ABSTRACT
There is now growing evidence that the immune contexture influences cancer progression and clinical outcome of patients with non-small cell lung cancer (NSCLC). If chemotherapy is widely used to treat patients with advanced-stage NSCLC, it remains unclear how it could modify the immune contexture and impact its prognostic value. Here, we analyzed two retrospective cohorts, respectively composed of 122 stage III-N2 NSCLC patients treated with chemotherapy before surgery and 39 stage-matched patients treated by surgery only. In patients treated with neoadjuvant chemotherapy, the histological characteristics, the expression of PD-L1 protein, and the tumor immune microenvironment (CD8+ T cells, DC-LAMP+ mature dendritic cells, and CD68+ macrophages) were evaluated and their prognostic value assessed together with standard clinical parameters. By analyzing pre- and post-treatment specimens, we did not find any changes in the PD-L1 expression. We also found that the tumor immune contexture in patients treated with neoadjuvant chemotherapy exhibited a similar pattern that the one found in chemotherapy-naive patients, with comparable densities of tumor-infiltrating CD8+ and DC-LAMP+ cells and a similar spatial organization. The percentage of residual viable tumor cells and the immune pattern (CD8+ and DC-LAMP+ cell densities) were significantly associated with the clinical outcome and allowed the identification of short- and long-term survivors, respectively. In multivariate analysis, the immune pattern was found to be the strongest independent prognostic factor. In conclusion, this study decrypts the complex interplay between cancer and immune cells in patients undergoing chemotherapy and supports potential beneficial synergistic effect of immunotherapy and chemotherapy.
KEYWORDS: Histological response, immune contexture, neoadjuvant chemotherapy, non-small cell lung cancer, survival
Abbreviations
- AUC
area under the curve
- CIS
cisplatin
- DC
dendritic cell
- DFS
disease-free survival
- DSS
disease-specific survival
- GC
gemcitabine
- IQR
interquartile range
- NSCLC
non-small cell lung cancer
- OS
overall survival
- PAC
paclitaxel
- TLS
tertiary lymphoid structure
- VIN
vinorelbine
Introduction
Locally advanced NSCLC are treated with surgery, radiotherapy, and/or adjuvant or neoadjuvant chemotherapy.1,2 In patients treated with neoadjuvant chemotherapy, the clinical response to treatment allows the identification of patients who will likely benefit from surgery. After resection, histological characteristics such as pathological downstaging as well as low percentage of viable tumor cells were found to be associated with prolonged survival.3,4 Unfortunately, these criteria are not fully reliable and new prognostic markers are needed to identify patients with high risk of relapse and help clinicians to define the most appropriate therapeutic strategy.
In primary and metastatic tumors, the immune contexture has been associated with a strong prognostic value5-7 and we previously showed that high densities of tumor-infiltrating CD8+ T cells were associated with improved overall survival in stage I–III NSCLC treated by surgery.8,9 Moreover, the concomitant presence of mature dendritic cells (DCs), reflecting the presence of tertiary lymphoid structures (TLS) within tumor tissues, identified group of patients with the best outcome.10 Patients with advanced stages of the disease are mainly treated with neoadjuvant chemotherapy and the peculiar tumor microenvironment after such treatment remains poorly characterized. If chemotherapy drugs have been designed to induce tumor cell death,11 they also can boost or hamper protective immune responses by modifying the composition and function of the tumor immune contexture.11-14 The aim of the present study was to characterize the tumor and immune compartments of NSCLC patients treated with neoadjuvant chemotherapy and evaluate their association with clinical outcome.
At first, we analyzed and compared two cohorts of NSCLC patients treated or not with neoadjuvant chemotherapy to decipher the impact of such treatment on the characteristics of the tumor and its immune contexture. Our results validated previously published studies showing a prognostic value of the clinical and histological responses after chemotherapy.3,4 Furthermore, we discovered that patients treated with chemotherapy exhibited a similar intra-tumor immune reaction, in terms of densities and organization, than the one found in untreated patients. PD-L1 expression by tumor and immune cells was not modified by chemotherapy treatment either. Finally, the immune pattern was found to be the strongest independent prognostic factor in patients that have been treated with neoadjuvant chemotherapy.
Materials and methods
Patients
We retrospectively analyzed 122 stage III-N2 NSCLC patients treated with platinum-based neoadjuvant chemotherapy, followed by lung resection and full nodal dissection at Hôtel-Dieu hospital, Paris. Surgery was performed after chemotherapy in patients with objective response or stable disease.15 Overall, tumor and lymph node objective clinical responses to neoadjuvant chemotherapy were assessed by CT-scan. The main clinical features of the patients are listed in Table 1. Among those 122 patients, we had access to 21 pairs of lymph nodes collected before and after neoadjuvant chemotherapy. We also analyzed 39 stage III-N2 NSCLC patients undergoing primary surgery at Hôtel-Dieu hospital but not treated with neoadjuvant chemotherapy for the following reasons: histological proof of N2 disease only at pathological examination of the surgically resected specimens (clinical N0-N1 disease), presence of co-morbidities not compatibles with chemotherapy or informed refusal (Table S1). All experiments were performed with the agreement of the Ile-de-France II ethics committee (CPP#2008–133 and #2012–06–12).
Table 1.
Baseline characteristics of the 122 patients with stage III NSCLC treated with neoadjuvant chemotherapy and univariate analysis of factors possibly affecting the survival.
