Abstract
The present study was aimed at establishing whether the mTOR pathway and its downstream effector p70S6K in CA3 pyramidal neurons are under the modulation of the cholinergic input to trigger the formation of long term memories, similar to what we demonstrated in CA1 hippocampus. We performed in vivo behavioral experiments using the step down inhibitory avoidance test in adult Wistar rats to evaluate memory formation under different conditions. We examined the effects of rapamycin, an inhibitor of mTORC1 formation, scopolamine, a muscarinic receptor antagonist or mecamylamine, a nicotinic receptor antagonist, on short and long term memory formation and on the functionality of the mTOR pathway. Acquisition was conducted 30 min after i.c.v. injection of rapamycin. Recall testing was performed 1h, 4h or 24h after acquisition. We found that (1) mTOR and p70S6K activation in CA3 pyramidal neurons were involved in long term memory formation; (2) rapamycin significantly inhibited mTOR and of p70S6K activation at 4h, and long term memory impairment 24h after acquisition; (3) scopolamine impaired short but not long term memory, with an early increase of mTOR/p70S6K activation at 1h followed by stabilization at longer times; (4) mecamylamine and scopolamine co-administration impaired short term memory at 1h and 4h and reduced the scopolamine-induced increase of mTOR/p70S6K activation at 1h and 4h; (5) mecamylamine and scopolamine treatment did not impair long term memory formation; (6) unexpectedly, rapamycin increased mTORC2 activation in microglial cells. Our results demonstrate that in CA3 pyramidal neurons the mTOR/p70S6K pathway is under the modulation of the cholinergic system and is involved in long-term memory encoding, and are consistent with the hypothesis that the CA3 region of the hippocampus is involved in memory mechanisms based on rapid, one-trial object–place learning and recall. Furthermore, our results are in accordance with previous reports that selective molecular mechanisms underlie either short term memory, long term memory, or both. Furthermore, our discovery that administration of rapamycin increased the activation of mTORC2 in microglial cells supports a reappraisal of the beneficial/adverse effects of rapamycin administration.
Keywords: acetylcholine, long term memory, scopolamine, mecamylamine, muscarinic receptor, nicotinic receptor
1. INTRODUCTION
The hippocampus is critical for learning and memory. Age related decline in hippocampal function may underlie impaired memory abilities in about half of the population over 60 years of age (Hedden & Gabrieli, 2004; Small, Tsai, DeLaPaz, Mayeux, & Stern 2002). The hippocampal regions CA1, CA3 and dentate gyrus, although interconnected via the trisynaptic pathway, display striking anatomical differences (Amaral & Witter, 1989) and show distinct functions, contributing to specific types of information processing such as novelty detection, encoding, short-term memory, intermediate-term memory and retrieval. In particular, CA3 and CA1 pyramidal neurons perform distinct, yet complementary, functions in the processing of spatial and contextual information (Vazdarjanova & Guzowski, 2004). The CA3 hippocampus supports processes associated with the rapid formation of spatial or contextual memory (Kesner, Lee, & Gilbert, 2004; Lee & Kesner, 2002; Lee & Kesner, 2003; Nakazawa et al., 2003). However, CA3 lesions, or experimentally-induced dysfunctions of CA3, also impair spatial memory (Lee & Kesner, 2004; Nakazawa et al., 2003) and object-place associations (Hunsaker & Kesner, 2008; Langston, Stevenson, Wilson, Saunders, & Wood, 2010). Intrahippocampal CA3 information processing is also important for memory-based behavior and can modulate activity in the CA1 (O'Reilly, Alarcon, & Ferbinteanu, 2014). The relative contribution of CA3 and CA1 regions to memory is not completely understood. A recent review of the learning and memory literature suggested (Stokes, Kile, & Ekstrom, 2015) that CA3/DG and CA1 have distinct and separate roles in the representation of a spatial context during the formation of memories.
Long term memory requires protein synthesis; mTOR signalling is of crucial importance in this process, especially at the level of neuronal synaptodendritic compartment (Giovannini & Lana, 2016). Activation of mTOR/p70S6K pathway in CA1 hippocampal pyramidal neurons is instrumental to the process of formation of a long term inhibitory avoidance (IA) memory, and the cholinergic input through muscarinic and nicotinic receptor blockade impairs short term, but not long term IA memory (Lana et al., 2013).
IA is an emotionally-arousing paradigm (Giovannini et al., 2005, 2008; Izquierdo et al., 1997a; Lana et al., 2013; Maren, 2001) that involves a spatial memory component as the animal remembers the location where the noxious stimulus was given during acquisition (Cammarota, Bevilaqua, Medina, & Izquierdo, 2007), an explicit, associative component to the context, and an operant-like conditioning component to the shock as the animal may avoid the aversive stimulus (Wilensky, Schafe, & LeDoux, 2000). The IA response is a learning task that depends upon the activation of the hippocampal cholinergic system (Giovannini et al., 2005), as shown by the impairment by pre-training (Giovannini, Bartolini, Bacciottini, Greco, & Blandina, 1999; Izquierdo et al., 1998a) or post-training administration of muscarinic receptor antagonists (Giovannini, Bartolini, Bacciottini, Greco, & Blandina, 1999; Izquierdo et al., 1998b; McGaugh & Izquierdo, 2000). The recall test, performed at different times after acquisition, offers insight into the mechanisms involved in short term (Izquierdo et al., 1998a; Izquierdo et al., 1998b) and long term memory (Izquierdo et al., 2002). The step-down IA is acquired in one trial by activation of different brain structures by sensorial stimuli, including spatial and visual perceptions, pain, and fear (Izquierdo, 1989; Izquierdo and Medina, 1997b).
In this paper we will extend our analysis on mTOR pathway dynamics in the CA3 region of the hippocampus during the formation of an IA memory. By comparing the similarities and differences between CA1 and CA3 we will be better able to define the relative contribution of these two hippocampal regions in the encoding of an IA memory. Furthermore, we will define whether in CA3 pyramidal neurons the mTOR pathway is modulated by the cholinergic input and whether activation of this pathway triggers the encoding of long term memories in a similar manner to what we had demonstrated in CA1 hippocampus (Lana et al., 2013).
2. METHODS
2.1. Animals
Male adult (3 months old) Wistar rats, weighing 200-225 g, were (Harlan Nossan, Milano, Italy) housed in macrolon cages until experiment with ad libitum food and water and maintained on a 16h light – 8h dark cycle with light at 7:00 am. The room temperature was 23 ± 1°C. All rats were kept for at least 1 week in the animal house facility of the University of Florence before initiating the experiment and were frequently handled. All animal manipulations were carried out according to the European Community guidelines for animal care. All efforts were made to minimize animal sufferings and to use only the number of animals necessary to produce reliable scientific data. No alternatives to in vivo techniques are available for this type of experiments.
2.2. Surgery
For the intracerebroventricular (i.c.v.) injection of rapamycin or mecamylamine, rats were deeply anaesthethized with Zoletyl 100, i.p. and placed in a stereotaxic frame (Stellar, Stoelting Co., Wood Dale, IL, USA) for surgery. A stainless steel cannula was implanted in the right lateral ventricle (coordinates from bregma: AP:-1.5; L: -1.5; H: 4.0 mm). Coordinates were taken from (Paxinos & Watson, 1982) and are relative to bregma and dural surface. The cannula was secured to the parietal bone with acrylic dental cement and the skin sutured closed. After surgery the rats were treated with Amplital 5 mg/rat s.c.. Injection of rapamycin or mecamylamine was performed i.c.v. 7 days after surgery.
2.3. Drug treatments
Rapamycin (Calbiochem, EMD Biosciences, La Jolla, CA, USA), an inhibitor of mTORC1, was dissolved in a H2O/DMSO solution (50% H2O, 50% DMSO) and administered i.c.v. (1.5 nmol/5 μl of vehicle, 5.49 μg/kg) to rats 30 min before acquisition of the step down IA test. Control animals received injection of vehicle alone (H2O/DMSO solution, 5 μl). The amount of rapamycin was chosen in order to obtain a tissue concentration ranging between 2-3 μM assuming the drug distributes evenly throughout the brain (1-1.5 g total). Scopolamine hydrochloride (SIGMA, St. Louis, MO, USA), a non-selective antagonist of cholinergic muscarinic receptors, was dissolved in saline and administered i.p. (1.5 mg/kg) 30 min before the acquisition trial of the step down IA test. Mecamylamine (SIGMA, St. Louis, MO, USA), a non-selective antagonist of cholinergic nicotinic receptors, was dissolved in saline and administered i.c.v (15 nmol/5 μl of saline, 10.04 μg/kg) 40 min before acquisition of the step down IA test.
For i.c.v. injection a micro syringe was connected to the cannula and rapamycin or mecamylamine was injected over a 2 min period. The syringe was then left in place for one additional min to avoid back diffusion of the solution.
2.4. Step down inhibitory avoidance test
In the step down IA test the rats, put on an elevated platform placed by one wall of an arena, learn to associate exploration of the adjacent compartment with a foot shock delivered through the floor grid. On a subsequent exposure to the same environment, the animal will avoid to step down onto the floor grid or will increase the latency before stepping down. We used a standard step down apparatus placed in a soundproof room. Rats were handled and habituated to the experimenter and to the handling procedure the day before the acquisition trial. Rats were positioned on an elevated platform placed in a dark compartment facing an open arena equipped with an electrified floor grid. The time spent to step down onto the grid where the aversive stimulus (10 electric shocks, 20 ms/0.5 mA/5Hz) was delivered was recorded (Acquisition Latency). After the aversive stimulus, rats were immediately removed from the arena and placed in their home cage for consolidation (encoding). Recall trial, performed 1h, 4h or 24h after the acquisition trial, was identical to the acquisition trial, except that the foot shock was omitted. In the recall trial the time spent in the dark compartment before stepping down onto the arena was also recorded (“Recall Latency”). All naïve and vehicle-treated rats acquired the behaviour. As previously published (Lana et al., 2013) a 300 s cutoff time was imposed on recall test latencies.
2.5. Experimental groups
Rats were randomly subdivided into different experimental groups. Rats in the first group received i.c.v. injection of rapamycin 30 min before the acquisition trial, those in the second group received i.p. injection of scopolamine 30 min before the acquisition trial, those in the third group received i.c.v. injection of mecamylamine plus an i.p. injection of scopolamine 40 min and 30 min, respectively, before the acquisition trial, those in the fourth group were control rats that received the vehicle (CTR). Within these experimental groups rats were further subdivided into subgroups: 1) rats that did not undergo the recall trial (ACQ); 2) rats that underwent the recall trial 1h after acquisition; 3) 4h after acquisition; 4) 24h after acquisition. Each experimental group consisted of at least 3 animals.
2.6. Immunohistochemistry
Immediately after testing, rats allocated in any experimental group were deeply anesthetized and perfused with ice-cold paraformaldehyde (4% in phosphate-buffered saline, pH 7.4) through the ascending aorta. The brains were post-fixed in paraformaldehyde O/N at 4 °C and cryoprotected in 18% sucrose/PBS solution for at least 48h. Coronal sections (40 μm-thick) were cut with a cryostat, placed in 1 ml of anti-freezer solution and stored at −20 °C until immunohistochemistry. Phospho-mTOR (P-mTOR) and phospho-p70S6K (P-p70S6K) immunohistochemistry was performed on brain coronal slices with the free-floating method (Giovannini et al., 2001; Giovannini et al., 2003).
