Abstract
Background
Atrial fibrillation (AF) adds significant risk of stroke and thromboembolism in patients on hemodialysis (HD). The aim of this study was to investigate the prevalence of AF in a population-based cohort of HD patients and practice patterns of antithrombotic therapy for stroke prevention in AF.
Methods
The Vienna InVestigation of AtriaL fibrillation and thromboembolism in patients on hemodialysis (VIVALDI), an ongoing prospective observational cohort study, investigates the prevalence of AF and the risk of thromboembolic events in HD patients in Vienna, Austria. We analyzed cross-sectional data of 626 patients (63.4% men, median age 66 years, approx. 73% of HD patients in Vienna), who provided informed consent. A structured interview with each patient was performed, recent and archived ECGs were viewed and medical histories were verified with electronic records.
Results
The overall prevalence of AF was 26.5% (166 patients, 71.1% men, median age 72 years) of which 57.8% had paroxysmal AF, 3.0% persistent AF, 32.5% permanent AF, and 6.6% of patients had newly diagnosed AF. The median CHA2DS2-VASc Score was 4 [25th-75th percentile 3–5]. In multivariable analysis, AF was independently associated with age (odds ratio: 1.05 per year increase, 95% confidence interval: 1.03–1.07), male sex (1.7, 1.1–2.6), history of venous thromboembolism (2.0, 1.1–3.6), congestive heart failure (1.7, 1.1–2.5), history of or active cancer (1.5, 1.0–2.4) and time on HD (1.08 per year on HD, 1.03–1.13). Antithrombotic treatment was applied in 84.4% of AF patients (anticoagulant agents in 29.5%, antiplatelet agents in 33.7%, and both in 21.1%). In AF patients, vitamin-K-antagonists were used more often than low-molecular-weight heparins (30.1% and 19.9%).
Conclusions
The prevalence of AF is high amongst HD patients and is associated with age, sex, and distinct comorbidities. Practice patterns of antithrombotic treatment indicate a lack of consensus for stroke prevention in HD patients with AF.
Introduction
Atrial fibrillation (AF) is a common cardiac arrhythmia that affects 1–2% of the general population, and increases the risk of stroke [1,2]. In patients with end-stage renal disease (ESRD) receiving hemodialysis (HD), AF is an underestimated comorbidity. Although it is recognized as a causal factor for thromboembolic ischemic stroke and is associated with increased mortality in HD patients [3,4], stroke prevention using antithrombotic agents is complicated by an excessive risk of bleeding in ESRD patients [5]. In the general population, stroke prevention in AF with oral anticoagulation with vitamin-K-antagonists (VKA) can reduce the incidence of stroke by 60% [6]. However, in HD patients there is currently no clear evidence for how to achieve stroke prevention [7], and large differences exists in the current practice for use of antithrombotic agents in HD patients [8].
The reported prevalence of AF in HD patients is 3.8 to 27% [9–14]. This large variability in AF prevalence may be explained by regional differences [8], but may also be biased by the different study designs and data capture methods. The overall higher prevalence of AF compared to the general population is hypothesized to be associated with the HD procedure itself,[15] which can lead to an increase of cardiac dimensions and decrease of ejection fraction, resulting in AF development [12]. Thus, the prevalence of AF in HD patients may depend upon hemodialysis-specific patient characteristics as well as on treatment modalities.
In this population-based cohort of HD patients, we investigated the prevalence of AF, analyzed the association of AF with clinico-epidemiological factors, and collected data on practice patterns of antithrombotic treatment strategies applied in HD patients for stroke prevention in AF.
Patients and Methods
The Vienna InVestigation of AtriaL fibrillation and thromboembolism in hemDIialysis patients (VIVALDI) is a cohort study with aims to gather population-based epidemiologic data on the prevalence of AF, thromboembolic events and use of antithrombotic treatments in HD patients and prospective data on the incidence of stroke and thromboembolism, as well as the incidence of new AF, bleeding, hospitalization, cardiovascular events, thrombotic shunt complications, and mortality. The study consists of a cross-sectional baseline investigation and a prospective observational evaluation of study outcomes and has approval of the local ethics committees. VIVALDI is conducted in accordance with the declaration of Helsinki and its later amendments.
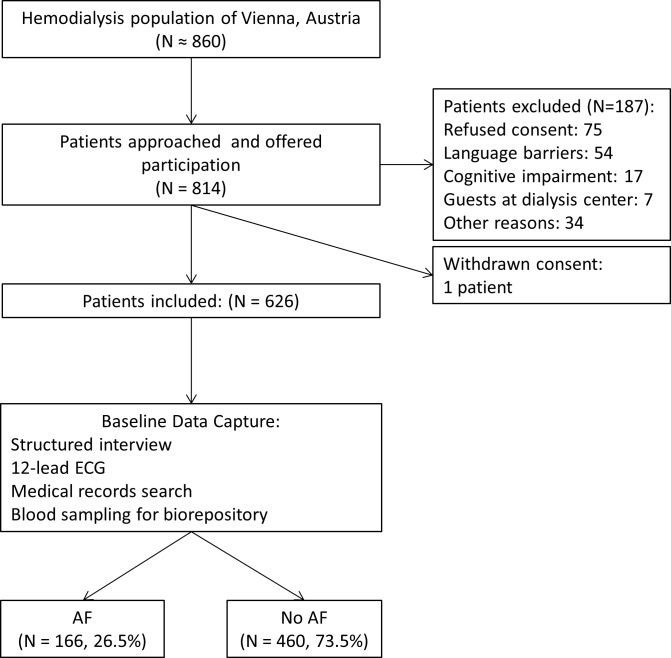
Patient treated at the seven major HD centers in the city of Vienna, Austria, were eligible for recruitment. The study cohort was recruited between April 2014 and July 2015. Patients aged 18 years or above, with an independent diagnosis of ESRD requiring long-term ambulatory HD treatment, able to provide signed and dated informed consent, and willing to comply with all study procedures were eligible for inclusion. Patients were excluded, if they were pregnant, lactating, suspected of pregnancy, incapable to consent, hospitalized as an in-patient, or serving a prison term at the time of enrolment. The steps of patient enrolment and corresponding patient numbers are provided in Fig 1. Out of approximately 860 patients receiving HD in Vienna (population of 1.7 million in 2014–2015), Austria, 814 (94.6%) were personally approached and 626 patients (~73% of the total HD population in the city) met the inclusion criteria and consented to participate. The prospective observation period is still ongoing.