| OS |
|||||
|---|---|---|---|---|---|
| Characteristic | Number (%) | DSSp-value* | DFSp- value* | p- value* | 5 y (%) |
| Sex | |||||
| Male | 99 (81%) | 0.38 | 0.30 | 0.27 | 27.9 |
| Female | 23 (19%) | 42.4 | |||
| Age (years) | 1 | 0.64 | 0.26 | ||
| Median [IQR] | 58 [51.8–66.0] | ||||
| ≤58 | 30.9 | ||||
| >58 | 32.0 | ||||
| Smoking history | 0.65 | 0.70 | 0.54 | ||
| No | 17 (14%) | 29.6 | |||
| Yes | 105 (86%) | 32.3 | |||
| Median pack/year [IQR] | 40 [30–60] | ||||
| Symptoms | 0.80 | 0.60 | 0.29 | ||
| Yes | 84 (69%) | 28.4 | |||
| No | 38 (31%) | 27.9 | |||
| FEV1 (%) | 0.26 | 0.27 | 0.17 | ||
| Median [IQR] | 83 [71.2–96.7] | ||||
| ≤83 | 64 (52%) | 22.2 | |||
| >83 | 58 (48%) | 40.8 | |||
| Performance status | 0.37 | 0.79 | 0.62 | ||
| 0–1 | 92 (75%) | 31.58 | |||
| 2–3 | 30 (25%) | 29.18 | |||
| Comorbidity | 0.72 | 0.89 | 0.99 | ||
| No | 22 (18%) | 27.8 | |||
| Yes | 100 (82%) | 32.2 | |||
| Moderate | 79 | ||||
| Severe | 21 | ||||
| Side of the tumor | 0.57 | 0.64 | 0.56 | ||
| Left | 40 (33%) | 36.4 | |||
| Right | 82 (67%) | 28.2 | |||
| Size of the tumor (mm; imaging evaluation) | 0.26 | 0.37 | 0.21 | ||
| Median [IQR] | 50 [40–60] | 15.8 | |||
| ≤50 | 61 (50%) | 37.8 | |||
| >50 | 61 (50%) | ||||
| TNM stage before neoadjuvant chemotherapy§ | 0.61 | 0.51 | 0.86 | ||
| IIIA | 111 (91%) | 32 | |||
| IIIB | 11 (9%) | 20.2 | |||
| Drug combination (neoadjuvant chemotherapy) | 0.84 | 0.79 | 0.684 | ||
| Cisplatin+gemcitabine | 56 (46%) | 31 | |||
| Cisplatin+vinorelbine | 42 (34%) | 27 | |||
| Cisplatin+paclitaxel | 17 (14%) | 55.4 | |||
| Platinum salt+other | 7 (6%) | 34.3 | |||
| Number of cycles | 0.12 | 0.06 | 0.004 | ||
| ≤2 | 53 (44%) | 19.5 | |||
| >2 | 69 (56%) | 41.1 | |||
| Neoadjuvant radiotherapy | NA | NA | NA | ||
| Yes | 3 (2%) | NR | |||
| No | 119 (98%) | 31.3% | |||
| Clinical-specific response on the tumor | 0.00031 | 0.000098 | 0.00051 | ||
| Stable disease | 45 (37%) | 13.5 | |||
| Objective response | 77 (63%) | 39.9 | |||
| Clinical-specific response on the lymph node | 0.000033 | 0.0018 | 0.0009 | ||
| Stable disease | 44 (36%) | 14.8 | |||
| Objective response | 78 (64%) | 39.7 | |||
| Overall clinical response (T+N) | 0.00025 | 0.0032 | 0.00059 | ||
| Yes | 88 (72%) | 38.4 | |||
| No | 34 (28%) | 11.3 | |||
| Intervention | 0.051 | 0.26 | 0.025 | ||
| Lobectomy | 54 (44%) | 42.7 | |||
| Bilobectomy | 12 (10%) | NR | |||
| Pneumonectomy | 56 (46%) | 23.5 | |||
| Resection quality | 0.15 | 0.072 | 0.062 | ||
| R0 | 114 (93%) | 32.2 | |||
| R1–2 | 8 (7%) | NR | |||
| Histological type | 0.12 | 0.027 | 0.047 | ||
| Adenocarcinoma | 51 (42%) | 52.2 | |||
| Squamous cell carcinoma | 52 (43%) | 24.5 | |||
| Large cell carcinoma | 19 (15%) | 14.7 | |||
| Pathological downstaging (lymph nodes) | 0.0052 | 0.019 | 0.0041 | ||
| Yes (pN0–N1) | 51 (42%) | 41.4 | |||
| No (pN2) | 71 (58%) | 23.6 | |||
| Pathological downstaging (global) | 0.020 | 0.042 | 0.0068 | ||
| Yes | 42 (34%) | 37.5 | |||
| No | 80 (66%) | 27 | |||
| ypT | 30(24.5%) | 0.66 | 0.042 | 0.012 | 51.8 |
| T0–T1 | |||||
| T2–T4 | 92 (75.5%) | 25.7 | |||
| Pathological stage# | 0.0047 | 0.0098 | 0.016 | ||
| 0–I | 25 (21%) | 55.8 | |||
| II–IV | 97 (79%) | 25.7 | |||
| Percentage of viable cancer cells | 0.025 | 0.084 | 0.013 | ||
| 0–10% | 27 (22%) | 51.4 | |||
| >10% | 95 (78%) | 27.5 | |||
The log-rank test was used. To be able to conduct univariate analyses with a categorical variable, they were coded before analysis:
TNM stage before neoadjuvant chemotherapy: IIIA (T1N2 = 9; T2N2 = 84; T3N2 = 18); IIIB (T4N2 = 11).
TNM stage after neoadjuvant chemotherapy: 0 (T0N0 = 8); I (T1N0 = 11; T2N0 = 6); II (T0N1 = 1; T1N1 = 2; T2N1 = 3; T3N0 = 8); IIIA (T0N2 = 2; T1N2 = 6; T2N2 = 32; T3N1 = 7; T3N2 = 20); IIIB (T4N0 = 1; T4N1 = 10; T4N2 = 3); IV (T2N1M1 = 1; T3N2M1 = 1).
IQR, interquartile range; NA, not applicable; NR, not reached.Bold: Significant p values.
Histological analyses of NSCLC samples
All eosin-hematoxylin-saffron (HES) slides containing tumor area were blindly reviewed for semi-quantitative evaluation of the percentage of viable tumor cells, necrosis, and stromal fibrosis (D.D., A.L., and R.R.). The histological response was based on the percentage of viable tumor cells and the following groups were defined: complete response (0% viable tumor cells), major response (1% to 10% viable tumor cells), and partial or no response (>10% viable tumor cells).16 N stage decrease (N2 to N1 or N0) was considered as pathological nodal downstaging.
Immunohistochemistry
Serial 5-μm tissue sections were deparaffinized, rehydrated, and pretreated for antigen retrieval. The sections were incubated with 3% hydrogen peroxide and then 5% human serum before adding the appropriate primary antibodies followed by secondary antibodies. Antibodies used are listed in Table S2. Enzymatic activity was revealed using 3,3′-diaminobenzidine (DAB, Dako), 3-amino-9-ethylcarbazole (AEC, Vector Laboratories) and blue alkaline phosphatase substrate kit (Vector Laboratories) as previously described.6,8 For single staining, sections were counterstained with hematoxylin. DC-LAMP positive DCs and CD8 positive cells were counted by three observers (R.R., A.L., and J.G.) and CD68+ macrophages were quantified using CaloPix (Tribvn) as previously described.10 The percentage of PD-L1 positive cells was evaluated semi-quantitatively on tumor cells by three independent observers (D.D., A.L., and J.B.). PD-L1 expression on lymphoid cells was also assessed and quantified semi-quantitatively.
Statistical analyses
Overall survival (OS), disease-free survival (DFS), and disease-specific survival (DSS) were evaluated with the Kaplan–Meier method and compared using the log-rank test. For PD-L1 expression, CD8+, DC-LAMP+, and CD68+ cell densities, the “minimum P value” approach was used to determine the best separation of Kaplan–Meier curves referring to the outcome. Correction and 10-fold cross-validations were performed17,18 and cutoffs at quartile values were also used (Table S3). Independent prognostic parameters identified at univariate analysis (p < 0.05) were introduced in a multivariate Cox-proportional hazards regression model to identify independent prognostic factors. The predictive performance of individual marker was assessed by Harrell's concordance index (c-index) and time-dependent c-index (Cτ) derived from time-dependent ROC curve analysis. Mann–Whitney and Kruskal–Wallis tests were used to compare the density of infiltrating immune cells between different groups of patients. p < 0.05 was considered statistically significant. All analyses were performed with Prism 5 (GraphPad), Statview (Abacus Systems), and R software (http://www.r-project.org/).
Results
The clinical response after neoadjuvant chemotherapy predicts survival in stage III-N2 NSCLC
All 122 patients had a N2 disease before chemotherapy treatment (Table 1). At the completion of the study, 69 patients were dead (78% from recurrence) and 53 patients were alive (mean follow-up = 42 mo).
Overall, tumor and lymph node objective clinical responses to neoadjuvant chemotherapy were associated with longer OS (p = 0.00059, p = 0.00051, and p = 0.0009, respectively), DFS (p = 0.0032, p = 0.000098, and p = 0.0018, respectively), and DSS (p = 0.00025, p = 0.00031, and p = 0.000033, respectively) (Table 1).