Day 1. Coronal brain sections were placed in 24-wells plates and were washed (3 times, 5 min) in PBS-0.3% Triton X-100 (PBS-TX), incubated for 15 min in PBS-TX containing 0.75% H2O2 and blocked with 1.5% normal goat serum and 0.05% NaN3 in PBS-TX (Blocking Buffer) for 1h. Sections were then incubated overnight (O/N) at 4°C with polyclonal rabbit primary antibody against phospho-(Ser2448)-mTOR (Abcam, Cambridge, UK) 1:100 in Blocking Buffer or with polyclonal rabbit primary antibody against phospho-(Thr389)-p70S6K (Cell Signaling, Danvers, MA, USA) 1:100 in Blocking Buffer. These antibody are specific for the phosphorylated moiety of the enzymes, and therefore recognize activated mTOR and activated p70S6K. Day 2. Slices were washed (3 times, 5 min) and then incubated in biotinylated goat anti-rabbit secondary antibody (Vector Laboratories, Burlingame, CA), diluted 1:300 in Blocking Buffer for 2h at room temperature, then for 90 min in avidin-biotin-peroxidase complex (Vectastain ABC complex, Vector Laboratories) diluted 1:100 in Blocking Buffer and staining was developed using 3,3'-diaminobenzidine (DAB) staining kit (Vector Laboratories) 2-3 min with NiCl as an enhancer. After extensive washings slices were mounted onto gelatine-coated slides and examined using an Olympus BX40 microscope equipped with an Olympus DP 50 (Olympus, Milan, Italy) digital camera. In order to verify the integrity of the cytoarchitecture of the structures under investigation some of the slices were counterstained using standard Nissl staining methods. Proper immunohistochemical controls were performed omitting the primary antibodies and developing the slices as above using anti-rabbit secondary antibodies only. No signal due to nonspecific labelling from the secondary antibodies used was ever detected.
2.7. Quantitative analysis and statistics
All quantification analyses were performed blind by two different experimenters and the results were averaged. Three coronal slices (spaced by 150 μm, starting at about −2.8 mm from bregma) containing the dorsal hippocampus were immunostained. The region of interest (ROI) in CA3, containing Str. Pyramidalis and Str. Radiatum, was consistently captured at 20x magnification using an Olympus digital camera. Phospho-mTOR and phospho-p70S6K immunoreactive neurons were counted in pyramidal CA3 using Image J choosing the same area in all slices and counts were expressed as number of cells/mm2. Data were then analyzed using GraphPad Prism 5.0 (GraphPad Software, Inc. S. Diego, CA, USA).
Step down test latencies were expressed in seconds (sec) and were presented as mean ± SEM and the effect of drugs administration on step down acquisition or recall latencies at 1h, 4h or 24h after acquisition was evaluated using two-way ANOVA followed by Bonferroni post test. The effect of drugs on phosphorylation of mTOR or p70S6K in CA3 pyramidal neurons was evaluated on the means ± SEM using two-way ANOVA followed by Bonferroni post test or one-way ANOVA followed by Newman Keuls post hoc test, as appropriate. Statistical significance was set at P<0.05.
3. RESULTS
This investigation determined whether the mTOR/p70S6K cascade was activated in the CA3 region of the hippocampus by the acquisition of short and long term IA memory.
3.1 Rapamycin administration impairs long term memory
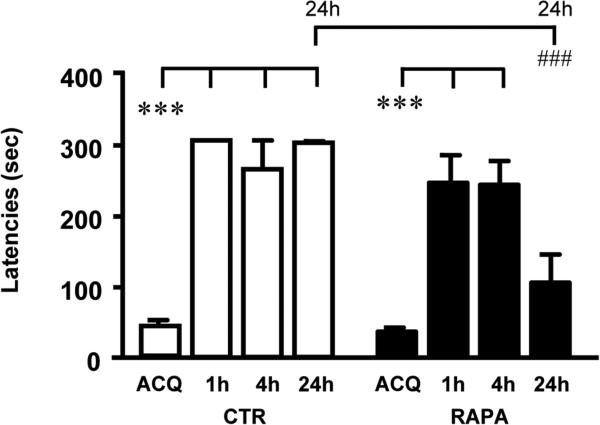
We performed preliminary experiments in naïve rats and in rats implanted with an i.c.v. cannula to verify whether the surgical operation might affect the animal's behavior in the step down inhibitory avoidance test. As previously demonstrated (Giovannini et al., 2005; Lana et al., 2013), acquisition and recall latencies did not differ significantly between naïve and cannula-implanted, but not infused, control rats (data not shown). Control rats injected with vehicle (5 μl) through the i.c.v. cannula acquired the short and long term IA memory at all time points tested, as shown by the significantly longer recall latencies at 1h, 4h and 24h after acquisition (One-way ANOVA, F(3,37)=76.78, P<0.001; ***P<0.001, CTR 1h, CTR 4h, and CTR 24h vs CTR ACQ, Newman-Keuls multiple comparison test; Figure 1, white columns). Recall latencies measured in control rats at 1h (299 ± 1 sec), 4h (260 ± 39.7 sec) and 24h (297 ± 1.4 sec) after acquisition did not differ significantly one another (One way ANOVA, n.s.). Rapamycin (RAPA, 1.5 nmol/5 μl, i.c.v., 30 min before acquisition) did not affect mobility and exploratory behavior during acquisition of the task, as shown by the similar acquisition latencies in controls (CTR ACQ; 41.5 ± 7.9 sec; Figure 1) and in rapamycin treated rats (RAPA ACQ; 36.9 ± 5.6 sec; t(27) = 0.49, P=0.6275, n.s., Student's t test). The time course of the effect of rapamycin on memory encoding was evaluated performing the recall test at 1h, 4h and 24h after acquisition in different groups of animals, with a cutoff time of 300 sec. As already demonstrated (Lana et al., 2013), we confirmed here that administration of rapamycin significantly impaired long term IA memory formation at 24h (−65% vs CTR 24h), but not at 1h (−19% vs CTR 1h) or 4h (−19% vs CTR 4h) after acquisition test (Figure 1, black columns). Indeed, the one-way ANOVA demonstrated that in rapamycin treated rats 1h and 4h recall latencies were significantly higher than acquisition latency (One-way ANOVA, F(3,43)=16.71, P<0.001; ***P<0.001, RAPA 1h, RAPA 4h vs RAPA ACQ, Newman-Keuls multiple comparison test, Figure 1, black columns). Rapamycin administration significantly impaired recall of the task at 24h after acquisition. Statistical analysis carried out by two-way ANOVA with Treatment and Recall Time as the two variables, revealed that there was a significant main effect for Treatment (Treatment, F(1,74)=17.55, P<0.001), Time (F(3,74)=41.32, P<0.001), and a significant Interaction Treatment × Time (F(3,74)=6.828, P<0.001). Most importantly, Bonferroni post test showed that in rats treated with rapamycin, recall latency at 24h after acquisition (RAPA 24h, 104.9 ± 38.9 sec) was significantly lower than in control animals at 24h (CTR 24h, 297 ± 1.4 sec, ***P<0.001, Figure 1). However, it is possible to hypothesize that, increasing the cutoff time to 10 min, differences between rapamycin treated animals and controls might have emerged on short term IA memory formation.
Figure 1.
Step down inhibitory avoidance test of control rats (CTR, white columns) and rapamycin treated rats (RAPA, black columns): acquisition (ACQ) and recall latencies at 1h, 4h and 24h are reported in seconds (300 s cut-off time of was applied). Control rats acquired short term and long term inhibitory avoidance memory, as demonstrated by the significantly longer latencies in the recall trial at 1h, 4h and 24h (***P<0.001, CTR ACQ vs CTR 1h, CTR 4h and CTR 24h). Rapamycin treated rats acquired short term inhibitory avoidance memory at 1h and 4h (***P<0.001, RAPA ACQ vs RAPA 1h and RAPA 4h). Administration of rapamycin significantly impaired long term IA memory formation at 24h (###P<0.001, RAPA 24h vs RAPA ACQ). CTR: ACQ n=12; 1h n=8; 4h n=7; 24h n=11; RAPA: ACQ n=17; 1h n=7; 4h n=11; 24h n=9.
3.2 Activation of mTOR-p70S6K in CA3 pyramidal neurons during memory encoding. Effect of rapamycin
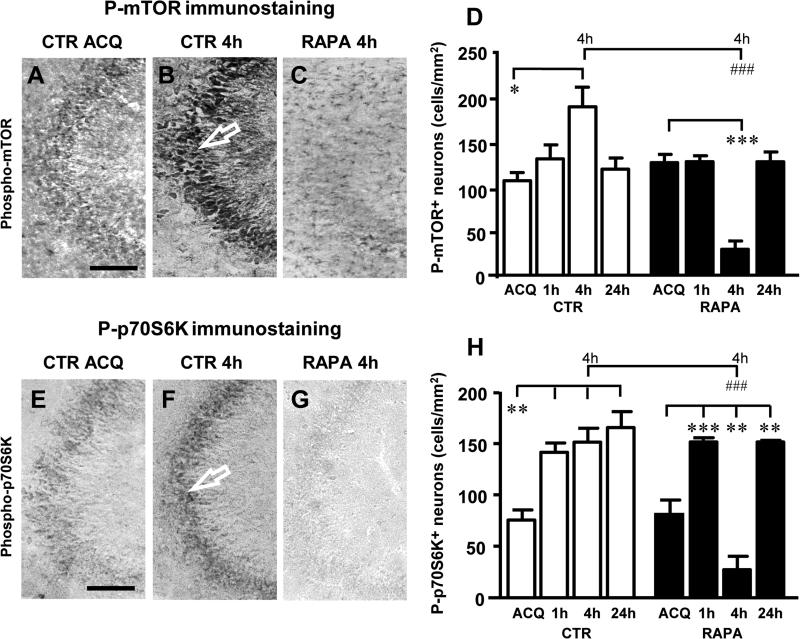
Activation of mTOR was detected by immunohistochemistry using a specific antibody against phospho-(Ser2448)-mTOR (Figure 2A-2C). The quantity of phospho-mTOR immunopositive CA3 pyramidal neurons immediately after the acquisition of the IA memory (Figure 2A, ACQ) did not differ between the controls and the rapamycin treated animals (CTR ACQ; 107.0 ± 9.07 sec RAPA ACQ; 127.7 ± 9.135 sec; P=0.1774, n.s., Student's t test, Figure 2D); this was taken as an indication of basal mTOR activation. Four h after acquisition, mTOR was significantly activated both in the cell body and apical dendrites of CA3 pyramidal neurons (Figure 2B, CTR 4h) in comparison to basal levels (Figure 2A, ACQ). The activation of mTOR was completely blocked by the administration of rapamycin (Figure 2C, RAPA 4h). Quantitative analysis of the time course of mTOR activation was carried out by counting phospho-mTOR immunopositive neurons in CA3 Str. Pyramidalis at acquisition and at 1h, 4h and 24h after acquisition, in the absence (Figure 2D, CTR, white columns) and presence of rapamycin (Figure 2D, RAPA, black columns). Statistical analysis (shown in detail in Table 1) of the time course of mTOR activation demonstrated that phospho-mTOR positive CA3 pyramidal neurons in control rats were not significantly more numerous at 1h after acquisition (+ 23%, n.s., CTR ACQ vs CTR 1h), increased significantly at 4h (+ 78%, *P<0.05, CTR ACQ vs CTR 4h) and then returned to basal levels at 24h (+ 12%, n.s., CTR ACQ vs CTR 24h).
Figure 2.
