrast, the reaction of alkyl substituted allylic electrophiles occurred with low γ/α-selectivities.
Table 4.
Scope of the Ir-catalyzed Asymmetric Allylic Substitution of Allylic Phosphates 1 with Dinenolate 2b.a–d
Reaction conditions: allylic phosphate 1 (0.2 mmol, 1.0 equiv), dinenolate 2b (0.4 mmol, 2.0 equiv), [Ir(cod)Cl]2 (3 mol %), (Sa,Sc,Sc)-L (6 mol %), KF (1.0 equiv), 18-crown-6 (1.0 equiv), THF (0.4 mL), 60 °C, 12 h.
Ratios of γ- to α-substitution were determined by 1H NMR analysis of the crude reaction mixtures.
Yields of isolated products 5 are listed (the average of at least two runs).
Enantioselectivities were determined by HPLC analysis using a chiral stationary phase.
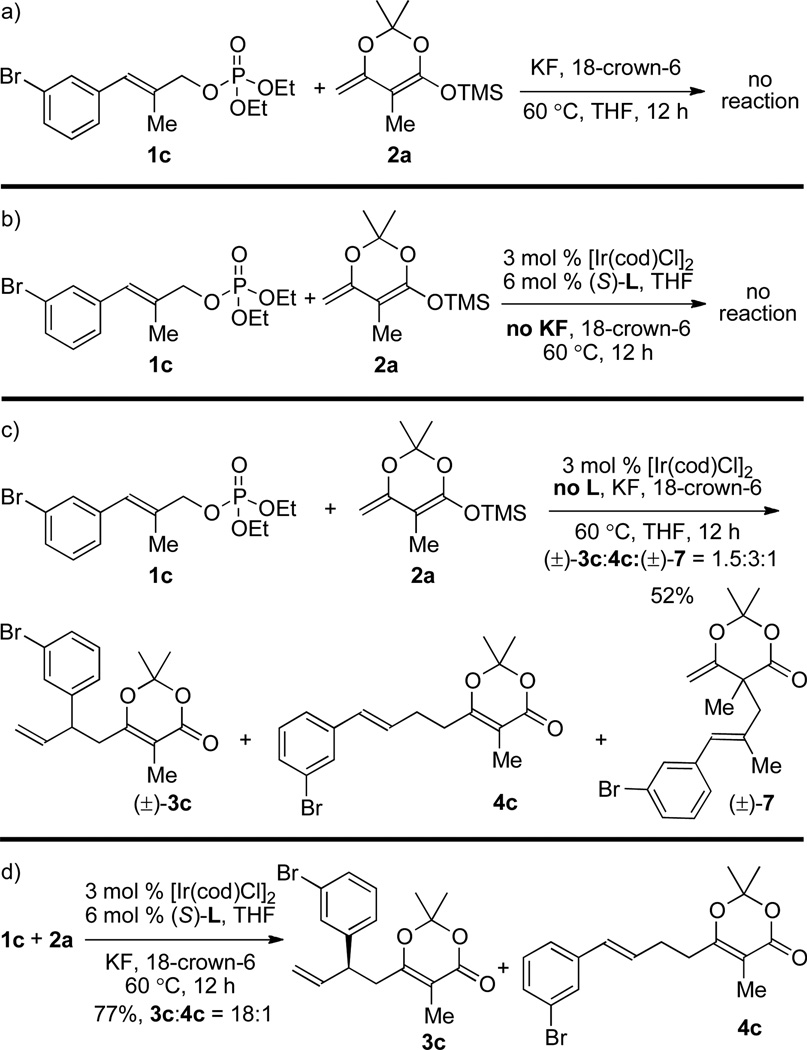
As demonstrated in Tables 1–4, allylic substitution of the trisubstituted allylic esters occurred in high regioselectivity and branched-to-linear selectivity only when conducted with the allylic phosphate. To reveal the relative influences of the leaving group, the ligand, and additives on selectivity of the reaction, the experiments summarized in Scheme 1 were conducted. In the absence of [Ir(cod)Cl]2 and the ligand (Sa,Sc,Sc)-L, no allylation occurred from the reaction of phosphate 1c with enol silane 2a (panel a, Scheme 1). No allylated product 3c was detected from reactions in the absence of KF (panel b, Scheme 1). However, [Ir(cod)Cl]2 catalyzes the allylic substitution of phosphate 1c with enol silane 2a without the phosphoramidite ligand. As shown in the panel c of Scheme 1, the allylation of 1c with enol silane 2a at 60 °C for 12 h gave a 1.5:3:1 mixture of (±)-3c, linear product 4c and the α-adduct (±)-7 in 52% combined yield in the presence of 3 mol % [Ir(cod)Cl]2, KF (1 equiv) and 18-crown-6 (1 equiv). The same reaction conducted with the catalyst generated from [Ir(cod)Cl]2 and the phosphoramidite ligand (Sa,Sc,Sc)-L occurred with much higher regioselectivity and branched selectivity (panel d, Scheme 1). These data indicate that the identity of the leaving group of the allylic electrophile alone does not control the site and branched-to-linear selectivities of these reactions. Instead, the combination of the phosphoramidite ligand L and the leaving group of the allylic electrophile is crucial to obtaining high regioselectivity at the nucleophile and high branched-to-linear selectivity at the electrophile.