The pathological staging after neoadjuvant chemotherapy predicts survival in stage III-N2 NSCLC
Most patients displayed histological modifications reflecting tumor regression process after neoadjuvant chemotherapy, including stromal fibrosis and tumor necrosis (Fig. 1A), as previously reported.3,19-22 After treatment 41.8% patients had a N0 (27.9%) or N1 (13.9%) disease.
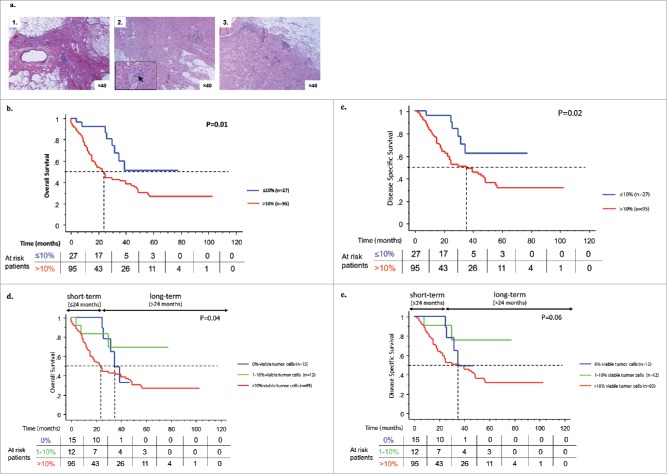
Figure 1.
Pathological response to neoadjuvant chemotherapy is associated with patients' survival. Representative images of the pathological responses observed after neoadjuvant chemotherapy: (A1) complete response (0% viable tumor cell), (A2) major response (1–10% viable tumor cells), and (A3) partial response (>10% viable tumor cells). Original magnification: ×40. Bottom left insert shows magnification of the tumor and black arrow indicates tumor cells. Kaplan–Meier curves for the duration of OS and DSS according to the percentage of viable tumor cells. Comparison were done between ≤ 10% versus >10% viable tumor cells (B and C) and between 0% versus 1–10% versus >10% viable tumor cells (D and E). Statistical comparison was performed by the log-rank test.
Complete (0% viable tumor cells), major (1 to 10% viable tumor cells; median = 3%), or partial (>10% viable tumor cells; median = 44%) histological response was found in 12%, 10%, or 78% of patients, respectively (Fig. 1A). Pathological N downstaging was associated with the histological response in the tumor (Fig. S1). Patients with complete or major histological response in the primary tumors also experienced N response and had a better survival (p = 0.0061) (Fig. S2). Necrosis and fibrosis were not found associated with survival.
In univariate analysis, the pathological staging and the percentage of viable cancer cells were significantly associated with the survival of patients (Table 1). Patients with 0 to 10% viable cancer cells had longer OS, DSS, and DFS than those with more than 10% viable cancer cells (p = 0.01, p = 0.02, and p = 0.05 Figs. 1B and C, S3A). Since patients with less than 10% living cancer cells had either complete or major response, we analyzed them separately. Patients with 0% cancer cell had the best survival 24 mo after surgery, but this difference was lost in the long-term follow-up (Figs. 1D and E, S3B). No major clinical difference was found between these two groups (Table S4).
An organized immune microenvironment is present in lung tumors after chemotherapy
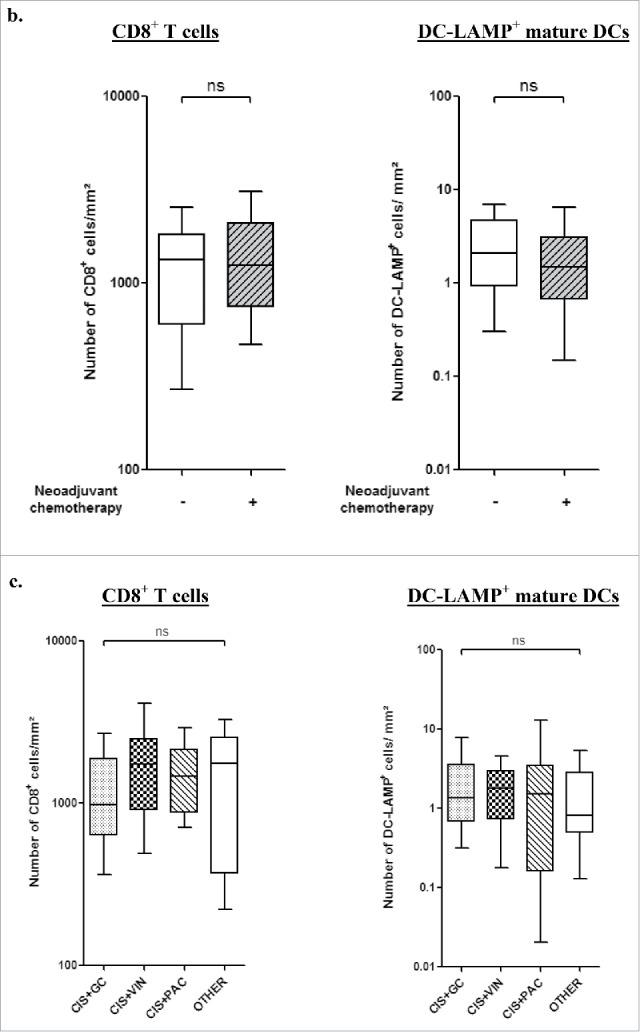