A-C: Phospho-mTOR immunostaining in CA3 hippocampal region of control and rapamycin treated rats: basal activation of mTOR at acquisition in a control rats (A), activation of mTOR 4h after acquisition in a control rats (B) and in a rapamycin treated rats (C). There was a consistent activation of mTOR in CA3 pyramidal neurons of control rats 4h after acquisition (arrow in panel B). This activation was strongly reduced in rapamycin treated rats at the same time point (C). Scale bar: 100 μm. D: Density of phospho-mTOR positive neurons (cells/mm2) in CA3 Str. Pyramidalis of control and rapamycin treated rats. In control rats there was a significant activation of mTOR at 4h after acquisition (*P<0.05, CTR 4h vs CTR ACQ, Newman-Keuls Multiple Comparison Test). Rapamycin administration significantly impaired this activation at 4h (###P<0.001, RAPA 4h vs CTR 4h, Bonferroni post test). CTR: ACQ n=4; 1h n=4; 4h n=6; 24h n=9; RAPA: ACQ n=3; 1h n=3; 4h n=5; 24h n=7. E-G: Phospho-p70S6K immunostaining in CA3 hippocampal region of control and rapamycin treated rats: basal activation of p70S6K at the time of acquisition in a control rat (E), activation of p70S6K 4h after acquisition in a control rat (arrow, F) and in a rapamycin treated rat (G). Activation of p70S6K in control rats 4h after acquisition was strongly reduced in rapamycin treated rats at the same time point. Scale bar: 100 μm. H: Density of phospho-p70S6K positive neurons (cells/mm2) in CA3 Str. Pyramidalis of control and rapamycin treated rats. In control rats there was a significant activation of p70S6K at 1h, 4h and 24h after acquisition (**P<0.01, CTR 1h, 4h and 24h vs CTR ACQ, Newman-Keuls Multiple Comparison Test). Rapamycin administration significantly reduced p70S6K activation at 4h (###P<0.001, RAPA 4h vs CTR 4h, Bonferroni post test). CTR: ACQ n=3; 1h n=5; 4h n=4; 24h n=3; RAPA: ACQ n=2; 1h n=4; 4h n=5; 24h n=4.
Table 1.
Summary of the time course of mTOR activation at 1h, 4h and 24h after acquisition of the inhibitory avoidance memory. Effect of rapamycin administration.
| Phospho-mTOR | ACQ | 1h | 4h | 24h | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N cell/mm2 | % vs CTR | N cell/mm2 | % vs ACQ | % vs CTR | N cell/mm2 | % vs ACQ | % vs CTR | N cell/mm2 | % vs ACQ | % vs CTR | |
| CTR | 107±9.1 | - | 132±15.9 | +23 n.s. | - | 191±22.5 | +79* | - | 120±12.7 | +12 n.s. | - |
| RAPA | 128±9.1 | +20 n.s. | 129±6.3 | +1 n.s. | −2 n.s. | 29±9.2 | −77*** | −85### | 128±11.0 | −1 n.s. | +6 n.s. |
Legend: N cell/mm2: number of phospho-mTOR positive pyramidal neurons/mm2 of CA3 (mean±SEM); % vs ACQ: percentage of N cell/mm2 at 1h, 4h and 24h compared to N cell/mm2 at ACQ; % vs CTR: percentage of N cell/mm2 compared to CTR at the same time point; n.s.: not significant.
Statistical analysis was performed within Treatments using One-way ANOVA. CTR: One-way ANOVA, F(3,22)= 4.874, P<0.05
P<0.05 CTR 4h vs CTR ACQ, Newman-Keuls Multiple Comparison test. RAPA: One-way ANOVA F(3,17)=22.02, P<0.001
P<0.001, RAPA 4h vs RAPA ACQ, Newman-Keuls Multiple Comparison test.
Statistical analysis within Time points was performed using Two-way ANOVA, using Treatment and Recall time as the two variables.
RAPA vs CTR: Treatment, F(1,33)=8.872, P<0.01; Time, F(3,33)=0.589, n.s.; Interaction Treatment × Time, F(3,33)=14.17, P<0.001
P<0.001 RAPA 4h vs CTR 4h, Bonferroni post test.
We hypothesized that mTOR activation found at 4h may be the consequence of the process of IA memory encoding triggered by the acquisition trial of the test. In order to verify this hypothesis we treated the rats with rapamycin 30 min before acquisition. We had previously demonstrated that rapamycin, by this time point, has already fully diffused from the injection point to the hippocampus (Lana et al., 2013). In rapamycin treated rats, long term IA memory was impaired and mTOR activation in CA3 pyramidal neurons was inhibited, particularly at 4h after the acquisition trial (as shown in Figure 2C, RAPA 4h).
Statistical analysis (shown in detail in Table 1) carried out by two-way ANOVA with Treatment and Recall time as the two independent variables, followed by Bonferroni post test, showed that activation of mTOR 4h after acquisition was significantly blocked by rapamycin (−85%, ###P<0.001, RAPA 4h vs h CTR 4h, Figure 2D and Table 1). It is interesting to note that in rapamycin treated rats activation of mTOR 4h after acquisition was also significantly lower than at acquisition (−77%,***P<0.001 RAPA 4h vs RAPA ACQ, Figure 2D and Table 1). At 24h time point, mTOR activation returned to basal levels (Figure 2D and Table 1).
We also evaluated the effect of IA acquisition and encoding on p70S6K activation in CA3 Str. Pyramidalis immediately after acquisition and at 1h, 4h or 24h. Activation of p70S6K was detected by immunohistochemistry using a specific antibody against phospho-(Thr389)-p70S6K immediately after acquisition in control rats, as an indication of p70S6K activation in basal conditions, and in rats administered with rapamycin (Figure 2E-2G). No differences were observed in p70S6K activation between control rats and rats injected with vehicle immediately after acquisition (not shown). Figure 2E-2G show examples of phospho-p70S6K in CA3 pyramidal neurons at acquisition (Figure 2E, ACQ), recall at 4h (Figure 2F, CTR 4h) and recall at 4h after rapamycin administration (Figure 2G, RAPA 4h). It is evident from the images, and from the statistical analysis (shown in detail in Table 2), that p70S6K was significantly activated 1h (+87%, **P<0.01, CTR 1h vs CTR ACQ), 4h (+100%, **P<0.01, CTR 4h vs CTR ACQ) and 24h (+118%, **P<0.01, CTR 24h vs CTR ACQ) after acquisition in most of CA3 pyramidal neurons (Figure 2H, white columns, CTR).
Table 2.
Summary of the time course of p70S6K activation at 1h, 4h and 24h after acquisition of the inhibitory avoidance memory. Effect of rapamycin administration.
| Phospho-p70S6K | ACQ | 1h | 4h | 24h | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N cell/mm2 | % vs CTR | N cell/mm2 | % vs ACQ | % vs CTR | N cell/mm2 | % vs ACQ | % vs CTR | N cell/mm2 | % vs ACQ | % vs CTR | |
| CTR | 76±9.7 | - | 142± 8.9 | +87** | - | 152±13.6 | +100** | - | 166±15.7 | +118** | - |
| RAPA | 81±14.0 | +7 n.s. | 152±4.2 | +88*** | +7 n.s. | 28±12 | −65** | −82### | 152±1.5 | +88** | −8 n.s. |
Legend: N cell/mm2: number of phospho-p70S6K positive pyramidal neurons/mm2 of CA3 (mean±SEM); % vs ACQ: percentage of N cell/mm2 at 1h, 4h and 24h compared to N cell/mm2 at ACQ; % vs CTR: percentage of N cell/mm2 compared to CTR at the same time point; n.s.: not significant.
Statistical analysis was performed within Treatments using One-way ANOVA. CTR: One-way ANOVA, F(3,14)=9.166, P<0.01; **P<0.01 CTR 1h, 4h and 24h vs CTR ACQ, Newman-Keuls Multiple Comparison test. RAPA: One-way ANOVA, F(3,14)=50.63, P<0.001, ***P<0.001 RAPA 1h vs RAPA ACQ, **P<0.01 RAPA 4h vs RAPA ACQ and RAPA 24h vs RAPA ACQ, Newman-Keuls Multiple Comparison test.
Statistical analysis within Time points was performed using Two-way ANOVA, using Treatment and Recall time as the two variables. RAPA vs CTR: Treatment, F(1,22)=15.14, P<0.001; Time, F(3,22)=26.29, P<0.001; Interaction Treatment × Time, F(3,22)=16.13, P<0.001; ###P<0.001 RAPA 4h vs CTR 4h, Bonferroni post test.
Statistical analysis (shown in detail in Table 2) demonstrated that in rapamycin treated rats (Figure 2H, black columns) activation of p70S6K was significantly increased at 1h (+87%, ***P<0.001, RAPA 1h vs RAPA ACQ) and 24h (+87%, **P<0.01, RAPA 1h vs RAPA ACQ) in comparison to basal levels. On the contrary, 4h after acquisition rapamycin blocked the activation of p70S6K, as shown in Figure 2G (RAPA 4h). Statistical analysis carried out by two-way ANOVA with Treatment and Recall time as the two independent variables showed that 4h after acquisition p70S6K activation was significantly lower not only in comparison to control rats (−82%, ###P<0.001, RAPA 4h vs CTR 4h, see also Table 2) but also in comparison to basal levels (−66%, **P<0.01, RAPA 4h vs RAPA ACQ).
3.3 Activation of mTOR-p70S6K in CA3 pyramidal neurons during memory encoding. Effect of cholinergic blockade by muscarinic and nicotinic receptors antagonists
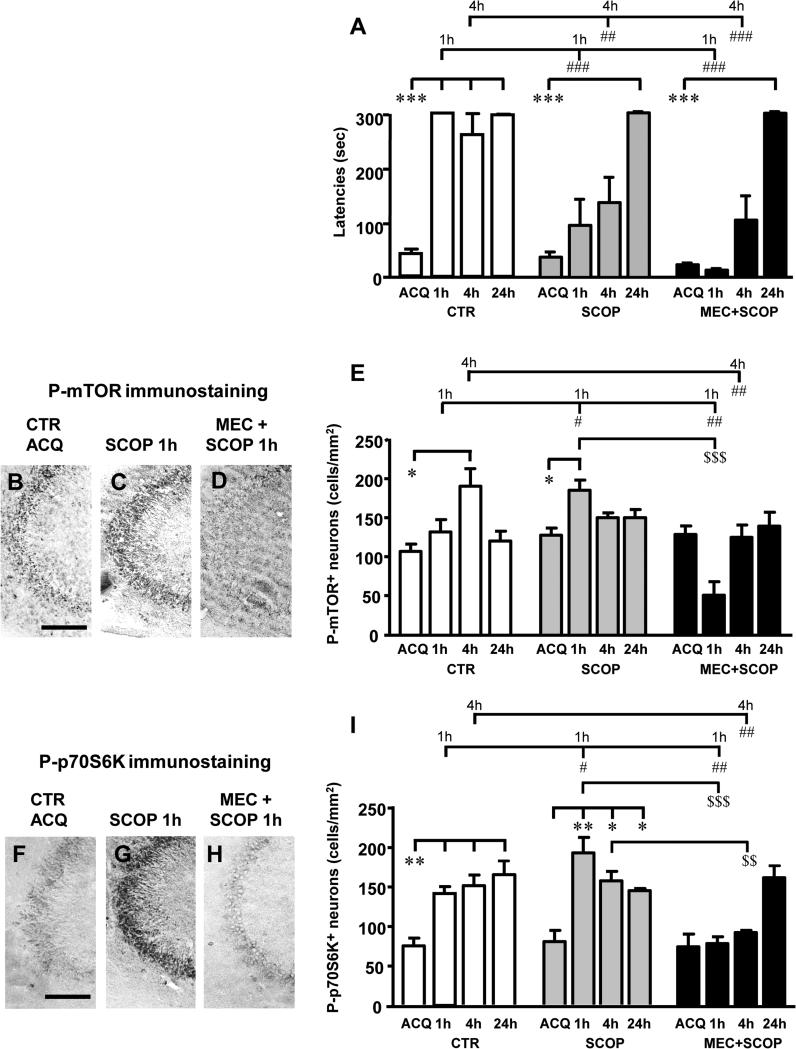
We had previously demonstrated in CA1 pyramidal neurons (Lana et al., 2013) that activation of intracellular pathways and the consequent increase of mTOR-p70S6K signaling which triggers long term memory formation is downstream of cholinergic muscarinic and/or nicotinic acetylcholine receptors. In order to understand whether this mechanism may have a functional role also in CA3 pyramidal neurons we administered scopolamine alone, or with mecamylamine, to rats before acquisition and then performed the step down IA recall test at 1h, 4h and 24h after acquisition. We investigated the activation of the mTOR-p70S6K pathway in CA3 pyramidal neurons during encoding of the inhibitory avoidance memory and after treatment with the cholinergic antagonists. We confirmed that administration of scopolamine alone (1.5 mg/kg, i.p., 30 min before acquisition), or together with mecamylamine (15 nmol/5 μl of saline, administered i.c.v. 40 min before acquisition), significantly impaired short term memory recall 1h and 4h after acquisition (Figure 3A, white columns: Two-way ANOVA with Treatment and Recall time as the two variables; Treatment, F(2,75)=19.27, P<0.001, Time, F(3,75)=47.75, P<0.001, Interaction Treatment × Time, F(6,75)=6.525, P<0.001; ***P<0.001, SCOP 1h vs CTR 1h, MEC+SCOP 1h vs CTR 1h, MEC+SCOP 4h vs CTR 4h; **P<0.01, SCOP 4h vs CTR 4h, Bonferroni post test). Long term memory encoding was not affected by scopolamine alone (Figure 3A, grey columns; One-way ANOVA, F(3,24)=7.674, P<0.01; ***P<0.001, SCOP 24h vs SCOP ACQ, Newman-Keuls multiple comparison test) or by scopolamine plus mecamylamine (Figure 3A, black columns; One-way ANOVA, F(3,23)=14.27, P<0.001; ***P<0.001, MEC+SCOP 24h vs MEC+SCOP ACQ, Newman-Keuls multiple comparison test).