Scheme 1.
Influence of the Ligand and Additives on the Selectivity.
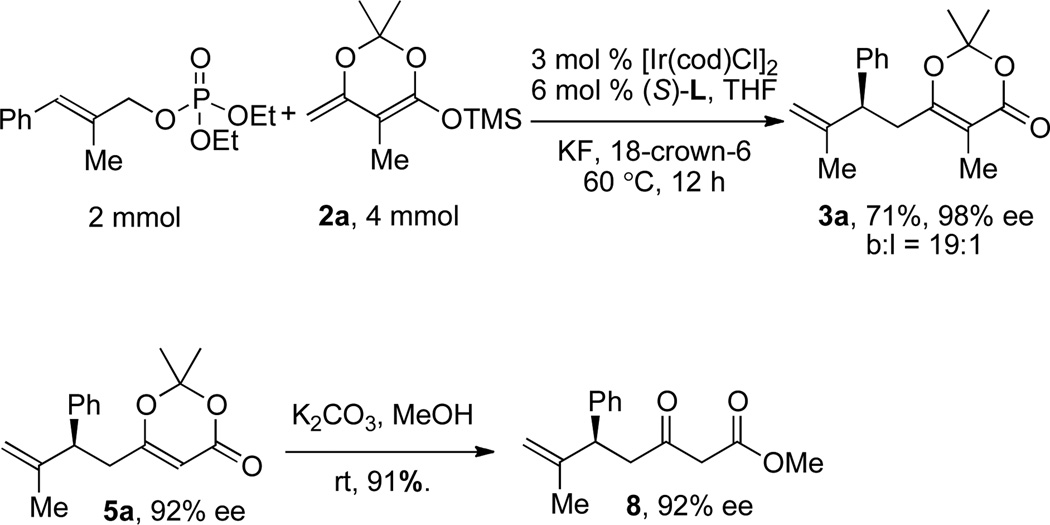
Finally, Scheme 2 shows that the reaction can be conducted on multi-mmol scale and that the product can be converted to the corresponding β-keto ester without eroding enantiomeric excess. The allylic substitution with 2 mmol allylic phosphate proceded smoothly to give allylated product 3a in 71% yield with 98% ee and a 19:1 branched-to-linear ratio under the same conditions as the reactions run on a smaller scale. The dioxinone moiety of 5a was converted to the β-keto ester by treatment of 5a (92% ee) with K2CO3 and MeOH at ambient temperature to give β-keto ester 8 in 91% yield with the same 92% ee as the reactant (Scheme 2).
Scheme 2.
A large-scale reaction and derivatization of compound 5a.
In conclusion, we have developed the first Ir-catalyzed enantioselective allylic alkylation of trisubstituted allylic electrophiles. By utilizing allylic phosphates as electrophiles, asymmetric allylic substitution of enol silanes derived from dioxinones gave allylated products in good yields with high enantioselectivities and branched-to-linear selectivities. The data presented here suggest that the combination of the phosphoramidite ligand and the phosphate leaving group is critical to obtain high regioselectivity and branched-to-linear selectivity.
The origins of these effects await detailed mechanistic studies, but we attribute the lack of reactions of linear, trisubstituted allylic esters to unfavorable thermodynamics for the oxidative addition of trisubstituted allylic esters to the Ir catalyst. Our previous studies have shown that the oxidative addition of a 1,2-disubstituted allyl methyl carbonate is endothermic and reversible.1f The oxidative addition of a 1,1,2-trisubstituted allylic ester should be even less favored thermodynamically because trisubstituted allylic electrophiles are sterically more hindered and would bind more weakly to the iridium than would a 1,2-disubstituted allylic electrophile. At the same time, it is known that the leaving group in the allyl electrophile influences the rate, and presumably thermodynamics, for oxidative addition.1c,e,11 Thus, we hypothesize that the identity of the leaving group and the nucleophile are crucial to observing these first enantioselective reactions of disubstituted allylic esters because the better leaving group make the reversible oxidative addition step more favored thermodynamically and the high nucleophilicity of the dioxinone effectively traps the small concentration of the allyliridium intermediate. Further studies to gain insight into the origin of the selectivity in this reaction and to expand the scope of nucleophile are currently underway in this laboratory.