Fig 1. Flow chart of the patient recruitment.
A trained study investigator interviewed each patient individually and recorded demographics, medical histories and HD parameters using the REDCap electronic data capture tools hosted at the Medical University of Vienna, Department of Medicine I, Clinical Division of Hematology and Haemostaseology [16]. Data quality was verified with treating nephrologists and the medical documentation of the participating dialysis centers. Cause of ESRD was established from the medical records at the dialysis center and classified according to categories of the Austrian Dialysis and Transplantation Registry [17]. A diagnosis of AF was recorded for symptomatic or asymptomatic AF in any one of the following (1) signs of AF on a recent 12-lead resting ECG within one month of recruitment, (2) documented AF episode during previous ECGs conducted routinely or in case of arrhythmia at the dialysis centers, or (3) recorded diagnosis of AF in medical records. Recently diagnosed AF was defined as recent new diagnosis of AF, where a specific type had not been established. Paroxysmal AF is recurrent self-terminating AF. Persistent AF is long-lasting AF that requires termination by cardioversion. In permanent AF, patients do not return to sinus rhythm or attempt cardioversion [18]. Time on HD was calculated as the sum of the current HD treatment period and periods of HD treatment before renal transplant in patients where applicable. Inter-dialytic weight gain was calculated as the difference between pre-dialysis weight and target dry body weight. A history of venous thromboembolism only includes non-catheter-associated deep vein thrombosis and pulmonary embolism.
Statistical methods
Descriptive parameters of the study population are given as absolute and relative frequencies or median values with 25th to 75th percentile, where appropriate. In order to compare the distribution of patient parameters between patients with and without AF, the dependent variable, we used the Mann-Whitney U test for ordinal and continuous independent variables or chi-squared test for categorical independent variables, where applicable. The odds ratios (OR) for association between AF and patient characteristics and comorbidities were calculated in univariable and multivariable logistic regression models. A two-tailed p-value below 0.05 was regarded as statistically significant. All calculations were conducted with SPSS (IBM SPSS for Windows, Version 23.0. Armonk, NY) and graphs were drawn with GraphPad Prism (GraphPad Software, Version 5.00 for Windows, San Diego CA).
Results
Study population
A total of 626 patients were recruited into the study, of which 397 (63.4%) were men. The median age was 66 years (25th to 75th percentile 55–75) and the median BMI was 25.7 kg/m2 (22.4–29.6). Diabetic nephropathy was the most frequent cause for ESRD (25.6%) followed by vascular nephropathy (19.3%). Further baseline characteristics of the study cohort are provided in Table 1.
Table 1. Characteristics of the study population at baseline.
| Full cohort | Non-AF cohort | AF cohort | p-value* | |
|---|---|---|---|---|
| Patients, n (%) | 626 (100) | 460 (73.5) | 166 (26.5) | — |
| Male sex (%) | 397 (63.4) | 279 (60.7) | 118 (71.1) | 0.017 |
| Age, median (25th– 75th percentile) | 66 (55–75) | 63.5 (50–73) | 71.5 (64–78) | <0.001 |
| BMI, median (25th– 75th percentile) | 25.7 (22.4–29.6) | 25.6 (22.2–29.4) | 25.9 (22.7–29.7) | 0.734 |
| Caucasian ethnicity (%) | 601 (96) | 435 (94.6) | 166 (100) | n.a. |
| African ethnicity (%) | 12 (1.9) | 12 (2.6) | 0 | n.a. |
| Asian, pacific islander ethnicity (%) | 13 (2.1) | 13 (2.8) | 0 | n.a. |
| Etiology of ESRD, n (%) | ||||
| Diabetic NP | 160 (25.6) | 117 (25.4) | 43 (25.9) | 0.906 |