Figure 3.
A: Step down inhibitory avoidance test of control rats (CTR, white columns), scopolamine treated rats (SCOP, grey columns) and scopolamine plus mecamylamine treated rats (MEC+SCOP, black columns). As previously reported control rats acquired short term (1h, 4h) and long term (24h) inhibitory avoidance memory (see Figure 1). Scopolamine treated rats and MEC+SCOP treated rats acquired long term inhibitory avoidance memory at 24h (***P<0.001, SCOP 24h vs SCOP ACQ, MEC+SCOP 24h vs MEC+SCOP ACQ, Newman-Keuls Multiple Comparison test). Administration of scopolamine alone or scopolamine plus mecamylamine significantly impaired short term IA memory formation at 1h and 4h (###P<0.001, SCOP 1h vs CTR 1h, MEC+SCOP 1h vs CTR 1h, MEC+SCOP 4h vs CTR 4h, ##P<0.01 SCOP 4h vs CTR 4h, Bonferroni post test). CTR: ACQ n=12; 1h n=8; 4h n=7; 24h n=11; SCOP: ACQ n=6; 1h n=7; 4h n=7; 24h n=5; MEC+SCOP: ACQ n=7; 1h n=5; 4h n=8; 24h n=4. B-D: Phospho-mTOR immunostaining in area CA3 of CTR, SCOP and MEC+SCOP rats. B: basal activation of mTOR at acquisition in a CTR rat; C: activation of mTOR at 1h after acquisition in a SCOP rat and in a MEC+SCOP rat (D). Scale bar: 100 μm. E: Density of phospho-mTOR positive neurons (cells/mm2) in CA3 hippocampal region of CTR, SCOP and MEC+SCOP rats. A significant activation of mTOR at 4h after acquisition in CTR rats was evident (*P<0.05, CTR 4h vs CTR ACQ, Newman-Keuls Multiple Comparison Test). The administration of scopolamine plus mecamylamine significantly impaired this activation (##P<0.01, MEC+SCOP 4h vs CTR 4h, Bonferroni post test). The administration of scopolamine alone caused a significant increment of mTOR activation at 1h in comparison to CTR rats (#P<0.05, SCOP 1h vs CTR 1h, Bonferroni post test) and the administration of scopolamine plus mecamylamine significantly impaired this over-activation ($$$P<0.001, MEC+SCOP 1h vs SCOP 1h, Bonferroni post test). The activation of mTOR at 1h in MEC+SCOP rats was lower than in CTR rats (##P<0.01, MEC+SCOP 1h vs CTR 1h). CTR: ACQ n=4; 1h n=4; 4h n=6; 24h n=9; SCOP: ACQ n=3; 1h n=5; 4h n=7; 24h n=4; MEC+SCOP: ACQ n=3; 1h n=5; 4h n=6; 24h n=3. F-H: Phospho-p70S6K immunostaining in area CA3 of CTR, SCOP and MEC+SCOP rats. F: basal activation of p70S6K at acquisition in a CTR rat; G: activation of p70S6K at 1h after acquisition in a SCOP rat and in a MEC+SCOP rat (H). Scale bar: 100 μm. I: Density of phospho-p70S6K positive neurons (cells/mm2) in area CA3 of CTR, SCOP and MEC+SCOP rats. In CTR rats there was a significant activation of p70S6K at 1h, 4h and 24h after acquisition (**P<0.01, CTR 1h, CTR 4h and CTR 24h vs CTR ACQ, Newman-Keuls Multiple Comparison Test). The administration of scopolamine plus mecamylamine significantly impaired this activation at 1h and 4h (##P<0.01, MEC+SCOP 1h vs CTR 1h and MEC+SCOP 4h vs CTR 4h, Bonferroni post test). The administration of scopolamine alone increased significantly p70S6K activation at 1h in comparison to CTR rats (#P<0.05, SCOP 1h vs CTR 1h, Bonferroni post test) and the administration of scopolamine plus mecamylamine significantly impaired this over-activation ($$$P<0.0015, MEC+SCOP 1h vs SCOP 1h, Bonferroni post test). CTR: ACQ n=3; 1h n=5; 4h n=4; 24h n=3; SCOP: ACQ n=3; 1h n=6; 4h n=6; 24h n=3; MEC+SCOP: ACQ n=3; 1h n=5; 4h n=5; 24h n=3.
The effect of scopolamine alone (SCOP 1h) or together with mecamylamine (MEC+SCOP 1h) on mTOR activation in CA3 pyramidal neurons 1h after acquisition, in comparison to basal levels of mTOR (ACQ), is shown in Figure 3B-E. Panels 3B-3D represent the immunostaining of phospho-mTOR in CA3 under the three experimental conditions: CTR, SCOP 1h and MEC + SCOP 1h. A quantitative analysis of the time course of the effect of cholinergic receptor antagonists on mTOR activation in CA3 pyramidal neurons is shown in Figure 3E (CTR, white columns; scopolamine alone, grey columns; scopolamine plus mecamylamine, black columns).
Statistical analysis (shown in detail in Table 3) demonstrated that scopolamine administration significantly increased mTOR activation in CA3 pyramidal neurons at 1h after acquisition in comparison to basal levels (Figure 3E, +45%, *P<0.05, SCOP 1h vs SCOP ACQ). The effect of scopolamine on mTOR activation at 1h after acquisition was significantly blocked by mecamylamine (−73%, $$$P<0.001 MEC+SCOP 1h vs SCOP 1h), which significantly decreased phospho-mTOR to levels below basal levels (−61%, *P<0,05, MEC+SCOP 1h vs MEC+SCOP ACQ). Statistical analysis demonstrated that treatment with scopolamine plus mecamylamine significantly blocked mTOR activation, as compared to controls at 4h (−32%, ##P<0.01, MEC+SCOP 4h vs CTR 4h).
Table 3.
Summary of the time course of mTOR activation at 1h, 4h and 24h after acquisition of the inhibitory avoidance memory. Effect of cholinergic antagonists.
| Phospho-mTOR | ACQ | 1h | 4h | 24h | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N cell/mm2 | % vs CTR | N cell/mm2 | % vs ACQ | % vs CTR | % vs SCOP | N cell/mm2 | % vs ACQ | % vs CTR | % vs SCOP | N cell/mm2 | % vs ACQ | % vs CTR | % vs SCOP | |
| CTR | 107±9.1 | - | 132± 15.9 | +23 n.s. |
- | - | 191±22.5 | +79* | - | - | 120±12.7 | +12 n.s. |
- | - |
| SCOP | 129±10.7 | +21 n.s. |
186±12.9 | +44* | +41# | - | 150±6.1 | +16 n.s. |
−21 n.s. |
- | 150±10.6 | +16 n.s. |
+25 n.s. |
- |
| MEC+SCOP | 130±9.8 | +21 n.s. |
51±17.2 | −61* | −61## | −73$$$ | 125±15.9 | −4 n.s. | −35## | −17 n.s. |
139±17.9 | +7 n.s. | +16 n.s. |
−7 n.s. |
Legend: N cell/mm2: number of phospho-mTOR positive pyramidal neurons/mm2 of CA3 (mean±SEM); % vs ACQ: percentage of N cell/mm2 at 1h, 4h and 24h compared to N cell/mm2 at ACQ; % vs CTR: percentage of N cell/mm2 compared to CTR at the same time point; n.s.: not significant.
Statistical analysis was performed within Treatments using One-way ANOVA. CTR: One-way ANOVA, F(3,22)=4.874, P<0.05; *P<0.05 CTR 4h vs CTR ACQ, Newman-Keuls Multiple Comparison test. SCOP: One-way ANOVA, F(3,18)=5.033, P<0.05; *P<0.05 SCOP 1h vs SCOP ACQ, Newman-Keuls multiple comparison test. MEC+SCOP: One-way ANOVA, F(3,16)=6.098, P<0.01; *P<0.05 MEC+SCOP 1h vs MEC+SCOP ACQ, Newman-Keuls multiple comparison test.
Statistical analysis within Time points was performed using Two-way ANOVA, using Treatment and Recall time as the two variables. SCOP vs CTR & MEC+SCOP vs CTR: Treatment, F(2,47)=7.062, P<0.01; Time, F(3,47)=2.817, P<0.05; Interaction Treatment × Time, F(6,47)=5.478, P<0.001; #P<0.05 SCOP 1h vs CTR 1h, ##P<0.01 MEC+SCOP 1h vs CTR 1h and MEC+SCOP 4h vs CTR 4h, Bonferroni post test. MEC+SCOP vs SCOP: Treatment, F(1,28)=18.000, P<0.001; Time, F(3,28)=1.297, n.s.; Interaction Treatment × Time, F(3,28)=9.764, P<0.001; $$$P<0.001 MEC+SCOP 1h vs SCOP 1h, Bonferroni post test.
The effect of scopolamine alone (SCOP 1h) or with mecamylamine (MEC+SCOP 1h) on p70S6K activation in CA3 pyramidal neurons is shown in Figure 3F-I. Panels 3F-3H represent the immunostaining of phospho-p70S6K in CA3 under the three experimental conditions, CTR, SCOP 1h and MEC + SCOP 1h. The quantitative analysis of the time course of the effect of cholinergic receptor antagonists on p70S6K activation in CA3 pyramidal neurons is shown in Figure 3I (CTR, white columns; SCOP alone, grey columns; SCOP plus mecamylamine, black columns). Administration of scopolamine (1.5 mg/kg, i.p., 30 min before acquisition) significantly increased activation of p70S6K in CA3 neurons 1h (+154%, **P<0.01, SCOP 1h vs SCOP ACQ, Figure 3I and Table 4), 4h (+108%, *P<0.05, SCOP 4h vs SCOP ACQ, Figure 3I and Table 4), and 24h (+91%, *P<0.05, SCOP 24h vs SCOP ACQ, Figure 3I and Table 4) after acquisition. Interestingly, at 1h after acquisition, activation of p70S6K was significantly higher than in control rats at the same time point (+36%, #P<0.05 SCOP 1h vs CTR 1h). We also found that administration of scopolamine plus mecamylamine significantly reduced p70S6K activation in CA3 neurons at 1h and 4h after acquisition in comparison to control rats and to rats treated with scopolamine alone. Statistical analysis (shown in detail in the legend of Table 4) demonstrated that mecamylamine significantly blocked p70S6K activation at 1h after acquisition (−44%, ##P<0.01, MEC+SCOP 1h vs CTR 1h, and −59%, ***P<0.001 MEC+SCOP 1h vs SCOP 1h) and 4h after acquisition (−38%, ##P<0.01, MEC+SCOP 4h vs CTR 4h, and −41%, $$P<0.01 MEC+SCOP 4h vs SCOP 4h).