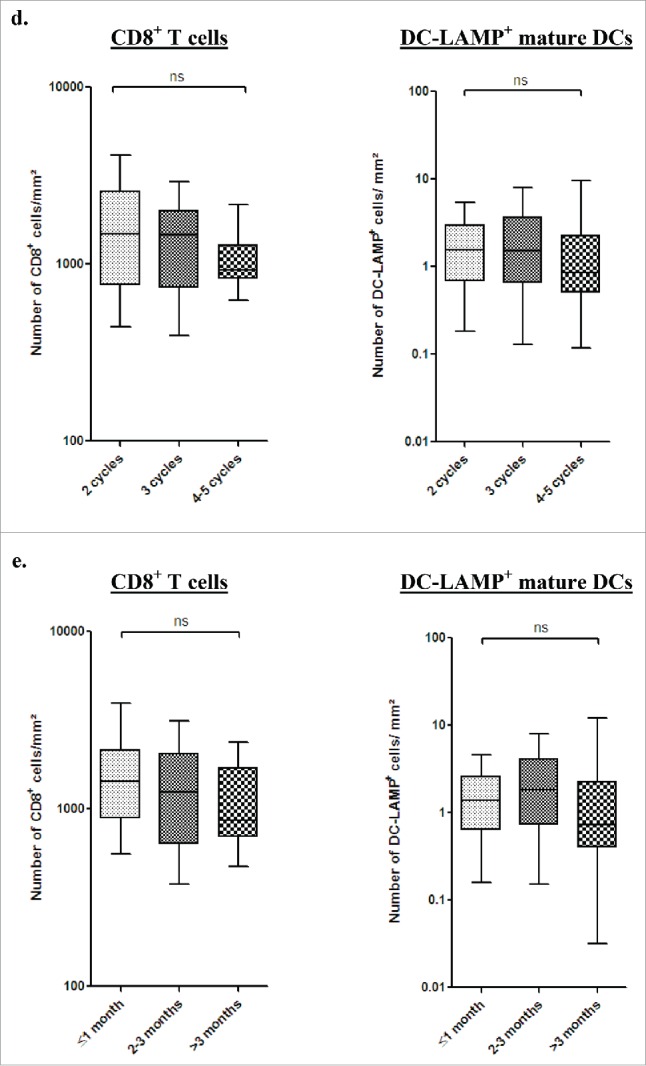
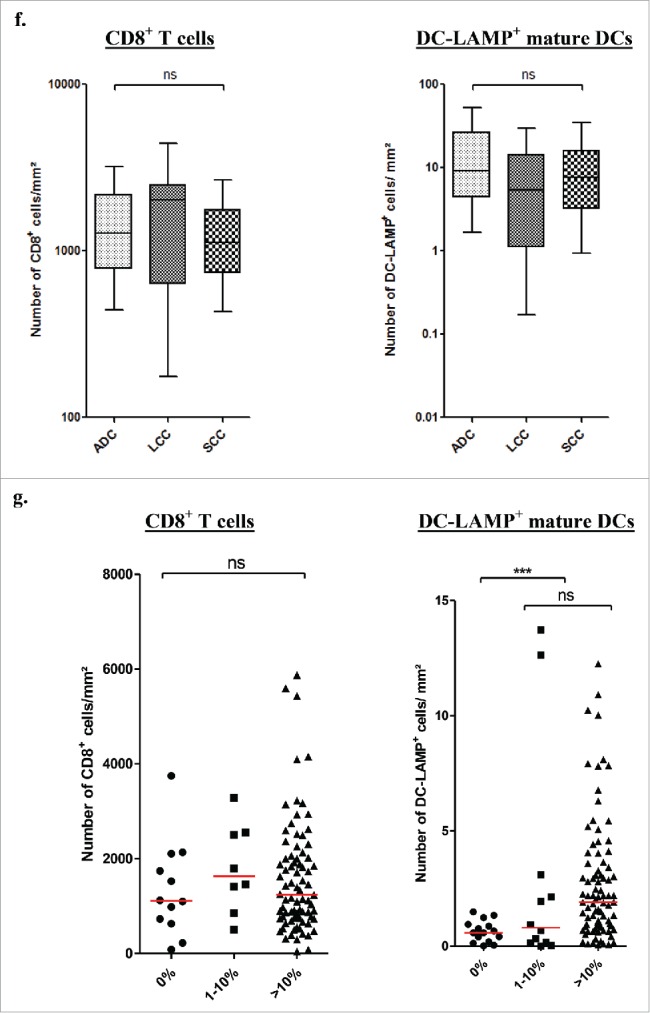
As previously reported in early stage NSCLC,8,23 TLS were observed in the tumor microenvironment after neoadjuvant chemotherapy (Fig. 2A). Similar densities of CD8+ and DC-LAMP+ cells were found in stage III-N2 patients treated with chemotherapy and in patients not treated prior surgery (Fig. 2B). In chemotherapy-treated patients, CD8+ or DC-LAMP+ cell densities were not associated with the drug combinations (Fig. 2C), the number of cycles (Fig. 2D), the delay between the end of the chemotherapy and the surgery (Fig. 2E), or the histological type (Fig. 2F).
Figure 2.
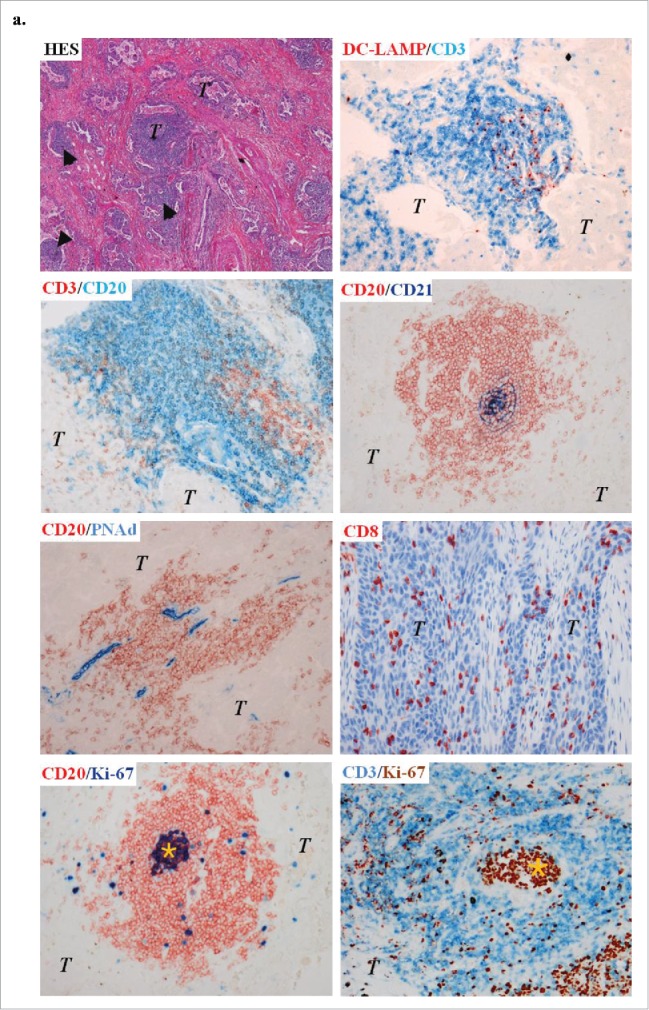
Presence of immune cells in stage III-N2 NSCLC treated or not with neoadjuvant chemotherapy. (A) In treated patients, immune cells are organized in TLS composed of T, B, and mature DCs. TLS are surrounded by high-endothelial venules PNAd+. Some B-cell follicles contained Ki-67+ proliferating germinal center. CD8+ cells are localized in the stroma and in the tumor nests. Ki-67+ T cells are also found inside and outside the TLS. Original magnification: ×200. Head arrows indicate the tertiary-lymphoid structures and germinal centers are marked with an asterisk. T, tumor; HES, hematoxylin-eosin-saffron. CD8+ and DC-LAMP+ immune cell densities in stage III-N2 NSCLC treated or not with neoadjuvant chemotherapy. (B) CD8+ and DC-LAMP+ cell densities in treated and untreated patients. CD8+ and DC-LAMP+ cells according to drug combination (C), number of chemotherapy cycles (D), interval of time between chemotherapy and surgery (E), or histological type. (F) ns, non-significant, Kruskal–Wallis test. ADC, adenocarcinoma; CIS, cisplatin; GC, gemcitabine; LCC, large cell carcinoma; PAC, paclitaxel; SCC, squamous cell carcinoma; VIN, vinorelbine. Densities of CD8+ and DC-LAMP+ immune populations according to the percentage of living tumor cells (0%, 1–10%, or >10% viable cancer cells) (G). Bars represent the median. Mann–Whitney (B) and Kruskal–Wallis tests were performed (C–G); ns, non-significant; ***p < 0.0001.
Figure 2.
Continued.
Figure 2.
Continued.
Figure 2.
Continued.
We further analyzed the immune microenvironment characteristics according to the percentage of viable cancer cells after chemotherapy. We found that tumors without viable cancer cells exhibit a significantly lower density of DC-LAMP+ cells (p < 0.0001, Figs. 2G and S4) whereas no difference was found when analyzing the CD8+ cell densities (p > 0.05, Fig. 2G).
