Abstract
Background
Masked hypertension (MHT), defined as normal office blood pressure (BP) but high ambulatory BP, has been associated with increased cardiovascular risk. Although MHT has been associated with obesity, there is limited knowledge on the prevalence and covariates of MHT in obese cohorts.
Methods
Office and ambulatory BP recordings and other cardiovascular risk factors were assessed in 323 obese participants included in the fat-associated cardiovascular dysfunction study (mean age 48.9±9.0 years, 55% women, mean BMI 32.3±4.4 kg/m2). Office BP 130–139/85–89 mmHg was considered high-normal. Subclinical arterial damage was identified as carotid–femoral pulse wave velocity more than 10 m/s by applanation tonometry or carotid plaque by ultrasound (maximal intima–media thickness ≥1.5 mm).
Results
MHT was present in 17.1% of the population. Patients with MHT had a higher prevalence of metabolic syndrome, high-normal office BP, and were more often male compared with the normotensive (NT) individuals (all P<0.05), but were younger and had lower prevalence of diabetes and subclinical arterial damage than the sustained hypertensive group (all P<0.05). In multinomial logistic regression analysis, MHT was associated with the presence of metabolic syndrome and high-normal office BP compared with NT individuals, and lower pulse wave velocity and fewer carotid plaques than sustained hypertension (all P<0.05).
Conclusion
In obese patients, MHT was associated with the presence of metabolic syndrome and high-normal office BP compared with NT individuals, but less subclinical arterial damage than sustained hypertensive patients.
Keywords: arterial damage, blood pressure, masked hypertension, metabolic syndrome, obesity, pulse wave velocity
Introduction
Patients with masked hypertension (MHT) have been shown to carry a double risk of cardiovascular events compared with normotensive (NT) individuals, comparable to that of patients with sustained hypertension (SHT) 1–3. MHT has been associated with target organ damage in the heart, arteries, kidney, and brain in previous reports 3–9. Several cardiovascular risk factors such as obesity, male sex, exercise-induced hypertension, smoking, diabetes, and family history of cardiovascular disease have also been found to cluster in MHT 3. Obesity is associated with metabolic changes, which may involve glucose and lipid metabolism, the fibrinolytic system, the autonomic nervous system, and the endothelial function 10. These changes all promote cardiovascular inflammation and the development of cardiovascular structural and functional changes 11. From this, a higher prevalence of MHT could be expected in obesity. However, few studies have reported on the prevalence and covariates of MHT within obese cohorts. This was the aim of the present study.
Methods
Study population
The fat-associated cardiovascular dysfunction (FATCOR) study has been on-going since 2009 aiming at identifying determinants of subclinical target organ damage in obese patients without known cardiovascular disease 12,13. The FATCOR study is a collaboration between a general practitioner center specializing in the management of obese patients, Alfahelse AS, and Department of Heart Disease, Haukeland University Hospital, both situated in Bergen, Norway. Inclusion criterion for the study was a BMI more than 27.0 kg/m2 in healthy individuals aged 30–65 years. Exclusion criteria were previous myocardial infarction, gastrointestinal disorders (including previous gastric bypass or sleeve operations), severe psychiatric illness, or inability to understand Norwegian language 12. For the present analysis, all individuals who had measurements of both office blood pressure (BP) and ambulatory BP were included, a total of 339 participants. The FATCOR study was approved by the Regional Ethics Committee. All participants signed written informed consent according to the declaration of Helsinki.
Blood pressure measurements
A standardized clinical examination by the general practitioner at Alfahelse AS included the measurement of office BP following the European Society of Hypertension guidelines 14 using a validated digital automatic Omron M4 sphygmomanometer (Omron Healthcare Co., Ltd, Hoofddorp, the Netherlands) and appropriate cuff size in relation to arm circumference. BP was measured three times at 1-min intervals after patients had rested in the seated position for at least 5 min. Office BP was taken as the average of the two last measurements in the individual patient. High office BP was defined as systolic BP greater than or equal to 140 mmHg and/or diastolic BP greater than or equal to 90 mmHg. High-normal office BP was defined as systolic BP 130–139 mmHg and/or diastolic BP 85–89 mmHg.