Table 4.
Summary of the time course of p70S6K activation at 1h, 4h and 24h after acquisition of the inhibitory avoidance memory. Effect of cholinergic antagonists.
| Phospho-p70S6K | ACQ | 1h | 4h | 24h | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N cell/mm2 | % vs CTR | N cell/mm2 | % vs ACQ | % vs CTR | % vs SCOP | N cell/mm2 | % vs ACQ | % vs CTR | % vs SCOP | N cell/mm2 | % vs ACQ | % vs CTR | % vs SCOP | |
| CTR | 76±9.7 | - | 142± 8.9 | +87** | - | - | 152±13.6 | +100** | - | - | 166±15.7 | +118** | - | - |
| SCOP | 77±16.0 | +1 n.s. |
193±19.4 | +151** | +36# | - | 158±11.9 | +105* | +4 n.s. |
- | 145±2.8 | +88* | −13 n.s. |
- |
| MEC+SCOP | 75±17.5 | −1 n.s. |
80±8.2 | +7 n.s. | −44## | −59$$$ | 94±2.6 | +25 n.s. |
−38## | −41$$ | 163±15.1 | +117*** | −2 n.s. |
+12 n.s. |
Legend: N cell/mm2: number of phospho-p70S6K positive pyramidal neurons/mm2 of CA3 (mean±SEM); % vs ACQ: percentage of N cell/mm2 at 1h, 4h and 24h compared to N cell/mm2 at ACQ; % vs CTR: percentage of N cell/mm2 compared to CTR at the same time point; n.s.: not significant
Statistical analysis was performed within Treatments using One-way ANOVA. CTR: One-way ANOVA, F(3,14)=9.166, P<0.01; **P<0.01 CTR 1h, 4h and 24h vs CTR ACQ, Newman-Keuls Multiple Comparison test. SCOP: One-way ANOVA, F(3,17)=5.604, P<0.01; *P<0.05 SCOP 4h vs SCOP ACQ, and SCOP 24h vs SCOP ACQ, **P<0.01 SCOP 1h vs SCOP ACQ, Newman-Keuls multiple comparison test. MEC+SCOP: One-way ANOVA, F(3,15)=17.22, P<0.001; ***P<0.001 MEC + SCOP 24h vs MEC + SCOP ACQ, Newman-Keuls multiple comparison test.
Statistical analysis within Time points was performed using Two-way ANOVA, using Treatment and Recall time as the two variables. SCOP vs CTR & MEC+SCOP vs CTR: Treatment, F(2,37)=8.231, P<0.01; Time, F(3,37)=17.77, P<0.001; Interaction Treatment × Time, F(6,37)=4.44, P<0.01; #P<0.05 SCOP 1h vs CTR 1h; ##P<0.01 MEC+SCOP 1h vs CTR 1h, and MEC+SCOP 4h vs CTR 4h, Bonferroni post test. MEC+SCOP vs SCOP: Treatment, F(1,26)=13.30, P<0.01; Time, F(3,26)=9.4 13, P<0.001; Interaction Treatment × Time, F(3,26)=7.486, P<0.001; $$$P<0.001 MEC+SCOP 1h vs SCOP 1h and $$P<0.01 MEC+SCOP 4h vs SCOP 4h Bonferroni post test.
3.4 Administration of rapamycin increases activation of mTOR in microglia
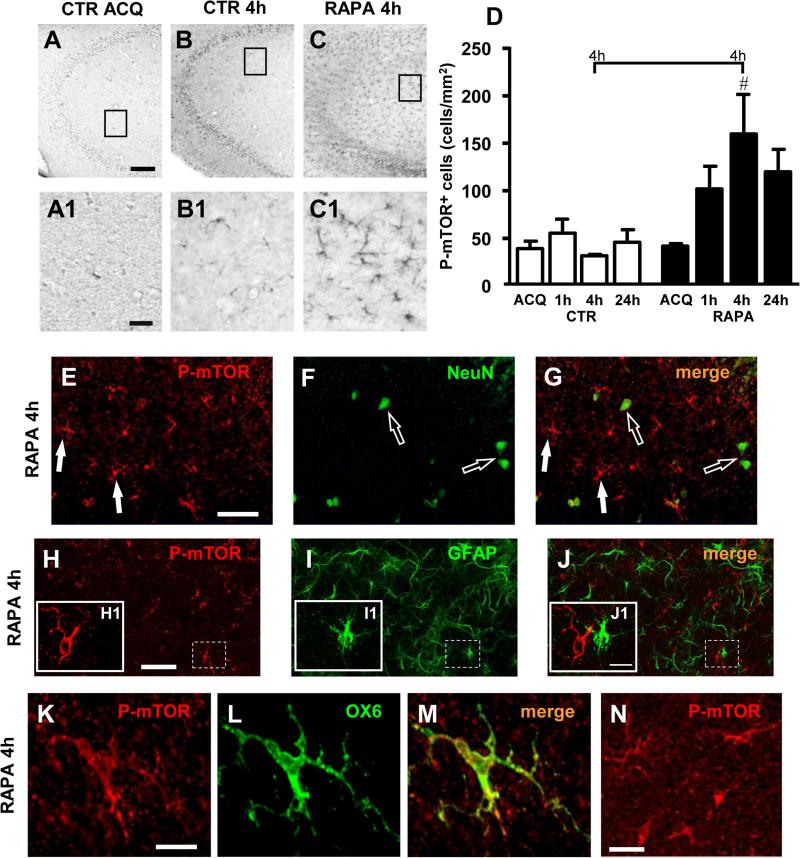
A serendipitous, unexpected effect evoked by administration of rapamycin was the significant activation of mTOR in cells located mainly in the Str. Radiatum of CA3 (Figure 4C-4C1). Panels 4A-4C show phospho-mTOR immunostaining in area CA3 under the three experimental conditions, CTR ACQ, CTR 4h and RAPA 4h, and panels A1-C1 are magnifications of the framed areas in the above images. Quantitative analysis of these phospho-mTOR positive cells in the Str. Radiatum of CA3 (Figure 4D) shows that mTOR activation had a time-dependent increase starting already at 1h (1h 30 min after rapamycin administration, +83%, RAPA 1h vs CTR 1h, n.s.). The effect reached statistical significance at 4h (4 h 30 min after administration of rapamycin, +408%, RAPA 4h vs CTR 4h), and returned towards basal levels at 24h (+161%, RAPA 24h vs CTR 24h, n.s.; 24.30 h after administration of rapamycin). Statistical analysis was performed by Two-way ANOVA with Treatment and Recall time as the two variables: Treatment, F(1,18)=9.584, P<0.001, Time, F(3,18)=1.372, n.s., Interaction Treatment × Time, F(3,18)=1.687, n.s. (#P<0.05, RAPA 4h vs CTR 4h, Bonferroni post test). No activation of p70S6K was found at any of the times tested (data not shown, but see also Figure 2G).
Figure 4.
A-C: Phospho-mTOR immunostaining in CA3 hippocampal region of control and rapamycin treated rats: phospho-mTOR positive cells in CA3 Str. Radiatum at acquisition time in a control rat (A), at 4h after acquisition in a control rat (B) and at 4h after acquisition in a rapamycin treated rat (C). Enlargement of framed areas in panels A-C are shown in panels A1-C1. In rapamycin treated rats at 4h after acquisition (C, C1) phospho-mTOR positive cells in CA3 Str. Radiatum appear more numerous than in control rats at the acquisition time (A, A1) and in control rats at 4h after acquisition (B, B1). Scale bars: 100 μm (A-C), 30 μm (A1-C1). D: Phospho-mTOR positive cells density (cells/mm2) in CA3 Str. Radiatum of control and rapamycin treated rats. At 4h after acquisition there is a significative increment of phospho-mTOR positive cells in rapamycin treated rats in comparison with control rats (#P<0.05, RAPA 4h vs CTR 4h, Bonferroni post test). CTR: ACQ n=3; 1h n=4; 4h n=3; 24h n=3; RAPA: ACQ n=3; 1h n=3; 4h n=3; 24h n=4. E-G: Representative confocal microscopy images of double fluorescent immunostaining for phospho-mTOR (red) and NeuN (green) in a rapamycin treated rat 4h after acquisition. Open arrows indicate neurons, arrows indicate mTOR positive cells. Scale bar: 50 μm. H-J1: Representative confocal microscopy images of double fluorescent immunostaining for phospho-mTOR (red) and GFAP (green) in a rapamycin treated rat 4h after acquisition. Enlargement of framed areas in panels H-J are shown in panels H1-J1. Phospho-mTOR immunostaining did not colocalize with GFAP immunostaining. Scale bars: 50 μm (H-J), 15 μm (H1-J1). K-N: Representative confocal microscopy images of double fluorescent immunostaining for phospho-mTOR (red) and OX6 (green) in a rapamycin treated rat 4h after acquisition. In some cells as the one shown in panels K-M complete colocalization between phospho-mTOR and OX6 immunostaining was present. In other cells like those in panel N there was no colocalization between the two markers. Scale bars: 20 μm (K-M), 50 μm (N).
From the morphology and localization of the mTOR immunopositive cells (Figure 4C1) it was possible to determine that these immunopositive cells were not neurons but rather glial cells. Indeed, double labelling confocal microscopy immunohistochemistry of phospho-mTOR (Figure 4E, arrows), and neurons (labelled with anti-NeuN antibody, Figure 4F, open arrows), unambiguously demonstrated that phospho-mTOR-positive cells found in the Str. Radiatum of CA3 after administration of rapamycin were not neurons (Figure 4G).
Therefore, to unambiguously define whether mTOR activation caused by rapamycin administration occurred in microglia or astrocytes, we set up a double immunolabelling of phospho-mTOR and GFAP to label astrocytes. Double labelling confocal microscopy of activated mTOR (Figure 4H, red) and GFAP (Figure 4I, green) showed that phospho-mTOR did not colocalize with GFAP (Figure 4J, and inset J1), demonstrating unambiguously that mTOR-positive cells were not astrocytes. Using double labelling confocal microscopy for phospho-mTOR (Figure 4K) and OX6, a marker of activated microglia (Figure 4L, green), we found colocalization of phospho-mTOR (Figure 4K, red) in OX6-positive cells (Figure 4M, merge, yellow-orange). As shown in Figure 4N, mTOR was activated in many cells with large pleomorphic bi-or tri-polar cell body, or in spindle or rod-shaped cells with modification in cellular structure that included de-ramification, shortening and twisting of cellular processes, not positive for OX6. In accordance to the literature (Herber et al., 2006; Miller & Streit, 2007; Nelson, Soma, & Lavi, 2002; Rezaie, Trillo-Pazos, Greenwood, Everall, & Male, 2002; Stence, Waite, & Dailey, 2001) these cells can be defined as reactive microglial cells. It is possible that the majority of phospho-mTOR positive cells are microglia in a precocious state of activation, not yet positive for OX6. A similar activation of mTOR in microglia was also found in CA1 Str. Radiatum (Supplementary material 1).