PD-L1 expression is not affected by neoadjuvant chemotherapy
We analyzed the PD-L1 expression in two different positive lymph nodes obtained before and after treatment with neoadjuvant chemotherapy and in matched resected lung tumor after chemotherapy when material was available (n = 21). PD-L1 expression was detected on tumors cells as well as on some infiltrating immune cells (Fig. 3), in accordance with the literature.24 We also found a positive correlation in the PD-L1 expression by tumor cells present in nodes before and after neoadjuvant chemotherapy (r = 0.618, p = 0.003), as well as in lymph node before treatment and in primary lung tumor after chemotherapy (r = 0.670, p = 0.001). We did not find any change in the PD-L1 expression on immune or tumor cells before and after treatment, suggesting that PD-L1 expression was not modified by chemotherapy.
Figure 3.

PD-L1 expression pattern in NSCLC. PD-L1 was found expressed on immune and tumor cells. Pictures show PD-L1 expression by tumor cells in lymph node before and after neoadjuvant chemotherapy and in primary lung tumor. Original magnifications: ×100 and ×200.
CD8+ and DC-LAMP+ cell densities, but neither CD68+ cell densities nor PD-L1 expression, are prognostic factors
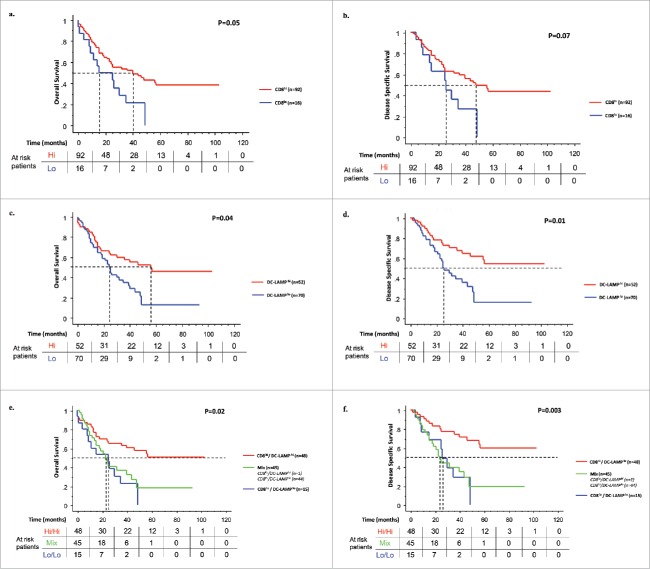
We did not find any significant association between PD-L1 expression by tumor or immune cells and clinical outcome of patients. However, we observed that CD8+ and DC-LAMP+ cell densities were associated with PD-L1 expression on tumor cells (r = 0.326, p = 0.004; r = 0.325, p = 0.003 for CD8 and DC-LAMP positive cells, respectively). We also found that CD68+ cell densities were not significantly associated with the survival of NSCLC patients treated with neoadjuvant chemotherapy (p = 0.311; Fig. S5).
High densities of CD8+ and DC-LAMP+ cells were associated with prolonged OS (Figs. 4A and C), DSS (Figs. 4B and D), and DFS (Figs. S6A and B). The median OS were 16 and 41 mo for patients with low and high CD8+ tumors, and 25 and 56 mo for low and high DC-LAMP+ tumors, respectively. No statistical difference was found when CD8+ T cells were analyzed separately in the center of the tumor and the invasive margin. The immune pattern (CD8+ and DC-LAMP+ cell density combination) allowed the identification of patients with the best survival (high/high immune pattern) (p = 0.02, p = 0.003, and p = 0.001 for OS, DSS, and DFS, respectively; Figs. 4E and F, S6c).
Figure 4.
CD8+ and DC-LAMP+ cell densities are associated with patients' survival. Kaplan–Meier curves illustrate the duration of OS and DSS according to the densities of CD8+ (A and B) and DC-LAMP+ cells (C and D). Red lines represent high densities of infiltrating immune cells and blue lines, low densities. Kaplan–Meier curves for the duration of OS and DSS according to a combined analysis of CD8+ and DC-LAMP+ cell densities (immune pattern) (E and F). For each marker, high densities in the tumor (hi, red line), low densities in the tumor (lo, blue line), and heterogeneous densities (Mix, green line) in patients treated with neoadjuvant chemotherapy are represented. Statistical comparisons were performed by the log-rank test and p-values corrected using the formula proposed by Altman et al. Cutoff values were 510 CD8+ cells/mm2 and 1.92 DC-LAMP+ cells/mm2.
The immune pattern is the strongest independent prognostic marker
Univariate analyses revealed that a variety of clinical, pathological, and immunological criteria (clinical-specific response on the lymph node, overall clinical response, pathological downstaging, intervention type, histological type, pathological downstaging, pathological stage, percentage of viable tumor cells, and immune pattern) were significantly associated with survival (Table 1 and Table S5). These parameters were subsequently analyzed using a Cox multivariate regression model. Three models were generated including clinical response (model 1), pathological stage (model 2), and percentage of viable tumor cells (model 3), because of significant interaction between them (Table 2). The immune pattern was found to be the strongest independent prognostic factor for OS (p < 0.001), DSS (p < 0.01), and DFS (p < 0.001) in all models (Table 2) with the better predictive performance (Fig. S7).
Table 2.
Multivariate analysis of overall survival (OS), disease-specific survival (DFS) and disease-free survival (DFS) according to clinical, pathological, and immune parameters.