| Vascular NP | 121 (19.3) | 82 (17.8) | 39 (23.5) | 0.113 |
| Glomerular nephritis | 82 (13.1) | 65 (14.1) | 17 (10.2) | 0.203 |
| Atrophic NP | 57 (9.1) | 41 (8.9) | 16 (9.6) | 0.781 |
| Cystic non-hereditary NP | 36 (5.8) | 26 (5.7) | 10 (6.0) | 0.860 |
| Hereditary NP | 31 (5.0) | 26 (5.7) | 5 (3.0) | 0.179 |
| Nephrectomy | 20 (3.2) | 10 (2.2) | 10 (6.0) | 0.016 |
| Iatrogenic/toxic NP | 28 (4.5) | 19 (4.1) | 9 (5.4) | 0.491 |
| Other causes | 91 (14.5) | 74 (16.1) | 17 (10.2) | 0.067 |
| Dialysis history, n (%) | ||||
| History of renal transplantation | 90 (14.4) | 64 (13.9) | 26 (15.7) | 0.582 |
| Previous peritoneal dialysis | 46 (7.3) | 35 (7.6) | 11 (6.6) | 0.606 |
| Current vascular access | ||||
| AV fistula | 329 (52.6) | 244 (53.0) | 85 (51.2) | 0.684 |
| AV graft | 72 (11.5) | 57 (12.4) | 15 (9.0) | 0.246 |
| Central venous catheter | 221 (35.3) | 157 (34.1) | 64 (38.6) | 0.307 |
| others | 4 (0.7) | 2 (0.4) | 2 (1.2) | 0.286 |
| Dialysis parameters, median (25th– 75th percentile) | ||||
| Remaining diuresis, ml | 500 (0–1000) | 500 (0–1000) | 350 (0–1000) | 0.295 |
| Inter-dialytic weight gain, kg | 2.0 (1.1–3.0) | 2.1 (1.1–3.1) | 1.9 (1.2–2.6) | 0.135 |
| Time on hemodialysis, years | 2.7 (1.0–5.0) | 2.5 (1.0–5.0) | 3.0 (1.1–6.0) | 0.084 |
| Comorbidities, n (%) | ||||
| History of stroke or TIA | 127 (20.3) | 82 (17.8) | 45 (27.1) | 0.011 |
| History of myocardial infarction | 105 (16.8) | 69 (15.0) | 36 (21.7) | 0.048 |
| Coronary heart disease | 233 (37.2) | 150 (32.6) | 83 (50.0) | <0.001 |
| Artificial heart valve | 43 (6.9) | 22 (4.8) | 21 (12.7) | 0.001 |
| Bioprosthetic valve | 31 (5.0) | 16 (3.5) | 15 (9.0) | 0.004 |
| Mechanical valve | 10 (1.6) | 5 (1.1) | 5 (3.0) | 0.071 |
| History of VTE | 61 (9.7) | 36 (7.8) | 25 (15.1) | 0.007 |
| Deep vein thrombosis | 44 (7.0) | 27 (5.9) | 17 (10.2) | 0.075 |
| Pulmonary embolism | 32 (5.1) | 18 (3.9) | 14 (8.4) | 0.037 |
| Peripheral artery disease | 197 (31.5) | 139 (30.2) | 58 (34.9) | 0.261 |
| Diabetes | 237 (37.9) | 168 (36.5) | 69 (41.6) | 0.244 |
| Hypertension | 576 (92.0) | 423 (92.0) | 153 (92.2) | 0.931 |
| Congestive heart failure | 184 (29.4) | 114 (24.8) | 70 (42.2) | <0.001 |
| Cancer history or active | 152 (24.3) | 96 (20.9) | 56 (33.7) | <0.001 |
| Smokers | 306 (48.9) | 230 (50.0) | 76 (45.8) | 0.351 |
| Long-term antithrombotic therapy, n (%) | ||||
| LMWH s.c. on non-HD days | 59 (9.4) | 26 (5.7) | 33 (19.9) | <0.001 |
| 20 mg o.d. | 4 (0.6) | 2 (0.4) | 2 (1.2) | <0.001 |
| 40 mg o.d. | 39 (6.2) | 18 (3.9) | 21 (12.7) | <0.001 |
| 60 mg o.d. | 11 (1.8) | 5 (1.1) | 6 (3.6) | <0.001 |
| 80 mg o.d. | 5 (0.8) | 1 (0.2) | 4 (2.4) | <0.001 |
| Vitamin K antagonist | 77 (12.3) | 27 (5.9) | 50 (30.1) | <0.001 |
| Antiplatelet agent | 345 (55.1) | 254 (55.2) | 91 (54.8) | 0.930 |
Table legend: AF–atrial fibrillation, BMI–body-mass-index, NP–nephropathy, AV–arteriovenous, TIA–transient ischemic attack, VTE–venous thromboembolism, LMWH–low-molecular-weight heparin, o.d.–once daily * Mann-Whitney U test or chi2 p-value for non-AF cohort versus AF cohort.
AF prevalence
A diagnosis of AF was recorded in 166 patients (26.5%). AF patients were predominantly men (71.1%) with a median age of 71.5 years (64–78). In 59.6% of patients with AF, AF developed after commencing HD treatment, while 40.4% had AF before ESRD. AF was present in 93 patients (56%) as paroxysmal AF, in 5 patients (3%) as persistent AF, in 53 patients (32%) as permanent AF, and in 15 patients (9%) as recently diagnosed or unknown type of AF.
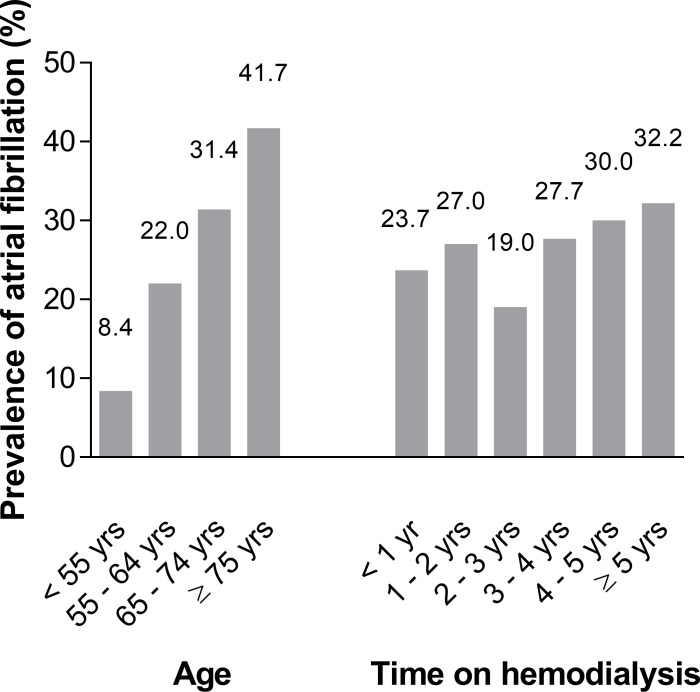
The prevalence of AF rose with increasing age, from 8.4% (14/166) in patients aged younger than 55 years, 22.0% (29/132) in those aged 55–64 years, 31.4% (55/175) in those aged 65–74 years, and 41.7% (68/163) in patients aged ≥ 75 years (Fig 2).