4. Discussion
The current study compared the dynamics of mTOR/p70S6K activation in the CA1 and CA3 regions of the hippocampus during the formation of IA memory with the goal of determining the differential contribution of these two hippocampal regions in the encoding of an IA memory. We discovered that the mTOR/p70S6K pathway in CA3 pyramidal neurons is activated by cholinergic input in order to trigger the formation of long term memory. Taken together with our previous study (Lana et al., 2013) this report confirmed the importance of both hippocampal areas in the encoding of IA memories.
Only long term memory was impaired following an almost complete blockade of mTOR and p70S6K activation by rapamycin. The first experiments that demonstrated the participation of mTOR in memory development in vivo were those of Parsons and coworkers (Parsons, Gafford, & Helmstetter, 2006) and Bekinschtein and coworkers (Bekinschtein et al., 2007), who showed that bilateral injection of rapamycin into the amygdala or the hippocampus impaired acquisition or consolidation of long-term fear memories. Rapamycin injection disrupts memory performance 24 h, but not 3 h, after training (Hoeffer et al., 2008), demonstrating that the animal can form the fear-associated STM.
The almost complete blockade of mTOR/p70S6K activation, present in CA3 pyramidal neurons but not in CA1 neurons (Lana et al., 2013) 4h after administration of rapamycin, is a phenomenon that distinguishes the dynamics of mTOR/p70S6K pathway activation/deactivation in the two regions of the hippocampus. This differential effect may be due to region-specific differences in the modulation of kinases and phosphatases after administration of rapamycin. Indeed, it has been previously demonstrated that the shifts between kinases and phosphatases activities in CA1 and CA3 pyramidal cells are differentially modulated (Gee et al., 2006), with phosphatases being more active in CA3 than in CA1 (Gee et al., 2006). We thus suggest that blockade of mTOR activation by rapamycin may have unmasked the higher activity of phosphatases that physiologically dephosphorylate mTOR. This effect seems to be more evident in CA3 than in CA1 (Gee et al., 2006). Our hypothesis is that the very low level of p70S6K phosphorylation at 4h is the direct consequence of two contrasting mechanisms: the inhibition of mTOR activation and of the higher activity of phosphatases. These two combined effects (inhibition of mTOR activation by rapamycin and high dephosphorylation activity) may be responsible for the almost complete inhibition of mTOR activation, and consequently of p70S6K activation, at the time 4h. The higher phosphatase activity in CA3 in comparison to CA1 (Gee et al., 2006) may underlie the maintenance of cellular homeostasis in CA3 neurons. Indeed, CA3 neurons, which receive many inputs not only from the dentate gyrus granule cells, but especially from collaterals of neighbouring CA3 neurons, may need to rapidly deactivate the mTOR/p70S6K pathway in order to avoid alterations in synaptic protein expression and synaptic strength due to recurrent collateral hyperactivation. This might explain why mice with hyperactivation of mTOR in mature CA3 neurons and dentate granule cells develop spontaneous seizures (Kwon et al., 2006). Hyperactivation of mTOR signaling has been also demonstrated in models of acute seizures in the rat (Zhang & Wong, 2012), in status epilepticus (Okamoto et al., 2010; Macias et al., 2013), and in in vitro models of epilepsy (Berdichevsky et al., 2013). Indeed, CA3 hippocampal neurons receive a dense network of recurrent glutamatergic collaterals whose stimulation-induced firing generates synchronized activity that may result in status epilepticus, leading to the activation of mTOR signaling (Zeng, Rensing, & Wong, 2009). The presence of high phosphatase activity in CA3 neurons may be responsible for interrupting the development of seizures.
Precise control of mTOR activity is necessary for proper memory encoding (Puighermanal, Busquets-Garcia, Maldonado, & Ozaita, 2012), since not only reduced but also enhanced activation of the mTOR signalling cascade impair memory (Troca-Marín, Alves-Sampaio, & Montesinos, 2012). Indeed, transgenic mice with constant activation of the hippocampal mTOR pathway (Ehninger et al., 2008; Goorden, van Woerden, van der Weerd, Cheadle, & Elgersma, 2007), and patients with tuberous sclerosis who have increase of mTORC1 activity, show memory deficits (Ehninger et al., 2008; Goorden, van Woerden, van der Weerd, Cheadle, & Elgersma, 2007). In this study we also demonstrated that short term memory encoding was not impaired by rapamycin administration, demonstrating that this type of memory is not influenced by the blockade of the mTORC1 activation and local protein synthesis inhibition.
Nevertheless, despite the large number of studies on mTOR, little is known on how intracellular mechanisms of feedback and/or feedforward activation of mTOR/p70S6K may act under physiological or pathological conditions in different, although strictly related and interconnected regions of the hippocampus to develop memory. To investigate whether the process of inhibitory avoidance memory formation might be triggered by the cholinergic system that activates the dynamics of mTOR signalling in CA3 pyramidal neurons, we correlated the results of the behavioural test with immunohistochemical analysis of mTOR/p70S6K activation at the different time points under the following experimental condition: blockade of the cholinergic signalling with scopolamine, a muscarinic AChRs antagonist, alone or together with mecamylamine, a nicotinic AChRs antagonist. We confirmed here that scopolamine, alone or with mecamylamine, induced an amnesic effect on short term memory at 1h and 4h after acquisition (Lana et al., 2013) but did not influence long term memory at 24h. Interestingly, we found increased activation of mTOR in hippocampal CA3 pyramidal neurons of scopolamine treated rats in comparison to control rats, a phenomenon also demonstrated in CA1 pyramidal neurons (Lana et al., 2013). A mechanistic explanation for this effect is that scopolamine, blocking the presynaptic inhibitory M2 mAChR, increases release of ACh in the synaptic cleft (Scali, Vannucchi, Pepeu, & Casamenti, 1995) which then impinges and activates the nAChR on the postsynaptic terminal, possibly causing downstream stimulation of the mTOR pathway. Scopolamine administration also induced a significant activation of p70S6K at 1h, 4h and 24h. Prolonged activation of p70S6K may be triggered by mTOR activation at 1h and may be responsible for long term memory formation in the presence of scopolamine. On the other hand, it is also possible that non-cholinergic neurotransmitter systems known to be involved in inhibitory avoidance memory formation (Izquierdo, Furini, & Myskiw, 2016), may be involved in an mTOR-independent regulation of p70S6K phosphorylation that may involve other intracellular signalling cascades such as PKC and ERK/p90RSK, (Gangarossa & Valjent, 2012).
The administration of scopolamine plus mecamylamine induced a profound short term memory impairment that was associated with a very strong inhibition of mTOR activation at 1h. p70S6K activation was not increased at 1h and 4h, but at 24h the levels of phospho-p70S6K were significantly higher than basal and did not differ from controls. We postulate that the residual activation of mTOR at 4h may be responsible for p70S6K activation between 4h and 24h and for the formation of the long term memory at 24h, or again that other, mTOR independent pathways, may be responsible for p70S6K activation (Gangarossa & Valjent, 2012). These results confirm the activation of the mTOR pathway via the cholinergic system input to CA3 neurons during the encoding of an inhibitory avoidance memory.
We demonstrated here that activation of mTOR/p70S6K pathway can be triggered by the stimulation of both mAChRs and nAChRs activated by ACh. We also demonstrated that in CA3 hippocampal neurons the mTOR/p70S6K wave of activation reached its maximum between 1h and 4h after the stimulation of the cholinergic system and exerted its role on the formation of long term memory. Taken together these results confirm the new concept that short term and long term memory formation are two processes that start together in the moment a new mnemonic stimulus is given to the animal and then proceed independently with distinct biochemical signalling pathway (Izquierdo et al., 1998a).
Furthermore, our data shed light on the common involvement not only of CA1, but also of CA3 pyramidal neurons in the formation of new inhibitory avoidance memories. The hippocampal area CA3 is central in the trisynaptic pathway through which the hippocampus is activated, being strictly interconnected to the other areas of the hippocampus by projections that derive from DG and project to CA1. This activation is at the basis of many types of memories such as the IA memory (Whitlock, Heynen, Shuler, & Bear, 2006), contextual fear conditioning (Ryan, Roy, Pignatelli, Arons, & Tonegawa, 2015), trace eyeblink conditioning (Gruart, Muñoz, & Delgado-García, 2006), and possibly object recognition memory (Clarke, Cammarota, Gruart, Izquierdo, & Delgado-García, 2010). The CA3 supports memory based on rapid, one-trial object–place, learning and recall. CA3 lesions produce chance performance on a one-trial object–place recall task (Kesner, Hunsaker, & Warthen, 2008) and other object–spatial tasks (Kesner & Rolls, 2015; Rolls & Kesner, 2006). Nevertheless, understanding what roles the different subfields of the hippocampus play remains critical in advancing our understanding of the neural basis of memory. Here we have demonstrated that the mTOR/p70S6K pathway in CA3 pyramidal neurons is activated during the encoding of an inhibitory avoidance memory, confirming the hypothesis that CA3 is especially needed in rapid one trial place memory (Rolls, 2016). It seems therefore that CA3 and CA1 show distinct but complementary roles in representation of spatial context during the formation of memories (Stokes, Kyle, & Ekstrom, 2015).
An unexpected, very interesting result obtained after the administration of rapamycin, was the activation of mTOR in cells located in the Str. Radiatum of CA3 as well as CA1 hippocampal regions. From the location and shape of these mTOR-positive cells, visualized by double labelling confocal microscopy, we demonstrated that they are microglial cells. Whilst activation of mTOR was evident in microglial cells at all times tested after administration of rapamycin, with a peak of activation at 4 h, no activation of p70S6K was ever found, at any of the times investigated. Rapamycin is a rather selective mTORC1 inhibitor and, according to the literature, at the concentration we used, rapamycin inhibits the activation of mTORC1, but does not significantly influence the activation of mTORC2 (Foster & Toschi, 2009). Indeed, mTORC2 responds only to prolonged and chronic rapamycin treatment, in part because rapamycin does not directly interfere with existing mTORC2 complex, but rather blocks the assembly of mTORC2 from newly synthesized Rictor and mTOR (Sarbassov et al., 2006). Therefore these data, taken together with the lack of p70S6K activation, indicate that administration of rapamycin increases activation of the mTORC2 complex in microglia cells.
The role of mTORC2 in cells and the cross link between the two mTOR complexes in the cells is not yet completely understood. It is well known that the evolutionarily conserved mTORC1 and mTORC2 are activated by different stimuli, have different composition and different downstream effectors (Gaubitz, Prouteau, Kusmider, & Loewith, 2016). Dysregulation of mTORC1 and mTORC2 signaling appears to have a crucial role in memory disorders. In recent papers it has been demonstrated that a negative feedback between mTORC1 and mTORC2 exists, and that potent activation of mTORC1 inhibits mTORC2 via a negative-feedback mechanism involving S6K1 (Harrington et al., 2004; Julien, Carriere, Moreau, & Roux, 2010). This negative feedback mechanism accounts for the increased mTORC2 signaling demonstrated following embryonic deletion of Rheb1 in neural progenitor cells, which abolishes mTORC1 signaling in developing brain (Zou et al., 2011). Interestingly, Liu and colleagues recently showed that in freshly isolated splenic B cells, rapamycin significantly increases Akt phosphorylation, a typical hallmark of mTORC2 activation. Similar results were also observed in mouse liver lysates: systemic rapamycin administration led to increased Akt-S473 phosphorylation (Liu, Guo, Gan, & Wei, 2014). We hypothesize here that in the hippocampus a similar negative feedback between mTORC1 and mTORC2 may take place, and the administration of rapamycin, by blocking mTORC1 formation, may consequently induce a similar overactivation of mTORC2.