| Model 1 |
Model 2 |
Model 3 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | HR | 95%CI | p-value | HR | 95%CI | p-value | HR | 95%CI | p-value | |
| OS | Overall clinical response | 1.76 | 1.05–1.95 | 0.031 | NA | NA | NA | NA | NA | NA |
| No vs. Yes | ||||||||||
| Overall pathological response | NA | NA | NA | 1.93 | 1.10–3.37 | 0.022 | NA | NA | NA | |
| No vs. Yes | ||||||||||
| Percentage of viable tumor cells | NA | NA | NA | NA | NA | NA | 2.87 | 1.32–6.23 | 0.0076 | |
| >10% vs. ≤ 10% | ||||||||||
| Intervention | 1.84 | 1.10–3.08 | 0.021 | 1.77 | 1.06–2.98 | 0.03 | 1.58 | 0.93–2.66 | 0.088 | |
| Pneumonectomy and bilobectomy vs. lobectomy | ||||||||||
| Histological type | 1.91 | 1.15–3.17 | 0.013 | 1.95 | 1.17–3.24 | 0.011 | 1.87 | 1.12–3.13 | 0.017 | |
| No SCC vs. SCC | ||||||||||
| Immune pattern | 0.0011 | 0.000061 | 0.000024 | |||||||
| Low vs. Mix+High | 0.56 | 0.39–0.79 | 0.49 | 0.34–0.69 | 0.46 | 0.32–0.66 | ||||
| Low+Mix vs. High | 0.31 | 0.15–0.63 | 0.24 | 0.12–0.48 | 0.21 | 0.10–0.44 | ||||
| DSS | Overall clinical response | 1.98 | 1.08–3.63 | 0.027 | NA | NA | NA | NA | NA | NA |
| No vs. Yes | ||||||||||
| Overall pathological response | NA | NA | NA | 2.17 | 1.15–4.09 | 0.017 | NA | NA | NA | |
| No vs. Yes | ||||||||||
| Percentage of viable tumor cells | NA | NA | NA | NA | NA | NA | 3.25 | 1.33–7.92 | 0.0096 | |
| >10% vs. ≤ 10% | ||||||||||
| Histological type | NA | NA | NA | NA | NA | NA | NA | NA | NA | |
| No SCC vs. SCC | ||||||||||
| Staging methodology | 0.80 | 0.44–1.46 | 0.46 | 0.73 | 0.41–1.3 | 0.28 | 0.89 | 0.48–1.62 | 0.69 | |
| Surgical vs. imaging | ||||||||||
| Immune pattern | 0.0082 | 0.00077 | 0.00021 | |||||||
| Low vs. Mix+High | 0.58 | 0.39–0.87 | 0.5 | 0.34–0.75 | 0.46 | 0.30–0.69 | ||||
| Low+Mix vs. High | 0.34 | 0.15–0.76 | 0.25 | 0.11–0.56 | 0.21 | 0.09–0.48 | ||||
| DFS | Overall clinical response | 1.87 | 1.07–3.25 | 0.027 | NA | NA | NA | NA | NA | NA |
| No vs. Yes | ||||||||||
| Overall pathological response | NA | NA | NA | 2.2 | 1.24–3.90 | 0.0069 | NA | NA | NA | |
| No vs. Yes | ||||||||||
| Percentage of viable tumor cells | NA | NA | NA | NA | NA | NA | NA | NA | NA | |
| >10% vs. ≤ 10% | ||||||||||
| Histological type | 2.24 | 1.29–3.89 | 0.04 | 2.35 | 1.35–4.08 | 0.0024 | NA | NA | NA | |
| No SCC vs. SCC | ||||||||||
| Immune pattern | 0.0008 | 0.000026 | NA | NA | NA | |||||
| Low vs. Mix+High | 0.53 | 0.36–0.77 | 0.46 | 0.32–0.66 | ||||||
| Low+Mix vs. High | 0.28 | 0.13–0.59 | 0.21 | 0.10–0.43 | ||||||
Model 1 contains overall clinical response as a predictor variable, model 2 contains overall pathological response and model 3 percentage of viable tumor cells. Pathological stage was not entered in models 2 and 3 because of high correlation with nodal downstaging and histopathological response.
CI, confidence interval; HR, hazard ratio; NA, not applicable.Bold: Significant p values.
Since histological response and immune pattern were two independent prognostic factors, we tested a new scoring approach for chemotherapy-treated patients that combined the percentage of viable tumor cells with the densities of tumor-infiltrating CD8+ and DC-LAMP+ cells. This approach revealed that during the first 24 mo, the percentage of viable cancer cells was the best prognostic factor (p < 0.01, Table 3) whereas the long-term survival beyond 36 mo was better predicted by the densities of CD8+ and DC-LAMP+ cells (p < 0.05, Table 3). Altogether, these results suggest that the percentage of viable tumor cells and the immune pattern are complementary prognostic factors that allowed a better stratification of patients treated by chemotherapy according to their clinical outcome.
Table 3.
Percentages of surviving patients at 24, 36, 60, and 96 mo, according to the percentage of viable cancer cells (0%, 1–10%, and >10%) and the immune pattern (low and high).
| Time (months) | |||||
|---|---|---|---|---|---|
| T1 | T2 |
||||
| Parameters |
Group |
24 |
36 |
60 |
96 |
| % viable tumor cells | 0% | 100% | 49% | NR | NR |
| 1–10% | 83% | 69% | 69% | NR | |
| >10% | 48% | 41% | 27% | 27% | |
| CD8+/DC-LAMP+ cells | High | 68% | 63% | 50% | 50% |
| Low | 53% | 23% | 0% | 0% | |
NR, not reached.
Discussion
In this study, we addressed the question of the effect of chemotherapy on cancer cells and immune contexture that both impact clinical outcome of patients with NSCLC. We confirmed that clinical and pathological responses were good indicators of survival after neoadjuvant chemotherapy in stage III-N2 NSCLC patients. As previously reported,3,19 less than 10% of viable tumor cells after neoadjuvant chemotherapy is a relevant threshold to identify patients with good or poor clinical outcome. Since it represents a reliable prognostic marker, we recommend to evaluate and report the percentage of living cancer cells after neoadjuvant chemotherapy.
The immune cell density and organization in patients who received neoadjuvant chemotherapy were similar than in chemotherapy-naive patients. These results are in contrast with some previous studies suggesting an impact of chemotherapy on the type11 and density12,13 of the immune infiltrate. One could explain that the delay between the last drug administration and the surgery (3 to 6 weeks) was sufficient to restore an organized immune microenvironment. A second possibility may be that the drug combination given in NSCLC did not modify the immune contexture. Interestingly, DC-LAMP+ cell number was lower in tumors without detectable viable malignant cells, suggesting that the presence of remaining living cancer cells was needed for the maintenance of a sustainable organized immune reaction. Obviously, it will be interesting to characterize the immune compartment longitunaly before and after chemotherapy in future studies to precisely define the effect of chemotherapy on the tumor immune contexture. This sequential analysis was not possible in the current study, as we did not have access to pre-treatment lung tumor samples.
We also demonstrated that high densities of CD8+ T cells and DC-LAMP+ mature DCs were independent survival markers after neoadjuvant chemotherapy. Low density of DC-LAMP+ cells in tumors was associated with poor survival even when density of CD8+ T cells was high, suggesting that CD8+ T cells alone were not capable to satisfactorily fulfill their antitumor role without mature DCs.25 These data support that mature DCs, mostly present within the TLS, are mandatory in the education of CD8+ T cells and potentialize their antitumor activity. The density of CD8+ T cells and mature DCs was found to be associated with PD-L1 expression. This finding is in accordance with previous reports showing that PD-L1 protein expression can be induced by type I and type II interferons.26 As previously reported,24 we also observed that, in addition to tumor cells, immune cells do express PD-L1. We found that the density of macrophages was not associated with OS, DSS, and DFS. The role of tumor-associated macrophages is not fully understood in NSCLC and the presence of CD68+ macrophages has been associated with opposite prognostic values depending on the study.9 To our knowledge, the prognostic value of CD68+ cells in late-stage NSCLC treated with neoadjuvant chemotherapy has never been reported. Even it might be challenging to discriminate between tumor-associated macrophages and necrosis-related macrophage infiltration, a more in-depth analysis of the clinical impact of tumor-associated macrophages is still needed using combination of different markers and multiplexed technologies.27
We showed that histological responses and immune pattern were complementary predictors of survival. Strikingly, the immune criterion was a powerful parameter to identify long-term survivors even among patients without satisfactory pathological response, supporting the idea that immune cells may control the local tumor proliferation but even more dramatically the spreading of residual tumor cells. In accordance with other studies, we showed that chemotherapy-induced short-term benefits27,28 and, in addition with data from clinical trial using immune-based therapies,29-32 our results strongly demonstrate that a strong adaptive immune reaction within the tumor is associated with long-term survival for the patients. This could bring some additional evidence to the significant long-term clinical benefits of the immune checkpoint blockade30,33 or the rituximab treatment,34 but with contrasted short-term effects. In NSCLC, immunotherapy is a very promising therapeutic approach as reflected by the satisfactory and long-lasting response rates obtained in recent trials targeting the PD1/PD-L1 immunosuppressive pathway.31,32,35-37 In accordance with these results, we found that a small number of living cancer cells was associated with an immediate advantage, but that the immune contexture was of major importance for the long-term effect. Even in patients without satisfactory pathological response, the immune pattern identified the long-term survivors. This finding highlights the fact that the immune contexture is highly relevant for predicting the long-term survival of patients treated with neoadjuvant chemotherapy.