Fig 2. Prevalence of atrial fibrillation in patients with increasing age and time on hemodialysis.
The prevalence of AF was 23.7% (40/169) in patients with ≤ 1 year of time on HD, 27.0% (31/115) with 1 to 2 years of time on HD, 19.0% (16/84) with 2 to 3 years time on HD, 27.7% (18/65) with 3 to 4 years time on HD, 30.0% (15/50) with 4 to 5 years of time on HD and 32.2% (46/143) with more than 5 years time on HD (Fig 2).
The distribution of AF for the baseline parameters sex, age, time on HD, a history of stroke or transient ischemic attack (TIA), coronary heart disease, venous thromboembolism (VTE), artificial heart valves, active or history of cancer, and congestive heart failure had significant trends in the Wilcoxon-Mann-Whitney U test and chi-squared test (Table 1). In univariable logistic regression, these factors were associated with the presence of AF (Table 2).
Table 2. Factors associated with prevalence of atrial fibrillation Model 1 (N = 626).
| Characteristics | Univariable OR (95%CI) | p | Multivariable OR (95% CI) | p |
|---|---|---|---|---|
| Male sex | 1.60 (1.09–2.34) | 0.017 | 1.73 (1.13–2.63) | 0.011 |
| Age, per year | 1.05 (1.04–1.07) | <0.001 | 1.05 (1.03–1.07) | <0.001 |
| Time on HD, per year | 1.02 (0.98–1.06) | 0.370 | 1.03 (0.99–1.08) | 0.135 |
| Stroke/TIA | 1.71 (1.13–2.60) | 0.011 | 1.36 (0.86–2.13) | 0.188 |
| Coronary heart disease | 2.07 (1.44–2.97) | <0.001 | 1.41 (0.95–2.10) | 0.091 |
| Artificial heart valve | 2.88 (1.54–5.40) | 0.001 | 1.80 (0.91–3.56) | 0.094 |
| VTE | 2.09 (1.21–3.60) | 0.008 | 1.99 (1.11–3.59) | 0.022 |
| Congestive heart failure | 2.21 (1.52–3.22) | <0.001 | 1.66 (1.10–2.51) | 0.015 |
| Cancer history/active | 1.93 (1.30–2.86) | 0.001 | 1.55 (1.02–2.36) | 0.042 |
In multivariable logistic regression with covariates identified as potentially associated with AF prevalence, male sex, increased age, history of VTE, cancer, and congestive heart failure were identified as independently associated with increased risk of AF diagnosis (Table 2). After excluding patients with AF diagnosed chronologically before ESRD for a second model of 559 patients, increased time on HD was associated with the presence of AF (Table 3).
Table 3. Model 2 of factors associated with atrial fibrillation, excluding patients with a diagnosis of atrial fibrillation before occurrence of ESRD (N = 559).
| Characteristics | Univariable OR (95%CI) | p | Multivariable OR (95%CI) | p |
|---|---|---|---|---|
| Male sex | 1.92 (1.18–3.14) | 0.009 | 2.18 (1.28–3.70) | 0.004 |
| Age, per year | 1.05 (1.03–1.06) | <0.001 | 1.05 (1.03–1.07) | <0.001 |
| Time on HD, per year | 1.06 (1.02–1.11) | 0.003 | 1.08 (1.03–1.13) | 0.001 |
| Stroke/TIA | 1.56 (0.93–2.60) | 0.090 | 1.14 (0.66–1.98) | 0.631 |
| Coronary heart disease | 2.03 (1.31–3.14) | 0.002 | 1.41 (0.87–2.27) | 0.160 |
| Artificial heart valve | 2.24 (1.02–4.89) | 0.043 | 1.44 (0.62–3.34) | 0.400 |
| VTE | 1.94 (1.00–3.75) | 0.049 | 1.89 (0.93–3.85) | 0.081 |
| Congestive heart failure | 1.59 (0.99–2.53) | 0.052 | 1.24 (0.75–2.03) | 0.403 |
| Cancer history/active | 1.65 (1.02–2.68) | 0.043 | 1.21 (0.72–2.03) | 0.481 |
Risk evaluation for stroke and bleeding in AF patients resulted in a median CHA2DS2-Vasc Score of 4 (25th to 75th percentile 3–5) and a median HAS-BLED Score of 4 (3–4). A strong indication for anticoagulation was given in 159 patients (95.8% of AF patients) who had a CHA2DS2-Vasc ≥2.
Anti-thrombotic therapy
The frequencies of antithrombotic medications in mono- and combination therapy are given in Table 4.
Table 4. Practice patterns of antithrombotics in patients on HD.
| Antithrombotic therapy | Full cohort, count (% of N = 626) | AF cohort, count (% of N = 166) |
|---|---|---|
| Vitamin-K-antagonist | 77 (12.3) | 50 (30.1) |
| Monotherapy | 51 (8.1) | 35 (21.1) |
| Combination with antiplatelet agent | 26 (4.2) | 15 (9.0) |
| Triple therapy (VKA + clopidogrel + aspirin) | 3 (0.5) | 0 |
| Low-molecular-weight heparin | 59 (9.4) | 33 (19.9) |
| Monotherapy | 27 (4.3) | 14 (8.4) |
| Combination with antiplatelet agent | 32 (5.1) | 19 (11.4) |
| Triple therapy (LMWH + clopidogrel + aspirin) | 6 (1.0) | 4 (2.4) |
| Antiplatelet | 345 (55.1) | 91 (54.8) |
| Monotherapy | 286 (45.7) | 56 (33.7) |
| Dual antiplatelet therapy | 50 (8.0) | 11 (6.7) |
| No antithrombotic | 203 (32.4) | 26 (15.6) |
For the dual pathway therapy in the full cohort, LMWH use was preferred over VKA (5.1% versus 4.2%, p = 0.013). The new generation P2Y12 inhibitors, ticagrelor and prasugrel, were not used in dual pathway antithrombotic therapy and direct oral anticoagulants were not used in any patient.