24-H ambulatory BP recording was performed using a noninvasive monitor (Diasys Integra II; Novacor, Cedex, France) set to auscultatory mode. An appropriately sized cuff for the nondominant arm was used, and the participants were instructed to relax their arm when the measurement was initiated. BP was measured every 20 min during daytime and every 30 min during night-time, yielding an average of 78 measurements per 24 h. The recording was repeated if less than 70% of the measurements were technically valid. High 24-h BP was defined as average 24-h systolic BP greater than or equal to 130 mmHg and/or average diastolic BP greater than or equal to 80 mmHg 14.
Study participants were grouped into BP categories combining office and ambulatory BP measurements. Participants were classified as NT when both office BP and ambulatory BP were normal and SHT if both were high. Participants with high office BP but normal ambulatory BP were classified as having white-coat hypertension (WCHT). The MHT participants had normal office BP but high ambulatory BP. All participants using BP medication were categorized as having SHT independent of the actual BP measurements, even though WCHT theoretically could be present in some of these treated participants.
Cardiovascular risk factors
A standardized questionnaire was used to gather self-reported information on the participants’ demographic and socioeconomic characteristics, and medical history including current use of medication and cardiovascular risk factors. The information was quality assured by the general practitioner. Fasting venous blood samples were obtained to measure serum lipids, fasting glucose, and serum creatinine. In nondiabetic patients, an oral glucose tolerance test was performed. Urine albumin–creatinine ratio was measured in a spot morning urine sample and defined as high as 3.4 mg/mmol 14.
Obesity was identified as BMI of at least 30 kg/m2 according to the guidelines by the WHO 15. Metabolic syndrome was diagnosed if at least three of the following features were present: (a) waist circumference of at least 88 cm in women and at least 102 cm in men, (b) office BP of at least 130/85 mmHg or antihypertensive treatment, (c) fasting serum glucose of at least 5.6 mmol/l or blood sugar lowering treatment, (d) fasting serum triglycerides of at least 1.7 mmol/l, and (e) fasting serum high-density-lipoprotein cholesterol less than 1.3 mmol/l in women and less than 1.03 mmol/l in men in accordance with the American Heart Association/National Heart, Lung and Blood Institute guidelines 16.
Diabetes mellitus was defined as a history of diabetes, use of antidiabetic medication, fasting glucose of at least 7.0 mmol/l, or postload plasma glucose after 2 h of more than 11.0 mmol/l. Impaired glucose tolerance was defined as serum glucose 7.8–11.0 mmol/l 2 h after oral intake of 75 g of glucose. Impaired fasting glucose was defined as fasting glucose of at least 6.1 mmol/l and less than 7.0 mmol/l. Insulin resistance was determined from homeostatic model assessment (HOMA-IR) 17.
Subclinical arterial damage
Carotid ultrasound was performed using a Phillips iE33 ultrasound machine (Phillips Healthcare, Best, the Netherlands) and an 11–3 MHz linear array transducer. Maximal carotid intima–media thickness (cIMT) was measured in B-mode on both the far and the near wall in the common carotid artery, carotid bulb, and internal carotid artery on both sides. A carotid plaque was defined as focal maximal cIMT greater than or equal to 1.5 mm 18.
Carotid–femoral pulse wave velocity (PWV) was measured using applanation tonometry (SphygmoCor; AtCor Medical, Sydney, West Ryde, Australia) according to guidelines 19. Pressure pulse waveforms were obtained transcutaneously from the right common carotid and femoral arteries with simultaneous recording of the electrocardiogram for synchronizing carotid and femoral pulse wave times as described previously 20. The proximal distance between the carotid site and the sternal notch and distal distance between the sternal notch and the femoral site were measured precisely. To find PWV, the proximal distance was subtracted from the distal distance and the net distance was divided by the transit time between the two recording sites determined in relation to the R wave on the ECG. PWV more than 10 m/s was defined as arterial organ damage 21, reflecting increased arterial stiffness.