Supplementary Material
Acknowledgments
Financial support provided by the National Institutes of Health (GM-58108) is gratefully acknowledged.
References
- 1.For seminal contributions, see: Takeuchi R, Kashio M. Angew. Chem., Int. Ed. 1997;36:263. Janssen JP, Helmchen G. Tetrahedron Lett. 1997;38:8025. Ohmura T, Hartwig JF. J. Am. Chem. Soc. 2002;124:15164. doi: 10.1021/ja028614m. Kiener CA, Shu C, Incarvito C, Hartwig JF. J. Am. Chem. Soc. 2003;125:14272. doi: 10.1021/ja038319h. Marković D, Hartwig JF. J. Am. Chem. Soc. 2007;129:11680. doi: 10.1021/ja074584h. Madrahimov ST, Marković D, Hartwig JF. J. Am. Chem. Soc. 2009;131:7228. doi: 10.1021/ja902609g. Madrahimov ST, Hartwig JF. J. Am. Chem. Soc. 2012;134:8136. doi: 10.1021/ja212217j. Madrahimov ST, Li Q, Sharma A, Hartwig JF. J. Am. Chem. Soc. 2015;137:14968. doi: 10.1021/jacs.5b08911.
- 2.For recent reviews of Ir-catalyzed asymmetric allylation, see: Helmchen G, Dahnz A, Dubon P, Schelwies M, Weihofen R. Chem. Commun. 2007:675. doi: 10.1039/b614169b. Helmchen G. In: Iridium Complexes in Organic Synthesis. Oro LA, Claver C, editors. Weinheim, Germany: Wiley-VCH; 2009. p. 211. Hartwig JF, Pouy MJ. Top. Organomet. Chem. 2011;34:169. Liu W-B, Xia J-B, You S-L. Top. Organomet. Chem. 2012;38:155. Tosatti P, Nelson A, Marsden SP. Org. Biomol. Chem. 2012;10:3147. doi: 10.1039/c2ob07086c. For a review of the use of phosphoramidite ligands in asymmetric catalysis, see: Teichert JF, Feringa BL. Angew. Chem., Int. Ed. 2010;49:2486. doi: 10.1002/anie.200904948.
- 3.For examples Cu-catalyzed enantioselective allylic substitution of 1,1,2-trisubstituted allyl electrophiles, See; Lee Y, Akiyama K, Gillingham DG, Brown MK, Hoveyda AH. J. Am. Chem. Soc. 2008;130:446. doi: 10.1021/ja0782192. Gao F, Carr JL, Hoveyda Amir. H. J. Am. Chem. Soc. 2014;136:2149. doi: 10.1021/ja4126565.
- 4.(a) Schafroth MA, Sarlah D, Krautwald S, Carreira EM. J. Am. Chem. Soc. 2012;134:20276. doi: 10.1021/ja310386m. [DOI] [PubMed] [Google Scholar]; (b) Hamilton JY, Sarlah D, Carreira EM. J. Am. Chem. Soc. 2013;135:994. doi: 10.1021/ja311422z. [DOI] [PubMed] [Google Scholar]; (c) Hamilton JY, Sarlah D, Carreira EM. Angew. Chem., Int. Ed. 2013;52:7532. doi: 10.1002/anie.201302731. [DOI] [PubMed] [Google Scholar]; (d) Krautwald S, Sarlah D, Schafroth MA, Carreira EM. Science. 2013;340:1065. doi: 10.1126/science.1237068. [DOI] [PubMed] [Google Scholar]; (e) Hamilton JY, Sarlah D, Carreira EM. J. Am. Chem. Soc. 2014;136:3006. doi: 10.1021/ja412962w. [DOI] [PubMed] [Google Scholar]; (f) Krautwald S, Schafroth MA, Sarlah D, Carreira EM. J. Am. Chem. Soc. 2014;136:3020. doi: 10.1021/ja5003247. [DOI] [PubMed] [Google Scholar]; (g) Hamilton JY, Hauser N, Sarlah D, Carreira EM. Angew. Chem. Int. Ed. 2014;53:10759. doi: 10.1002/anie.201406077. [DOI] [PubMed] [Google Scholar]; (h) Breitler S, Carreira EM. J. Am. Chem. Soc. 2015;137:5296. doi: 10.1021/jacs.5b01951. [DOI] [PubMed] [Google Scholar]; (i) Hamilton JY, Sarlah D, Carreira EM. Angew. Chem. Int. Ed. 2015;54:7644. doi: 10.1002/anie.201501851. [DOI] [PubMed] [Google Scholar]; (j) Sandmeier T, Krautwald S, Zipfel HF, Carreira EM. Angew. Chem. Int. Ed. 2015;54:14363. doi: 10.1002/anie.201506933. [DOI] [PubMed] [Google Scholar]