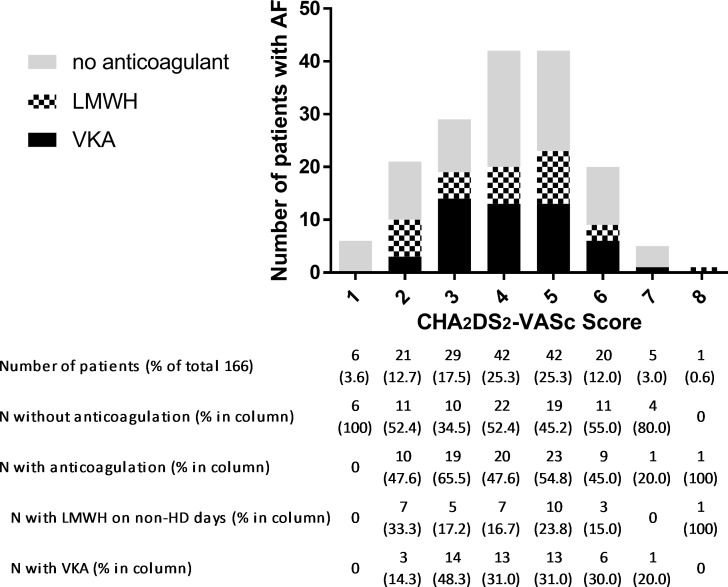
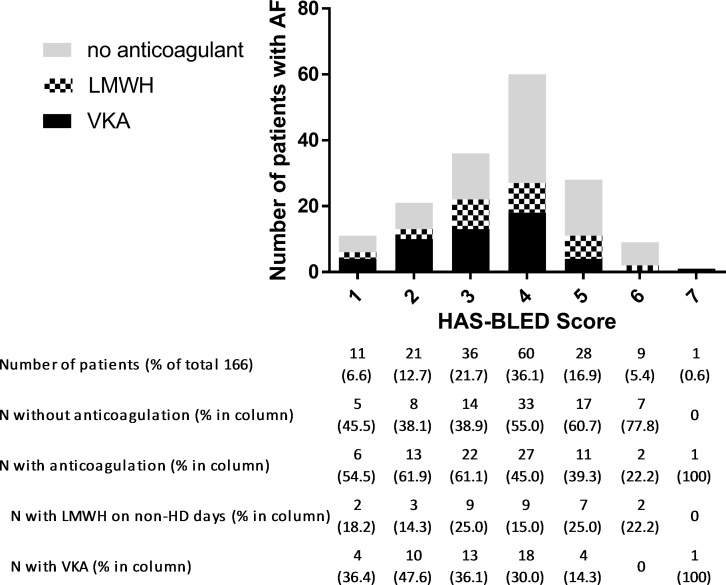
In the AF group, 84 patients (50.6%) received anticoagulation treatment. 50 patients (30.1%) were orally anticoagulated with VKA, 33 patients (19.9%) received LMWH on non-HD days for long-term stroke prevention. One patient (0.6%) received fondaparinux in the same indication. Fifty-six patients (33.7%) received antiplatelet agents instead of anticoagulants for stroke prevention. Twenty-six AF patients (15.6%) received no antithrombotic therapy at all, except the anticoagulation during HD sessions. There was no association of CHA2DS2-VASc scores with use or non-use of anticoagulation therapy (p = 0.938) (Fig 3). Patients with higher HAS-BLED scores were less likely to receive anticoagulation therapy (p = 0.019) (Fig 4).
Fig 3. Distribution of patients with AF across CHA2DS2-VASc Scores and frequency of corresponding anticoagulation treatment.
Fig 4. Distribution of patients with AF across HAS-BLED Scores and frequency of corresponding anticoagulation treatment.
Discussion
Patients with ESRD are at approximately 6-fold increased risk of stroke and systemic embolism compared to the general population [19–21]. One of the main culprits suspected of increasing the risk of stroke in ESRD patients is AF. In the cross-sectional evaluation of our population-based cohort study of 626 HD patients in Vienna, we found a 26.5% prevalence of AF.
Previous studies have determined the prevalence of AF in HD patients between 3.8 and 27% [9–13]. Studies with retrospective design or based on large insurance claims databases estimated a prevalence between 10 and 15% [13,14,22], while studies with prospective, patient-level data tended to find a higher prevalence of AF [11,12]. Prevalence rates derived from patient-level cohort studies can be suspected of selection bias, especially if a large number of centers contributes only few patients each, potentially over-representing AF patients. Nevertheless, from our findings we have to assume that the prevalence of AF is underestimated in large population-level cohorts. The data gathered in our investigation is entirely constituted of patient-level data, derived from personal patient interviews, structured review of the detailed medical records at the dialysis centers, and objective verification of AF based on standardized criteria. According to estimates from the Austrian Registry of Dialysis and Transplantation yearly report of 2013 [23], the cohort in our study covers ~ 73% of the population of HD patients in the city of Vienna (population 1.7 million).
In the general population and HD patients alike, AF is a disease of the elderly and is more frequent in men than in women [1,10,14]. In our cohort, the prevalence AF in patients 75 years of age and older reached over 40% and male sex was independently associated with 1.7-fold increased odds of AF diagnosis.