Statistics
Data management and statistical analysis was carried out using SPSS, version 22 statistical software (IBM SPSS Statistics, Chicago, Illinois, USA). The study population was divided into BP categories: NT, MHT, and SHT. Continuous variables are presented as mean±SD, whereas categorical variables are presented as percentages. The MHT group was compared with NT and SHT groups using the χ2-test for categorical values and analysis of variance for continuous variables with Scheffe’s post-hoc test. Univariable covariables of MHT were identified by logistic regression analyses within groups of participants with normal office BP (NT and MHT) and with elevated 24 h ambulatory BP (MHT and SHT). Multinomial logistic regression analysis using an MHT participant as a reference was used to identify independent covariables of MHT. The results were reported as odds ratio and 95% confidence intervals. A P-value up to 0.05 was considered statistically significant in all analyses.
Results
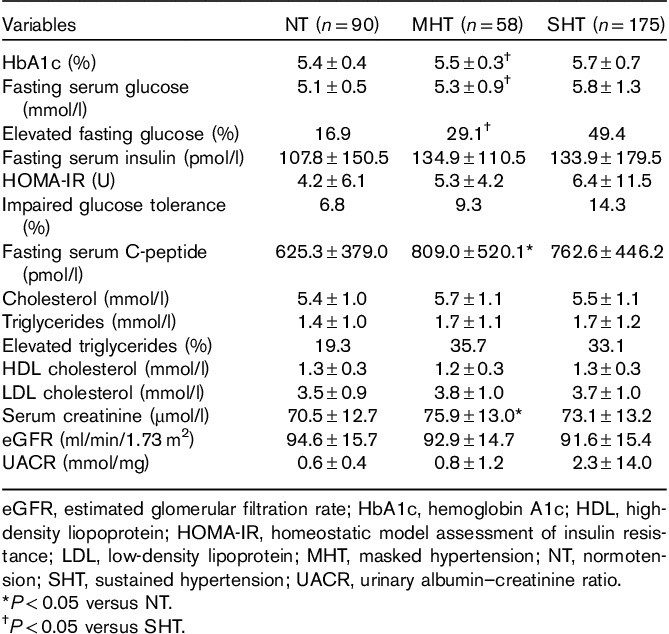
The prevalence of MHT was 17.1% in the total study population. Only 16 (4.7%) participants had WCHT, and this BP category was therefore excluded from further analysis. Among participants with normal office BP (NT and MHT groups), the prevalence of MHT was 39.2%. Within the MHT group five (9%), participants had 24-h systolic BP greater than or equal to 130 mmHg, 41 (71%) participants 24-h diastolic BP had greater than or equal to 80 mmHg, and 12 (21%) participants had both 24-h systolic BP greater than or equal to 130 mmHg and 24-h diastolic BP greater than or equal to 80 mmHg. Compared with the NT group, the MHT group had higher prevalences of men, metabolic syndrome, and high-normal office BP (Tables 1 and 2). Furthermore, compared with NT patients, PWV was higher among patients with MHT whereas carotid IMT and the prevalence of carotid plaque did not differ (Table 1 and Fig. 1). The MHT group had a higher fasting C-peptide than the NT group, although fasting serum glucose did not differ (Table 3). Compared with patients with SHT, patients with MHT were younger and had lower prevalences of metabolic syndrome, diabetes, and lower glucosylated hemoglobin A1c (Tables 1 and 3), as well as lower prevalences of arterial organ damage measured by high PWV, increased cIMT, and carotid plaque (Fig. 1).
Table 1.