Since mTOR is an ubiquitous protein, present not only in neurons, but also in astrocytes and microglia (Dello Russo, Lisi, Feinstein, & Navarra, 2013), the question that still remains to be addressed is why in our samples the overactivation of mTORC2 after rapamycin was present only in microglial cells. The two mTOR complexes have an ubiquitous cellular expression but no systematic study of their differential function, and of the regulation of their activity in specific brain areas or cells has so far been performed. The data on mTORC2 localization and role in cellular mechanisms are still sparse. It has been demonstrated that mTORC2 regulates a number of cellular processes such as cell proliferation, apoptosis and longevity (Bockaert & Marin, 2015), and it has also a key role in actin polymerization and lamellipodia formation (Hernandez-Negrete et al., 2007). In line with its ability to regulate actin cytoskeleton, mTORC2 has been involved in neutrophil chemotaxis (Liu & Parent, 2011). The specific activation of mTOR in microglial cells after rapamycin administration is in agreement with all these results, since it accounts for the negative feedback between mTORC1 and mTORC2 and also for the localization of mTORC2 in microglial cells that express lamellipodia and actin cytoskeleton activity having a specific function of movement in the nervous system. The questions still open are i) what may be the physiological significance of the feedback activation of mTORC2 when mTORC1 is inhibited and ii) may mTORC2 activation by rapamycin in microglia account for the therapeutic effects of the drug or iii) can this effect be considered as a new therapeutic target of rapamycin.
Conclusions
In this paper we have demonstrated that in the CA3 region of the hippocampus, activation of the mTOR pathway is necessary for the formation of a long term inhibitory avoidance memory. A serendipitous, possibly important finding was that after administration of rapamycin the activation of mTORC2 increases in microglial cells, poses the basis for a reappraisal of the beneficial/adverse effects of rapamycin administration.
Supplementary Material
Highlights.
mTOR/p70S6K activation in area CA3 are involved in long term memory formation
rapamycin inhibited mTOR/p70S6K activation at 4h and impaired long term memory at 24h
scopolamine activated mTOR/p70S6K at 1h and impaired short but not long term memory
mecamylamine reduced the scopolamine-induced increase of mTOR/p70S6K activation at 1h
rapamycin increased mTORC2 activation in microglial cells in Stratum Radiatum
Acknowledgements
We thank Dr. A. Melani for help in animal care and Miss. M.R. Malatacca, for help in immunohistochemistry and quantification analysis. This work was partly supported by Fondazione Ente Cassa di Risparmio Grant N. 2014/0663 and Grant 2015 from Università di Firenze. D.L. was supported by “Prize from Italian Society of Pharmacology”, with the contribution of MSD Italia, Prize Edition 2014. GLW was supported by U.S. Public Health Service, RO1 AG037320.
Abbreviations
- ACQ
acquisition
- ACh
acetylcholine
- DMSO
dimethyl sulfoxide
- IA
inhibitory avoidance
- i.c.v.
intracerebroventricular
- i.p.
intraperitoneal
- mTOR
mammalian Target of Rapamycin
- mAChR
muscarinic acetylcholine receptors
- mTORC1,2
mammalian Target of Rapamycin Complex1,2
- MEC
mecamylamine
- nAChR
nicotinic acetylcholine receptors
- p70S6K
p70S6Kinase
- P-mTOR
phospho-mTOR
- P-p70S6K
phospho-p70S6K
- RAPA
rapamycin
- SCOP
scopolamine
- SEM
standard error of the mean
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amaral DG, Witter MP. The three-dimensional organization of the hippocampal formation: a review of anatomical data. Neuroscience. 1989;31:571–591. doi: 10.1016/0306-4522(89)90424-7. [DOI] [PubMed] [Google Scholar]
- Bekinschtein P, Katche C, Slipczuk LN, Igaz LM, Cammarota M, Medina JH. mTOR signaling in the hippocampus is necessary for memory formation. Neurobiology of Learning and Memory. 2007;87:303–307. doi: 10.1016/j.nlm.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Berdichevsky Y, Dryer AM, Saponjian Y, Mahoney MM, Pimentel CA, Staley KJ. PI3K-Akt signaling activates mTOR-mediated epileptogenesis in organotypic hippocampal culture model of post-traumatic epilepsy. The Journal of Neuroscience. 2013;33:9056–9067. doi: 10.1523/JNEUROSCI.3870-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockaert J, Marin P. mTOR in Brain Physiology and Pathologies. Physiological Reviews. 2015;95:1157–1187. doi: 10.1152/physrev.00038.2014. [DOI] [PubMed] [Google Scholar]
- Cammarota M, Bevilaqua LRM, Medina JH, Izquierdo I. Studies of Short-Term Avoidance Memory. In: Bermudez-Rattoni F, editor. Neural Plasticity and Memory: From Genes to Brain Imaging (Chapter 10) CRC Press/Taylor & Francis; Boca Raton, FL, USA: 2007. [PubMed] [Google Scholar]
- Clarke JR, Cammarota M, Gruart A, Izquierdo I, Delgado-García JM. Plastic modifications induced by object recognition memory processing. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:2652–2657. doi: 10.1073/pnas.0915059107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dello Russo C, Lisi L, Feinstein DL, Navarra P. mTOR kinase, a key player in the regulation of glial functions: relevance for the therapy of multiple sclerosis. Glia. 2013;61:301–311. doi: 10.1002/glia.22433. [DOI] [PubMed] [Google Scholar]
- Ehninger D, Han S, Shilyansky C, Zhou Y, Li W, Silva AJ. Reversal of learning deficits in a Tsc2+/− mouse model of tuberous sclerosis. Nature Medicine. 2008;14:843–848. doi: 10.1038/nm1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster DA, Toschi A. Targeting mTOR with rapamycin: one dose does not fit all. Cell Cycle. 2009;8:1026–1029. doi: 10.4161/cc.8.7.8044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangarossa G, Valjent E. Regulation of the ERK pathway in the dentate gyrus by in vivo dopamine D1 receptor stimulation requires glutamatergic transmission. Neuropharmacology. 2012;63:1107–1117. doi: 10.1016/j.neuropharm.2012.06.062. [DOI] [PubMed] [Google Scholar]
- Gaubitz C, Prouteau M, Kusmider B, Loewith R. TORC2 Structure and Function. Trends in Biochemical Sciences. 2016;41:532–545. doi: 10.1016/j.tibs.2016.04.001. [DOI] [PubMed] [Google Scholar]
- Gee CE, Benquet P, Raineteau O, Rietschin L, Kirbach SW, Gerber U. NMDA receptors and the differential ischemic vulnerability of hippocampal neurons. The European Journal of Neuroscience. 2006;23:2595–2603. doi: 10.1111/j.1460-9568.2006.04786.x. [DOI] [PubMed] [Google Scholar]
- Giovannini MG, Bartolini L, Bacciottini L, Greco L, Blandina P. Effects of histamine H3 receptor agonists and antagonists on cognitive performance and scopolamine-induced amnesia. Behavioural Brain Research. 1999;104:147–155. doi: 10.1016/s0166-4328(99)00063-7. [DOI] [PubMed] [Google Scholar]
- Giovannini MG, Blitzer RD, Wong T, Asoma K, Tsokas P, Landau EM. Mitogen-activated protein kinase regulates early phosphorylation and delayed expression of Ca2+/calmodulin-dependent protein kinase II in long-term potentiation. The Journal of Neuroscience. 2001;21:7053–7062. doi: 10.1523/JNEUROSCI.21-18-07053.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannini MG, Efoudebe M, Passani MB, Baldi E, Bucherelli C, Blandina P. Improvement in fear memory by histamine-elicited ERK2 activation in hippocampal CA3 cells. The Journal of Neuroscience. 2003;23:9016–9023. doi: 10.1523/JNEUROSCI.23-27-09016.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannini MG, Pazzagli M, Malmberg-Aiello P, Della Corte L, Rakovska AD, Pepeu G. Inhibition of acetylcholine-induced activation of extracellular regulated protein kinase prevents the encoding of an inhibitory avoidance response in the rat. Neuroscience. 2005;136:15–32. doi: 10.1016/j.neuroscience.2005.07.046. [DOI] [PubMed] [Google Scholar]
- Giovannini MG, Cerbai F, Bellucci A, Melani C, Grossi C, Casamenti F. Differential activation of mitogen-activated protein kinase signalling pathways in the hippocampus of CRND8 transgenic mouse, a model of Alzheimer's disease. Neuroscience. 2008;153:618–633. doi: 10.1016/j.neuroscience.2008.02.061. [DOI] [PubMed] [Google Scholar]
- Giovannini MG, Lana D. mTOR Involvement in the Mechanisms of Memory. In: Maiese K, editor. Molecules to Medicine with mTOR: translating critical pathways into novel therapeutic strategies. Cellular and molecular signaling; Newark, NJ, USA: 2016. pp. 169–184. [Google Scholar]
- Goorden SM, van Woerden GM, van der Weerd L, Cheadle JP, Elgersma Y. Cognitive deficits in Tsc1+/− mice in the absence of cerebral lesions and seizures. Annals of Neurology. 2007;62:648–655. doi: 10.1002/ana.21317. [DOI] [PubMed] [Google Scholar]
- Gruart A, Muñoz MD, Delgado-García JM. Involvement of the CA3-CA1 synapse in the acquisition of associative learning in behaving mice. The Journal of Neuroscience. 2006;26:1077–1087. doi: 10.1523/JNEUROSCI.2834-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington LS, Findlay GM, Gray A, Tolkacheva T, Wigfield S, Lamb RF. The TSC1-2 tumor suppressor controls insulin-PI3K signaling via regulation of IRS proteins. The Journal of Cell Biology. 2004;166:213–223. doi: 10.1083/jcb.200403069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedden T, Gabrieli JD. Insights into the ageing mind: a view from cognitive neuroscience. Nature Reviews. Neuroscience. 2004;5:87–96. doi: 10.1038/nrn1323. [DOI] [PubMed] [Google Scholar]
- Herber DL, Maloney JL, Roth LM, Freeman MJ, Morgan D, Gordon MN. Diverse microglial responses after intrahippocampal administration of lipopolysaccharide. Glia. 2006;53:382–391. doi: 10.1002/glia.20272. [DOI] [PubMed] [Google Scholar]
- Hernandez-Negrete I, Carretero-Ortega J, Rosenfeldt H, Hernandez-Garcia R, Calderon-Salinas JV, Vazquez-Prado J. P-Rex1 links mammalian target of rapamycin signaling to Rac activation and cell migration. The Journal of Biological Chemistry. 2007;282:23708–23715. doi: 10.1074/jbc.M703771200. [DOI] [PubMed] [Google Scholar]
- Hoeffer CA, Tang W, Wong H, Santillan A, Patterson RJ, Klann E. Removal of FKBP12 enhances mTOR-Raptor interactions, LTP, memory, and perseverative/repetitive behavior. Neuron. 2008;60:832–845. doi: 10.1016/j.neuron.2008.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunsaker MR, Kesner RP. Evaluating the differential roles of the dorsal dentate gyrus, dorsal CA3, and dorsal CA1 during a temporal ordering for spatial locations task. Hippocampus. 2008;18:955–964. doi: 10.1002/hipo.20455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo I. Mechanism of action of scopolamine as an amnesic. Trends in Pharmacological Sciences. 1989;10:175–177. doi: 10.1016/0165-6147(89)90231-9. [DOI] [PubMed] [Google Scholar]
- Izquierdo I, Quillfeldt JA, Zanatta MS, Quevedo J, Schaeffer E, Medina JH. Sequential role of hippocampus and amygdala, entorhinal cortex and parietal cortex in formation and retrieval of memory for inhibitory avoidance in rats. The European Journal of Neuroscience. 1997a;9:786–793. doi: 10.1111/j.1460-9568.1997.tb01427.x. [DOI] [PubMed] [Google Scholar]