In search of other characteristics that may be associated with AF development in HD patients, we investigated the time on HD expressed as in HD age years. The risk of an AF diagnosis increased by 8% for every year a patient spent on HD treatment. We were further able to show that congestive heart failure was associated with AF diagnosis. Further cardiovascular conditions, which are generally considered risk factors for the development of AF, such as coronary heart disease, hypertension or valvular diseases, were not associated with AF. This may prove difficult in an HD cohort enriched with higher frequencies of comorbidities than the general population. It may be a specific characteristic of HD cohorts that recognized factors associated with AF in the general population may not apply. Surprisingly however, the risk of an AF diagnosis was independently increased in patients with a history of VTE. We ruled out a confounding relationship of catheter-associated or shunt-related thrombosis by only including non-catheter DVT and PE in the analysis. The interpretation of this novel relationship between a history of VTE and AF in HD patients will certainly require prospective analysis. The prevalence of AF was further associated with presence or history of malignancy. A causal connection through cardiotoxic cancer-therapy would have to be examined in a randomized trial setting. Prospective observational data could reveal if shared risk factors lead to confounding of the relationship between cancer and AF.
The high risk of stroke and bleeding in ESRD patients on HD and a complex risk profile including many risk factors for thromboembolic complications warrants a specific risk evaluation for AF patients on HD. The CHADS2, CHA2DS2-VASc, and HAS-BLED Scores, predict the risk of stroke and bleeding in the general population based on epidemiologic distribution of risk factors [24–26]. These risk evaluation approaches also have validity in HD patients [27–29], but because of the higher frequencies of risk factors amongst HD patients, these scores may lose the ability to distinguish between truly high and low risk patients. On the CHA2DS2-VASc scale 95.8% of the patients with AF in this cohort reached indication for continuous anticoagulation. Strict adherence to these generally established risk scores may lead to more aggressive anticoagulation treatment in HD patients. More recently, evidence from a Taiwanese nationwide cohort indicated that the risk of stroke in HD patients with AF may be overestimated [3] and that ethnicity may be a risk modifying factor. Cohorts of HD patients with AF are further never untreated cohorts, as the majority of patients receive LMWH during HD sessions to prevent clotting in the dialysis tubes.
There is currently no consensus on the appropriate use of antithrombotic agents for stroke prevention in HD patients. This is because patients on HD were excluded from trials on stroke prevention and not all results from non-randomized clinical studies show the same benefit of anticoagulation treatment for AF patients [30–34]. The treatment practices in our cohort showed 30.1% of AF patients receiving oral anticoagulant with VKA and 19.9% receiving LMWH on non-HD days for stroke prevention. Treatment practices from different cohorts had 25 to 58% use of VKA in AF patients, indicating that the prescription is very dependent on local preferences [8,28,34–38]. Due to renal elimination, LMWHs are essentially contraindicated in patients with a creatinine clearance < 30ml/min [39]. However, in the absence of evidence for a clear benefit of VKA in stroke prevention for AF, LMWH in prophylactic dose given on non-HD days may be an option in some cases of patients with very high risk of stroke. Despite lack of evidence for LMWH treatment as long-term stroke prevention in AF, almost 20% of AF patients in our cohort received this treatment. Over 50% of both AF and non-AF patients in this cohort received antiplatelet agents and treating physicians chose a cautious approach with LMWH over VKA in patients with indication for dual pathway antithrombotic therapy and tended to decide against anticoagulation in patients with higher HAS-BLED scores.
The present study has limitations in need of acknowledgment. The recruited cohort has a relatively small sample size, compared to national or insurance-claims registry studies and may thus be biased for the true prevalence of AF and associated medical conditions. However, this study was designed to capture an entire regional population of HD patients. We performed a cross-sectional analysis of 626 HD patients from the seven major HD centers in the city of Vienna and avoided a selection bias by recruiting a representative sample of approximately 73% of the regional HD population. The study design further ensured that the AF diagnosis derived from patient-level data, according to reproducible criteria for diagnosis. Asymptomatic, paroxysmal AF may, however, have been underdiagnosed. Since the HD centers are all within the city limits of Vienna, our cohort may represent a largely urban population and may therefore differ from more rural areas. Previous studies on Austrian populations of HD patients had similar demographic characteristics but lower prevalence rates of AF and lower use of VKA anticoagulants [22,36]. The risk of stroke and mortality associated with AF may lead to a decreased prevalence of AF in patients with long time on HD, otherwise known as healthy user bias. We cannot exclude that this form of selection bias may have led to further underestimation of the AF prevalence in our current analysis.
In conclusion, we confirmed that the prevalence of AF is high amongst ESRD patients on chronic hemodialysis. Prevalence data from retrospective and registry cohorts may underestimate the burden of AF in ESRD patients. The use of antithrombotic agents for stroke prevention in AF in our cohort indicates a lack of consensus for ideal antithrombotic therapy and therapy-guiding risk evaluation for thromboembolic complications and bleeding in HD patients may require more HD-specific risk factors.
Acknowledgments
The authors would like to acknowledge all clinical collaborators who assisted in patient recruitment at the dialysis centers.
Data Availability
Austrian law prohibits public availability of health-related personal data. Data are available upon reasonable request, submitted to Silvia Koder (silvia.koder@meduniwien.ac.at).