In conclusion, we showed that neoadjuvant chemotherapy in NSCLC strongly impacts the tumor compartment but not dramatically the immune contexture. Percentage of viable tumor cells and immune pattern were prognostic factors and the combination of both allowed identifying patients with the best outcome. Our results give new evidence for the rational of combining conventional chemotherapies and immunotherapies in order to improve the clinical outcome of patients with cancer.38
Supplementary Material
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
Authors want to thank Patricia Bonjour and Martine Bovet for technical assistance.
References
- 1.Goldstraw P, Ball D, Jett JR, Le Chevalier T, Lim E, Nicholson AG, Shepherd FA. Non-small-cell lung cancer. Lancet 2011; 378:1727-40; PMID:21565398; http://dx.doi.org/ 10.1016/S0140-6736(10)62101-0 [DOI] [PubMed] [Google Scholar]
- 2.Rosell R, Gomez-Codina J, Camps C, Maestre J, Padille J, Canto A, Mate JL, Li S, Roig J, Olazabal A et al.. A randomized trial comparing preoperative chemotherapy plus surgery with surgery alone in patients with non-small-cell lung cancer. N Engl J Med 1994; 330:153-8; PMID:8043059; http://dx.doi.org/ 10.1056/NEJM199401203300301 [DOI] [PubMed] [Google Scholar]
- 3.Pataer A, Kalhor N, Correa AM, Raso MG, Erasmus JJ, Kim ES, Behrens C, Lee JJ, Roth JA, Stewart DJ et al.. Histopathologic response criteria predict survival of patients with resected lung cancer after neoadjuvant chemotherapy. J Thorac Oncol 2012; 7:825-32; PMID:22481232; http://dx.doi.org/ 10.1097/JTO.0b013e318247504a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garrido P, Gonzalez-Larriba JL, Insa A, Provencio M, Torres A, Isla D, Sanchez JM, Cardenal F, Domine M, Barcelo JR et al.. Long-term survival associated with complete resection after induction chemotherapy in stage IIIA (N2) and IIIB (T4N0-1) non small-cell lung cancer patients: the Spanish Lung Cancer Group Trial 9901. J Clin Oncol 2007; 25:4736-42; PMID:17947721; http://dx.doi.org/ 10.1200/JCO.2007.12.0014 [DOI] [PubMed] [Google Scholar]
- 5.Fridman WH, Pages F, Sautes-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer 2012; 12:298-306; PMID:22419253; http://dx.doi.org/ 10.1038/nrc3245 [DOI] [PubMed] [Google Scholar]
- 6.Remark R, Alifano M, Cremer I, Lupo A, Dieu-Nosjean MC, Riquet M, Crozet L, Ouakrim H, Goc J, Cazes A et al.. Characteristics and clinical impacts of the immune environments in colorectal and renal cell carcinoma lung metastases: influence of tumor origin. Clin Cancer Res 2013; 19:4079-91; PMID:23785047; http://dx.doi.org/ 10.1158/1078-0432.CCR-12-3847 [DOI] [PubMed] [Google Scholar]
- 7.Remark R, Merghoub T, Grabe N, Litjens G, Damotte D, Wolchok JD, Merad M, Gnjatic S. In-depth tissue profiling using multiplexed immunohistochemical consecutive staining on single slide. Sci Immunol 2016; 1:aaf6925; http://dx.doi.org/ 10.1126/sciimmunol.aaf6925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dieu-Nosjean MC, Antoine M, Danel C, Heudes D, Wislez M, Poulot V, Rabbe N, Laurans L, Tartour E, de Chaisemartin L et al.. Long-term survival for patients with non-small-cell lung cancer with intratumoral lymphoid structures. J Clin Oncol 2008; 26:4410-7; PMID:18802153; http://dx.doi.org/ 10.1200/JCO.2007.15.0284 [DOI] [PubMed] [Google Scholar]
- 9.Remark R, Becker C, Gomez JE, Damotte D, Dieu-Nosjean MC, Sautes-Fridman C, Fridman WH, Powell CA, Altorki NK, Merad M et al.. The non-small cell lung cancer immune contexture. A major determinant of tumor characteristics and patient outcome. Am J Respir Crit Care Med 2015; 191:377-90; PMID:25369536; http://dx.doi.org/ 10.1164/rccm.201409-1671PP [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goc J, Germain C, Vo-Bourgais TK, Lupo A, Klein C, Knockaert S, de Chaisemartin L, Ouakrim H, Becht E, Alifano M et al.. Dendritic cells in tumor-associated tertiary lymphoid structures signal a Th1 cytotoxic immune contexture and license the positive prognostic value of infiltrating CD8+ T cells. Cancer Res 2014; 74:705-15; PMID:24366885; http://dx.doi.org/ 10.1158/0008-5472.CAN-13-1342 [DOI] [PubMed] [Google Scholar]
- 11.Zitvogel L, Apetoh L, Ghiringhelli F, Andre F, Tesniere A, Kroemer G. . The anticancer immune response: indispensable for therapeutic success? J Clin Invest 2008; 118:1991-2001; PMID:18523649; http://dx.doi.org/ 10.1172/JCI35180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsuchikawa T, Md MM, Yamamura Y, Shichinohe T, Hirano S, Kondo S. The immunological impact of neoadjuvant chemotherapy on the tumor microenvironment of esophageal squamous cell carcinoma. Ann Surg Oncol 2012; 19:1713-9; PMID:21822560; http://dx.doi.org/ 10.1245/s10434-011-1906-x [DOI] [PubMed] [Google Scholar]
- 13.Hornychova H, Melichar B, Tomsova M, Mergancova J, Urminska H, Ryska A. Tumor-infiltrating lymphocytes predict response to neoadjuvant chemotherapy in patients with breast carcinoma. Cancer Invest 2008; 26:1024-31; PMID:19093260; http://dx.doi.org/ 10.1080/07357900802098165 [DOI] [PubMed] [Google Scholar]
- 14.Tsuda N, Chang DZ, Mine T, Efferson C, Garcia-Sastre A, Wang X, Ferrone S, Ioannides CG. Taxol increases the amount and T cell activating ability of self-immune stimulatory multimolecular complexes found in ovarian cancer cells. Cancer Res 2007; 67:8378-87; PMID:17804754; http://dx.doi.org/ 10.1158/0008-5472.CAN-07-0327 [DOI] [PubMed] [Google Scholar]
- 15.Alifano M, Boudaya MS, Salvi M, Collet JY, Dinu C, Camilleri-Broet S, Regnard JF. Pneumonectomy after chemotherapy: morbidity, mortality, and long-term outcome. Ann Thorac Surg 2008; 85:1866-72; PMID:18498785; http://dx.doi.org/17374834 10.1016/j.athoracsur.2008.01.103 [DOI] [PubMed] [Google Scholar]
- 16.van Meerbeeck JP, Kramer GW, Van Schil PE, Legrand C, Smit EF, Schramel F, Tjan-Heijnen VC, Biesma B, Debruyne C, van Zandwijk N et al.. Randomized controlled trial of resection versus radiotherapy after induction chemotherapy in stage IIIA-N2 non-small-cell lung cancer. J Natl Cancer Inst 2007; 99:442-50; PMID:17374834; http://dx.doi.org/ 10.1093/jnci/djk093 [DOI] [PubMed] [Google Scholar]
- 17.Altman DG, Lausen B, Sauerbrei W, Schumacher M. Dangers of using “optimal” cutpoints in the evaluation of prognostic factors. J Natl Cancer Inst 1994; 86:829-35; PMID:8182763; http://dx.doi.org/ 10.1093/jnci/86.11.829 [DOI] [PubMed] [Google Scholar]