- Izquierdo I, Medina JH. Memory formation: The sequence of biochemical events in the hippocampus and its connection to activity in other brain structures. Neurobiology of Learning and Memory. 1997b;68:285–316. doi: 10.1006/nlme.1997.3799. [DOI] [PubMed] [Google Scholar]
- Izquierdo I, Medina JH, Izquierdo LA, Barros DM, de Souza MM, Mello e Souza T. Short- and long-term memory are differentially regulated by monoaminergic systems in the rat brain. Neurobiology of Learning and Memory. 1998a;69:219–224. doi: 10.1006/nlme.1998.3825. [DOI] [PubMed] [Google Scholar]
- Izquierdo I, Barros DM, Mello e Souza T, de Souza MM, Izquierdo LA, Medina JH. Mechanisms for memory types differ. Nature. 1998b;393:635–636. doi: 10.1038/31371. [DOI] [PubMed] [Google Scholar]
- Izquierdo LA, Barros DM, Vianna MR, Coitinho A, deDavid e Silva T, Izquierdo I. Molecular pharmacological dissection of short- and long-term memory. Cellular and Molecular Neurobiology. 2002;22:269–287. doi: 10.1023/A:1020715800956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo I, Furini CR, Myskiw JC. Fear Memory. Physiological Reviews. 2016;96:695–750. doi: 10.1152/physrev.00018.2015. [DOI] [PubMed] [Google Scholar]
- Julien LA, Carriere A, Moreau J, Roux PP. mTORC1-activated S6K1 phosphorylates Rictor on threonine 1135 and regulates mTORC2 signaling. Molecular and Cellular Biology. 2010;30:908–921. doi: 10.1128/MCB.00601-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesner RP, Lee I, Gilbert P. A behavioral assessment of hippocampal function based on a subregional analysis. Reviews in the Neurosciences. 2004;15:333–351. doi: 10.1515/revneuro.2004.15.5.333. [DOI] [PubMed] [Google Scholar]
- Kesner RP, Hunsaker MR, Warthen MW. The CA3 subregion of the hippocampus is critical for episodic memory processing by means of relational encoding in rats. Behavioural Neurosciences. 2008;122:1217–1225. doi: 10.1037/a0013592. [DOI] [PubMed] [Google Scholar]
- Kesner RP, Rolls ET. A computational theory of hippocampal function, and tests of the theory: new developments. Neuroscience and Biobehavioral Reviews. 2015;48:92–147. doi: 10.1016/j.neubiorev.2014.11.009. [DOI] [PubMed] [Google Scholar]
- Kwon CH, Luikart BW, Powell CM, Zhou J, Matheny SA, Parada LF. Pten regulates neuronal arborization and social interaction in mice. Neuron. 2006;50:377–388. doi: 10.1016/j.neuron.2006.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lana D, Cerbai F, Di Russo J, Boscaro F, Giannetti A, Giovannini MG. Hippocampal long term memory: effect of the cholinergic system on local protein synthesis. Neurobiology of Learning and Memory. 2013;106:246–257. doi: 10.1016/j.nlm.2013.09.013. [DOI] [PubMed] [Google Scholar]
- Langston RF, Stevenson CH, Wilson CL, Saunders I, Wood ER. The role of hippocampal subregions in memory for stimulus associations. Behavioural Brain Research. 2010;215:275–291. doi: 10.1016/j.bbr.2010.07.006. [DOI] [PubMed] [Google Scholar]
- Lee I, Kesner RP. Differential contribution of NMDA receptors in hippocampal subregions to spatial working memory. Nature Neuroscience. 2002;5:162–168. doi: 10.1038/nn790. [DOI] [PubMed] [Google Scholar]
- Lee I, Kesner RP. Differential roles of dorsal hippocampal subregions in spatial working memory with short versus intermediate delay. Behavioural Neuroscience. 2003;117:1044–1053. doi: 10.1037/0735-7044.117.5.1044. [DOI] [PubMed] [Google Scholar]
- Lee I, Kesner RP. Encoding versus retrieval of spatial memory: double dissociation between the dentate gyrus and the perforant path inputs into CA3 in the dorsal hippocampus. Hippocampus. 2004;14:66–76. doi: 10.1002/hipo.10167. [DOI] [PubMed] [Google Scholar]
- Liu L, Parent CA. Review series: TOR kinase complexes and cell migration. The Journal of Cell Biology. 2011;194:815–824. doi: 10.1083/jcb.201102090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Guo J, Gan W, Wei W. Dual phosphorylation of Sin1 at T86 and T398 negatively regulates mTORC2 complex integrity and activity. Protein & Cell. 2014;5:171–177. doi: 10.1007/s13238-014-0021-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macias M, Blazejczyk M, Kazmierska P, Caban B, Skalecka A, Jaworski J. Spatiotemporal characterization of mTOR kinase activity following kainic acid induced status epilepticus and analysis of rat brain response to chronic rapamycin treatment. PLoS One. 2013;8:e64455. doi: 10.1371/journal.pone.0064455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S. Is there savings for pavlovian fear conditioning after neurotoxic basolateral amygdala lesions in rats? Neurobiology of Learning and Memory. 2001;76:268–283. doi: 10.1006/nlme.2001.4042. [DOI] [PubMed] [Google Scholar]
- McGaugh JL, Izquierdo I. The contribution of pharmacology to research on the mechanisms of memory formation. Trends in Pharmacological Sciences. 2000;21:208–210. doi: 10.1016/s0165-6147(00)01473-5. [DOI] [PubMed] [Google Scholar]
- Miller KR, Streit WJ. The effects of aging, injury and disease on microglial function: a case for cellular senescence. Neuron Glia Biology. 2007;3:245–253. doi: 10.1017/S1740925X08000136. [DOI] [PubMed] [Google Scholar]
- Nakazawa K, Sun LD, Quirk MC, Rondi-Reig L, Wilson MA, Tonegawa S. Hippocampal CA3 NMDA receptors are crucial for memory acquisition of one-time experience. Neuron. 2003;38:305–315. doi: 10.1016/s0896-6273(03)00165-x. [DOI] [PubMed] [Google Scholar]
- Nelson PT, Soma LA, Lavi E. Microglia in diseases of the central nervous system. Annals of Medicine. 2002;34:491–500. doi: 10.1080/078538902321117698. [DOI] [PubMed] [Google Scholar]
- Okamoto OK, Janjoppi L, Bonone FM, Pansani AP, da Silva AV, Cavalheiro EA. Whole transcriptome analysis of the hippocampus: toward a molecular portrait of epileptogenesis. BMC Genomics. 2010;11:230. doi: 10.1186/1471-2164-11-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Reilly KC, Alarcon JM, Ferbinteanu J. Relative contributions of CA3 and medial entorhinal cortex to memory in rats. Frontiers in Behavioral Neurosciences. 2014;8:292. doi: 10.3389/fnbeh.2014.00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons RG, Gafford GM, Helmstetter FJ. Translational control via the mammalian target of rapamycin pathway is critical for the formation and stability of long-term fear memory in amygdala neurons. The Journal of Neuroscience. 2006;26:12977–12983. doi: 10.1523/JNEUROSCI.4209-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson G. The rat brain in stereotaxic coordinates. Academic Press; New York, NY, USA: 1982. [Google Scholar]
- Puighermanal E, Busquets-Garcia A, Maldonado R, Ozaita A. Cellular and intracellular mechanisms involved in the cognitive impairment of cannabinoids. Philosophycal Transactions of the Royal Society of London. Series B, Biological Sciences. 2012;367:3254–3263. doi: 10.1098/rstb.2011.0384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezaie P, Trillo-Pazos G, Greenwood J, Everall IP, Male DK. Motility and ramification of human fetal microglia in culture: an investigation using time-lapse video microscopy and image analysis. Experimental Cell Research. 2002;274:68–82. doi: 10.1006/excr.2001.5431. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Kesner RP. A computational theory of hippocampal function, and empirical tests of the theory. Progress in Neurobiology. 2006;79:1–48. doi: 10.1016/j.pneurobio.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Rolls ET. Pattern separation, completion, and categorisation in the hippocampus and neocortex. Neurobiology of Learning and Memory. 2016;129:4–28. doi: 10.1016/j.nlm.2015.07.008. [DOI] [PubMed] [Google Scholar]
- Ryan TJ, Roy DS, Pignatelli M, Arons A, Tonegawa S. Memory. Engram cells retain memory under retrograde amnesia. Science. 2015;348:1007–1013. doi: 10.1126/science.aaa5542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarbassov DD, Ali SM, Sengupta S, Sheen JH, Hsu PP, Sabatini DM. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Molecular Cell. 2006;22:159–168. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- Scali C, Vannucchi MG, Pepeu G, Casamenti F. Peripherally injected scopolamine differentially modulates acetylcholine release in vivo in the young and aged rats. Neuroscience Letters. 1995;197:171–174. doi: 10.1016/0304-3940(95)11914-i. [DOI] [PubMed] [Google Scholar]
- Small SA, Tsai WY, DeLaPaz R, Mayeux R, Stern Y. Imaging hippocampal function across the human life span: is memory decline normal or not? Annals of Neurology. 2002;51:290–295. doi: 10.1002/ana.10105. [DOI] [PubMed] [Google Scholar]
- Stence N, Waite M, Dailey ME. Dynamics of microglial activation: a confocal time-lapse analysis in hippocampal slices. Glia. 2001;33:256–266. [PubMed] [Google Scholar]
- Stokes J, Kyle C, Ekstrom AD. Complementary roles of human hippocampal subfields in differentiation and integration of spatial context. Journal of Cognitive Neuroscience. 2015;27:546–559. doi: 10.1162/jocn_a_00736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troca-Marín JA, Alves-Sampaio A, Montesinos ML. Deregulated mTOR-mediated translation in intellectual disability. Progress in Neurobiology. 2012;96:268–282. doi: 10.1016/j.pneurobio.2012.01.005. [DOI] [PubMed] [Google Scholar]
- Vazdarjanova A, Guzowski JF. Differences in hippocampal neuronal population responses to modifications of an environmental context: evidence for distinct, yet complementary, functions of CA3 and CA1 ensembles. The Journal of Neuroscience. 2004;24:6489–6496. doi: 10.1523/JNEUROSCI.0350-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlock JR, Heynen AJ, Shuler MG, Bear MF. Learning induces long-term potentiation in the hippocampus. Science. 2006;313:1093–1097. doi: 10.1126/science.1128134. [DOI] [PubMed] [Google Scholar]
- Wilensky AE, Schafe GE, LeDoux JE. The amygdala modulates memory consolidation of fear-motivated inhibitory avoidance learning but not classical fear conditioning. The Journal of Neuroscience. 2000;20:7059–7066. doi: 10.1523/JNEUROSCI.20-18-07059.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng LH, Rensing NR, Wong M. The mammalian target of rapamycin signaling pathway mediates epileptogenesis in a model of temporal lobe epilepsy. The Journal of Neuroscience. 2009;29:6964–6972. doi: 10.1523/JNEUROSCI.0066-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Wong M. Pentylenetetrazole-induced seizures cause acute, but not chronic, mTOR pathway activation in rat. Epilepsia. 2012;53:506–511. doi: 10.1111/j.1528-1167.2011.03384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou J, Zhou L, Du XX, Ji Y, Xu J, Xiao B. Rheb1 is required for mTORC1 and myelination in postnatal brain development. Developmental Cell. 2011;20:97–108. doi: 10.1016/j.devcel.2010.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.