Funding Statement
Support was provided by Österreichische Nationalbank Jubiläumsfond (Austrian Nationbank Anniversary fund) grant number 16433 awarded to IP [https://www.oenb.at/Ueber-Uns/Forschungsfoerderung/Jubilaeumsfonds.html] and Österreichische Gesellschaft für Innere Medizin (Austrian Society for Internal Medicine) Joseph-Skoda-Prize 2014 awarded to CA [http://www.oegim.at/aktuell/preise/josef-skoda-projektfoerderungspreis.html]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Go A, Hylek E, Phillips K. Prevalence of diagnosed atrial fibrillation in adults. JAMA. 2001;285: 2370–2375. [DOI] [PubMed] [Google Scholar]
- 2.Schnabel RB, Yin X, Gona P, Larson MG, Beiser AS, McManus DD, et al. 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham Heart Study: a cohort study. Lancet. 2015;386: 154–162. 10.1016/S0140-6736(14)61774-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shih C-J, Ou S-M, Chao P-W, Kuo S-C, Lee Y-J, Yang C-Y, et al. Risks of Death and Stroke in Patients Undergoing Hemodialysis With New-Onset Atrial Fibrillation: A Competing-Risk Analysis of a Nationwide Cohort. Circulation. 2016;133: 265–272. 10.1161/CIRCULATIONAHA.115.018294 [DOI] [PubMed] [Google Scholar]
- 4.Genovesi S, Vincenti A, Rossi E, Pogliani D, Acquistapace I, Stella A, et al. Atrial fibrillation and morbidity and mortality in a cohort of long-term hemodialysis patients. Am J Kidney Dis. 2008;51: 255–262. 10.1053/j.ajkd.2007.10.034 [DOI] [PubMed] [Google Scholar]
- 5.Molnar AO, Bota SE, Garg AX, Harel Z, Lam N, McArthur E, et al. The Risk of Major Hemorrhage with CKD. J Am Soc Nephrol. 2016;27: ePub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hart RG, Halperin JL. Atrial Fibrillation and Thromboembolism: A Decade of Progress in Stroke Prevention. Ann Intern Med. 1999;131: 688–695. [DOI] [PubMed] [Google Scholar]
- 7.January CT, Wann LS, Alpert JS, Calkins H, Cleveland JC, Cigarroa JE, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: A report of the American college of cardiology/American heart association task force on practice guidelines and the heart rhythm society. Circulation. 2014;130: e199–e267. 10.1161/CIR.0000000000000041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wizemann V, Tong L, Satayathum S, Disney A, Akiba T, Fissell RB, et al. Atrial fibrillation in hemodialysis patients: clinical features and associations with anticoagulant therapy. Kidney Int. 2010;77: 1098–106. 10.1038/ki.2009.477 [DOI] [PubMed] [Google Scholar]
- 9.Ohsawa M, Tanno K, Okamura T, Yonekura Y, Kato K, Fujishima Y, et al. Standardized Prevalence Ratios for Atrial Fibrillation in Adult Dialysis Patients in Japan. J Epidemiol. 2016;26: 272–6. 10.2188/jea.JE20150077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Atar I, Konaş D, Açikel S, Külah E, Atar A, Bozbaş H, et al. Frequency of atrial fibrillation and factors related to its development in dialysis patients. Int J Cardiol. 2006;106: 47–51. 10.1016/j.ijcard.2004.12.048 [DOI] [PubMed] [Google Scholar]
- 11.Genovesi S, Pogliani D, Faini A, Valsecchi MG, Riva A, Stefani F, et al. Prevalence of atrial fibrillation and associated factors in a population of long-term hemodialysis patients. Am J Kidney Dis. 2005;46: 897–902. 10.1053/j.ajkd.2005.07.044 [DOI] [PubMed] [Google Scholar]
- 12.Vazquez E, Sanchez-Perales C, Garcia-Garcia F, Castellano P, Garcia-Cortes M-J, Liebana A, et al. Atrial fibrillation in incident dialysis patients. Kidney Int. 2009;76: 324–30. 10.1038/ki.2009.185 [DOI] [PubMed] [Google Scholar]
- 13.Wetmore JB, Ellerbeck EF, Mahnken JD, Phadnis MA, Rigler SK, Spertus JA, et al. Stroke and the “Stroke Belt” in Dialysis: Contribution of Patient Characteristics to Ischemic Stroke Rate and Its Geographic Variation. J Am Soc Nephrol. 2013;24: 2053–2061. 10.1681/ASN.2012111077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Winkelmayer WC, Patrick AR, Liu J, Brookhart MA, Setoguchi S. The increasing prevalence of atrial fibrillation among hemodialysis patients. J Am Soc Nephrol. 2011;22: 349–57. 10.1681/ASN.2010050459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buiten MS, de Bie MK, Rotmans JI, Gabreëls BA, van Dorp W, Wolterbeek R, et al. The dialysis procedure as a trigger for atrial fibrillation: new insights in the development of atrial fibrillation in dialysis patients. Heart. 2014;100: 685–90. 10.1136/heartjnl-2013-305417 [DOI] [PubMed] [Google Scholar]
- 16.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research Electronic Data Capture (REDCap)—A metadata driven methodology and workflow process for providing translational research informatict support. J Biomed Inform. 2009;42: 377–81. 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stel VS, van Dijk PCW, van Manen JG, Dekker FW, Ansell D, Conte F, et al. Prevalence of co-morbidity in different European RRT populations and its effect on access to renal transplantation. Nephrol Dial Transplant. 2005;20: 2803–2811. 10.1093/ndt/gfi099 [DOI] [PubMed] [Google Scholar]
- 18.Camm a J, Kirchhof P, Lip GYH, Schotten U, Savelieva I, Ernst S, et al. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J. 2010;31: 2369–429. 10.1093/eurheartj/ehq278 [DOI] [PubMed] [Google Scholar]