- 18.Faraggi D, Simon R. A simulation study of cross-validation for selecting an optimal cutpoint in univariate survival analysis. Stat Med 1996; 15:2203-13; PMID:8910964; http://dx.doi.org/ 10.1002/(SICI)1097-0258(19961030)15:20%3c2203::AID-SIM357%3e3.0.CO;2-G [DOI] [PubMed] [Google Scholar]
- 19.Junker K, Thomas M, Schulmann K, Klinke F, Bosse U, Muller KM. Tumour regression in non-small-cell lung cancer following neoadjuvant therapy. Histological assessment. J Cancer Res Clin Oncol 1997; 123:469-77; http://dx.doi.org/ 10.1007/BF01192200 [DOI] [PubMed] [Google Scholar]
- 20.Yamane Y, Ishii G, Goto K, Kojima M, Nakao M, Shimada Y, Nishiwaki Y, Nagai K, Kohrogi H, Ochiai A. A novel histopathological evaluation method predicting the outcome of non-small cell lung cancer treated by neoadjuvant therapy: the prognostic importance of the area of residual tumor. J Thorac Oncol 2010; 5:49-55; PMID:20035185; http://dx.doi.org/ 10.1097/JTO.0b013e3181c0a1f8 [DOI] [PubMed] [Google Scholar]
- 21.Mouillet G, Monnet E, Milleron B, Puyraveau M, Quoix E, David P, Ducolone A, Molinier O, Zalcman G, Depierre A et al.. Pathologic complete response to preoperative chemotherapy predicts cure in early-stage non-small-cell lung cancer: combined analysis of two IFCT randomized trials. J Thorac Oncol 2012; 7:841-9; PMID:22722786; http://dx.doi.org/ 10.1097/JTO.0b013e31824c7d92 [DOI] [PubMed] [Google Scholar]
- 22.Liu-Jarin X, Stoopler M.B, Raftopoulos H, Ginsburg M, Gorenstein L, Borczuk AC. Histologic assessment of non-small cell lung carcinoma after neoadjuvant therapy. Mod Pathol 2003; 16:1102-8; PMID:14614049; http://dx.doi.org/ 10.1097/01.MP.0000096041.13859.AB [DOI] [PubMed] [Google Scholar]
- 23.Chatterjee S, Crozet L, Damotte D, Iribarren K, Schramm C, Alifano M, Lupo A, Cherfils-Vicini J, Goc J, Katsahian S et al.. TLR7 promotes tumor progression, chemotherapy resistance, and poor clinical outcomes in non-small cell lung cancer. Cancer Res 2014; 74:5008-18; PMID:25074614; http://dx.doi.org/ 10.1158/0008-5472.CAN-13-2698 [DOI] [PubMed] [Google Scholar]
- 24.Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger SN et al.. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 2014; 515:563-7; PMID:25428504; http://dx.doi.org/ 10.1038/nature14011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goc J, Germain C, Vo-Bourgais TK, Lupo A, Klein C, Knockaert S, de Chaisemartin L, Ouakrim H, Becht E, Alifano M et al.. Dendritic cells in tumor-associated tertiary lymphoid structures signal a Th1 cytotoxic immune contexture and license the good positive prognostic value of infiltrating CD8+ T cells. Cancer Res 2014; 74:705-15; PMID:24366885; http://dx.doi.org/11956257 10.1158/0008-5472.CAN-13-1342 [DOI] [PubMed] [Google Scholar]
- 26.Zou W, Wolchok JD, Chen L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: Mechanisms, response biomarkers, and combinations. Sci Transl Med 2016; 8:328rv324; PMID:26936508; http://dx.doi.org/11956257 10.1126/scitranslmed.aad7118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martin J, Ginsberg R.J, Venkatraman ES, Bains MS, Downey RJ, Korst RJ, Kris MG, Rusch VW. Long-term results of combined-modality therapy in resectable non-small-cell lung cancer. J Clin Oncol 2002; 20:1989-95; PMID:11956257; http://dx.doi.org/ 10.1200/JCO.2002.08.092 [DOI] [PubMed] [Google Scholar]
- 28.Blazer DG 3rd, Kishi Y, Maru DM, Kopetz S, Chun YS, Overman MJ, Fogelman D, Eng C, Chang DZ, Wang H et al.. Pathologic response to preoperative chemotherapy: a new outcome end point after resection of hepatic colorectal metastases. J Clin Oncol 2008; 26:5344-51; PMID:18936472; http://dx.doi.org/ 10.1200/JCO.2008.17.5299 [DOI] [PubMed] [Google Scholar]
- 29.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB et al.. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med 2010; 363:411-22; PMID:20818862; http://dx.doi.org/ 10.1056/NEJMoa1001294 [DOI] [PubMed] [Google Scholar]
- 30.Hodi FS, O'Day S.J, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC et al.. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 363:711-23; PMID:20525992; http://dx.doi.org/ 10.1056/NEJMoa1003466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brahmer JR, Tykodi S.S, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K et al.. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012; 366:2455-65; PMID:22658128; http://dx.doi.org/ 10.1056/NEJMoa1200694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB et al.. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012; 366:2443-54; PMID:22658127; http://dx.doi.org/ 10.1056/NEJMoa1200690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buque A, Bloy N, Aranda F, Castoldi F, Eggermont A, Cremer I, Fridman WH, Fucikova J, Galon J, Marabelle A et al.. Trial watch: Immunomodulatory monoclonal antibodies for oncological indications. Oncoimmunology 2015; 4:e1008814; PMID:26137403; http://dx.doi.org/ 10.1080/2162402X.2015.1008814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abes R, Gelize E, Fridman WH, Teillaud JL. Long-lasting antitumor protection by anti-CD20 antibody through cellular immune response. Blood 2010; 116:926-934; PMID:20439625; http://dx.doi.org/ 10.1182/blood-2009-10-248609 [DOI] [PubMed] [Google Scholar]
- 35.Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L et al.. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015; 372:2018-28; PMID:25891174; http://dx.doi.org/ 10.1056/NEJMoa1501824 [DOI] [PubMed] [Google Scholar]
- 36.Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer cell 2015; 27:450-61; PMID:25858804; http://dx.doi.org/ 10.1016/j.ccell.2015.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Topalian SL, Taube JM, Anders RA, Pardoll DM. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer 2016; 16:275-87; PMID:27079802; http://dx.doi.org/ 10.1038/nrc.2016.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Champiat S, Ileana E, Giaccone G, Besse B, Mountzios G, Eggermont A, Soria JC. Incorporating immune-checkpoint inhibitors into systemic therapy of NSCLC. J Thorac Oncol 2014; 9:144-53; PMID:24419410; http://dx.doi.org/ 10.1097/JTO.0000000000000074 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.