- 5.(a) Wu Q-F, He H, Liu W-B, You S-L. J. Am. Chem. Soc. 2010;132:11418. doi: 10.1021/ja105111n. [DOI] [PubMed] [Google Scholar]; (b) Ye K-Y, He H, Liu W-B, Dai L-X, Helmchen G, You S-L. J. Am. Chem. Soc. 2011;133:19006. doi: 10.1021/ja2092954. [DOI] [PubMed] [Google Scholar]; (c) Wu Q-F, Liu W-B, Zhuo C-X, Rong Z-Q, Ye K-Y, You S-L. Angew. Chem., Int. Ed. 2011;50:4455. doi: 10.1002/anie.201100206. [DOI] [PubMed] [Google Scholar]; (d) Wu Q-F, Zheng C, You S-L. Angew. Chem. Int. Ed. 2012;51:1680. doi: 10.1002/anie.201107677. [DOI] [PubMed] [Google Scholar]; (e) Liu W-B, Zheng C, Zhuo C-X, Dai L-X, You S-L. J. Am. Chem. Soc. 2012;134:4812. doi: 10.1021/ja210923k. [DOI] [PubMed] [Google Scholar]; (f) Zhuo C-X, Wu Q-F, Zhao Q, Xu Q-L, You S-L. J. Am. Chem. Soc. 2013;135:8169. doi: 10.1021/ja403535a. [DOI] [PubMed] [Google Scholar]; (g) Yang Z-P, Wu Q-F, You S-L. Angew. Chem. Int. Ed. 2014;53:6986. doi: 10.1002/anie.201404286. [DOI] [PubMed] [Google Scholar]; (h) Zheng C, Zhuo C-X, You S-L. J. Am. Chem. Soc. 2014;136:16251. doi: 10.1021/ja5080135. [DOI] [PubMed] [Google Scholar]; (i) Yang Z-P, Wu Q-F, Shao W, You S-L. J. Am. Chem. Soc. 2015;137:15899. doi: 10.1021/jacs.5b10440. [DOI] [PubMed] [Google Scholar]; (j) Zhang X, Yang Z-P, Hang L, You S-L. Angew. Chem. Int. Ed. 2015;54:1873. doi: 10.1002/anie.201409976. [DOI] [PubMed] [Google Scholar]; (k) Zhuo C-X, Chen Q, Liu W-B, Zhao Q, You S-L. Angew. Chem. Int. Ed. 2015;54:8475. doi: 10.1002/anie.201502259. [DOI] [PubMed] [Google Scholar]; (l) Cheng Q, Wang Y, You S-L. Angew. Chem. Int. Ed. 2016;55:3496. doi: 10.1002/anie.201511519. [DOI] [PubMed] [Google Scholar]; (m) Huang L, Dai L-X, You S-L. J. Am. Chem. Soc. 2016;138:5793. doi: 10.1021/jacs.6b02678. [DOI] [PubMed] [Google Scholar]
- 6.(a) Chen W, Hartwig JF. J. Am. Chem. Soc. 2012;134:15249. doi: 10.1021/ja306850b. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Chen W, Hartwig JF. J. Am. Chem. Soc. 2013;135:2068. doi: 10.1021/ja311363a. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Chen W, Hartwig JF. J. Am. Chem. Soc. 2014;136:377. doi: 10.1021/ja410650e. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Chen M, Hartwig JF. Angew. Chem., Int. Ed. 2014;53:8691. doi: 10.1002/anie.201403844. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Chen M, Hartwig JF. Angew. Chem. Int. Ed. 2014;53:12172. doi: 10.1002/anie.201406778. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Chen W, Chen M, Hartwig JF. J. Am. Chem. Soc. 2014;136:15825. doi: 10.1021/ja506500u. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Chen M, Hartwig JF. J. Am. Chem. Soc. 2015;136:13972. doi: 10.1021/jacs.5b09980. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Jiang X, Chen W, Hartwig JF. Angew. Chem., Int. Ed. 2016;55:5819. doi: 10.1002/anie.201600235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kanayama T, Yoshida K, Miyabe H, Takemoto Y. Angew. Chem., Int. Ed. 2003;42:2054. doi: 10.1002/anie.200250654. Polet D, Rathgeb X, Falciola CA, Langlois J-B, El HS, Alexakis A. Chem.—Eur. J. 2009;15:1205. doi: 10.1002/chem.200801879. Liu W-B, Reeves CM, Virgil SC, Stoltz BM. J. Am. Chem. Soc. 2013;135:10626. doi: 10.1021/ja4052075. Liu W-B, Reeves CM, Virgil SC, Stoltz BM. J. Am. Chem. Soc. 2013;135:17298. doi: 10.1021/ja4097829. Qu J, Roßberg L, Helmchen G. J. Am. Chem. Soc. 2014;136:1272. doi: 10.1021/ja411869r. Seehafer K, Malakar C, Bender M, Qu J, Liang C, Helmchen G. Eur. J. Org. Chem. 2016:493. For recent applications of Ir-catalyzed asymmetric allylic substitution in natural product syntheses, see: Deng J, Zhou S, Zhang W, Li J, Li R, Li A. J. Am. Chem. Soc. 2014;136:8185. doi: 10.1021/ja503972p. Jiang S-Z, Zeng X-Y, Liang X, Lei T, Wei K, Yang Y-R. Angew. Chem. Int. Ed. 2016;55:4044. doi: 10.1002/anie.201511549.
- 8.(a) Carroll MF, Bader AR. J. Am. Chem. Soc. 1953;75:5400. [Google Scholar]; (b) Hyatt JA, Feldman PL, Clemens RJ. J. Org. Chem. 1984;49:5105. [Google Scholar]; (c) Clemens RJ, Hyatt JA. J. Org. Chem. 1985;50:2431. [Google Scholar]; (d) Sato M, Sunami S, Sugita Y, Kaneko C. Chem. Pharm. Bull. 1994;42:839. [Google Scholar]; (e) Sato M, Sunami S, Sugita Y, Kaneko C. Heterocycles. 1995;41:1435. [Google Scholar]; (f) Singer RA, Carreira EM. J. Am. Chem. Soc. 1995;117:12360. [Google Scholar]; (g) Evans DA, Murry JA, Kozlowski MC. J. Am. Chem. Soc. 1996;118:5814. [Google Scholar]; (h) Krueger J, Carreira EM. J. Am. Chem. Soc. 1998;120:837. [Google Scholar]
- 9.(a) Sato M, Kanuma N, Kato T. Chem. Pharm. Bull. 1984;32:106. [Google Scholar]; (b) Sato M, Ogasawara H, Kato T. Chem. Pharm. Bull. 1984;32:2602. [Google Scholar]; (c) West FG, Fisher PV, Gunawardena GU, Mitchellt S. Tetrahedron Lett. 1993;34:4583. [Google Scholar]; (d) Hart AC, Phillips AJ. J. Am. Chem. Soc. 2006;128:1094. doi: 10.1021/ja057899a. [DOI] [PubMed] [Google Scholar]; (e) Crimmins MT, Smith AC. Org. Lett. 2006;8:1003. doi: 10.1021/ol0601601. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Custar DW, Zabawa TP, Scheidt KA. J. Am. Chem. Soc. 2008;130:804. doi: 10.1021/ja710080q. [DOI] [PubMed] [Google Scholar]; (g) Hoye T, Danielson ME, May AE, Zhao H. J. Org. Chem. 2010;75:7052. doi: 10.1021/jo101598y. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Yavari I, Bayat MJ, Skoulika S. Synlett. 2013:2591. [Google Scholar]
- 10.(a) Gu C-L, Liu L, Wang D, Chen Y-J. J. Org. Chem. 2009;74:5754. doi: 10.1021/jo900977y. [DOI] [PubMed] [Google Scholar]; (b) Lei B, Fallis A. Can. J. Chem. 1991;69:1450. [Google Scholar]; (c) Dugger RW, Heathcock CH. J. Org. Chem. 1980;45:1181. [Google Scholar]
- 11.Kinouchi W, Saeki R, Kawashima H, Kobayashi Y. Tetrahedron Lett. 2015;56:2265. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.