- 19.Seliger SL, Gillen DL, Longstreth WT, Kestenbaum B, Stehman-Breen CO. Elevated risk of stroke among patients with end-stage renal disease. Kidney Int. 2003;64: 603–609. 10.1046/j.1523-1755.2003.00101.x [DOI] [PubMed] [Google Scholar]
- 20.Wang HH, Hung SY, Sung JM, Hung KY, Wang JD. Risk of stroke in long-term dialysis patients compared with the general population. Am J Kidney Dis. 2014;63: 604–611. 10.1053/j.ajkd.2013.10.013 [DOI] [PubMed] [Google Scholar]
- 21.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu C. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351: 1296–305. 10.1056/NEJMoa041031 [DOI] [PubMed] [Google Scholar]
- 22.Wiesholzer M, Harm F, Tomasec G, Barbieri G, Putz D, Balcke P. Incidence of stroke among chronic hemodialysis patients with nonrheumatic atrial fibrillation. Am J Nephrol. 2001;21: 35–9. [DOI] [PubMed] [Google Scholar]
- 23.Kramar R. Österreichisches Dialyse- und Transplantationsregister. In: Jahresbericht 2013 [Internet]. 2015 [cited 8 Mar 2016]. Available: www.nephro.at/oedr2013/oedr2013.htm
- 24.Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of Clinical Classification Schemes Results From the National Registry of Atrial Fibrillation. 2015;285: 2864–2870. [DOI] [PubMed] [Google Scholar]
- 25.Lip GYH, Nieuwlaat R, Pisters R, Lane DA, Crijns HJGM. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: The Euro Heart Survey on atrial fibrillation. Chest. 2010;137: 263–272. 10.1378/chest.09-1584 [DOI] [PubMed] [Google Scholar]
- 26.Pisters R, Lane DA, Nieuwlaat R, De Vos CB, Crijns HJGM, Lip GYH. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: The euro heart survey. Chest. 2010;138: 1093–1100. 10.1378/chest.10-0134 [DOI] [PubMed] [Google Scholar]
- 27.Chao T-F, Liu C-J, Wang K-L, Lin Y-J, Chang S-L, Lo L-W, et al. Incidence and prediction of ischemic stroke among atrial fibrillation patients with end-stage renal disease requiring dialysis. Heart Rhythm. 2014;11: 1752–9. 10.1016/j.hrthm.2014.06.021 [DOI] [PubMed] [Google Scholar]
- 28.Friberg L, Benson L, Lip GYH. Balancing stroke and bleeding risks in patients with atrial fibrillation and renal failure: the Swedish Atrial Fibrillation Cohort study. Eur Heart J. 2015;36: 297–306. 10.1093/eurheartj/ehu139 [DOI] [PubMed] [Google Scholar]
- 29.Yang H, Chen Y-H, Hsieh T-F, Chuang S-Y, Wu M-J. Prediction of Mortality in Incident Hemodialysis Patients: A Validation and Comparison of CHADS2, CHA2DS2, and CCI Scores. PLoS One. 2016;11: e0154627 10.1371/journal.pone.0154627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Winkelmayer WC, Liu J, Setoguchi S, Choudhry NK. Effectiveness and safety of warfarin initiation in older hemodialysis patients with incident atrial fibrillation. Clin J Am Soc Nephrol. 2011;6: 2662–2668. 10.2215/CJN.04550511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shen JI, Montez-Rath ME, Lenihan CR, Turakhia MP, Chang TI, Winkelmayer WC. Outcomes after warfarin initiation in a cohort of hemodialysis patients with newly diagnosed atrial fibrillation. Am J Kidney Dis. 2015;66: 677–688. 10.1053/j.ajkd.2015.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shah M, Avgil Tsadok M, Jackevicius CA, Essebag V, Eisenberg MJ, Rahme E, et al. Warfarin use and the risk for stroke and bleeding in patients with atrial fibrillation undergoing dialysis. Circulation. 2014;129: 1196–203. 10.1161/CIRCULATIONAHA.113.004777 [DOI] [PubMed] [Google Scholar]
- 33.Carrero JJ, Evans M, Szummer K, Spaak J, Lindhagen L, Edfors R, et al. Warfarin, kidney dysfunction, and outcomes following acute myocardial infarction in patients with atrial fibrillation. JAMA. 2014;311: 919–28. 10.1001/jama.2014.1334 [DOI] [PubMed] [Google Scholar]
- 34.Chan KE, Lazarus JM, Thadhani R, Hakim RM. Warfarin use associates with increased risk for stroke in hemodialysis patients with atrial fibrillation. J Am Soc Nephrol. 2009;20: 2223–2233. 10.1681/ASN.2009030319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Olesen JB, Lip GYH, Kamper A-L, Hommel K, Køber L, Lane DA, et al. Stroke and bleeding in atrial fibrillation with chronic kidney disease. N Engl J Med. 2012;367: 625–35. 10.1056/NEJMoa1105594 [DOI] [PubMed] [Google Scholar]
- 36.Knoll F, Sturm G, Lamina C, Zitt E, Lins F, Freistätter O, et al. Coumarins and survival in incident dialysis patients. Nephrol Dial Transplant. 2012;27: 332–7. 10.1093/ndt/gfr341 [DOI] [PubMed] [Google Scholar]
- 37.Genovesi S, Rossi E, Gallieni M, Stella A, Badiali F, Conte F, et al. Warfarin use, mortality, bleeding and stroke in haemodialysis patients with atrial fibrillation. Nephrol Dial Transplant. 2015; 491–498. 10.1093/ndt/gfu334 [DOI] [PubMed] [Google Scholar]
- 38.Wetmore JB, Mahnken JD, Mukhopadhyay P, Hou Q, Ellerbeck EF, Rigler SK, et al. Medication Usage in Dialysis Patients. Am J Kidney Dis. 2011;58: 73–83. 10.1053/j.ajkd.2011.02.387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nagge J, Crowther M, Hirsh J. Is impaired renal function a contraindication to the use of low-molecular-weight heparin? Arch Intern Med. 2002;162: 2605–2609. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Austrian law prohibits public availability of health-related personal data. Data are available upon reasonable request, submitted to Silvia Koder (silvia.koder@meduniwien.ac.at).