Clinical characteristics in groups with normotensive, masked hypertension, and sustained hypertensive
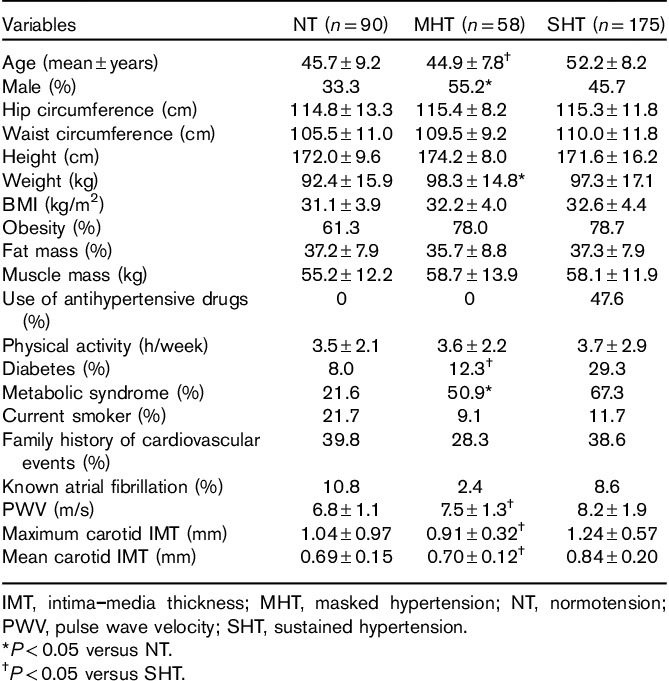
Table 2.
Office and ambulatory blood pressure in groups with normotension, masked hypertension, and sustained hypertension
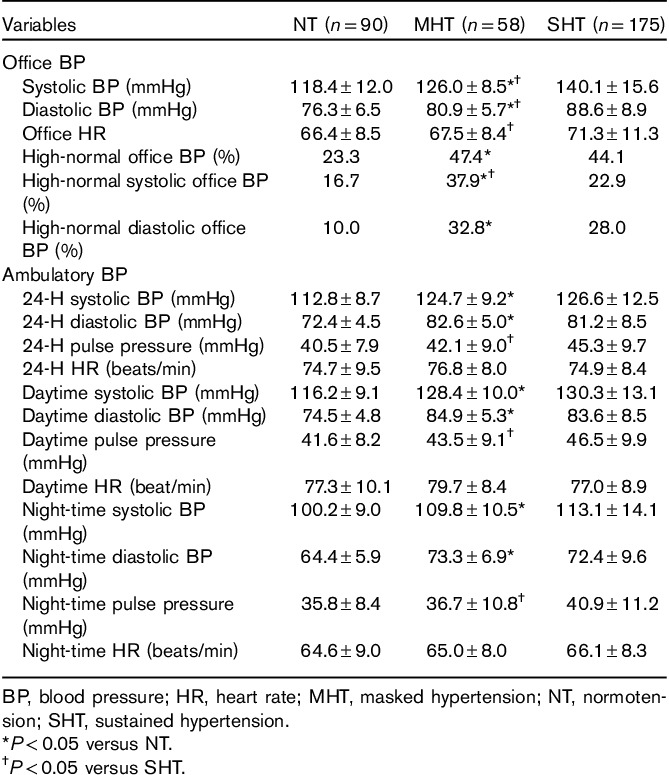
Fig. 1.
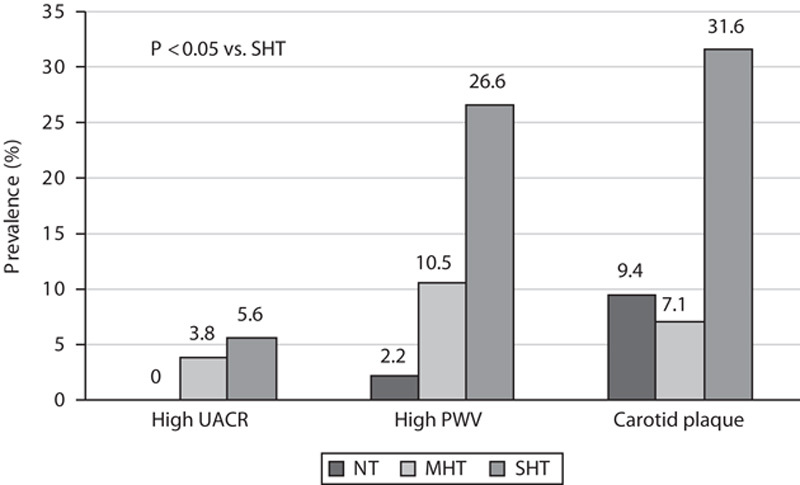
Prevalences of subclinical arterial disease in masked hypertension (MHT) compared with normotensive (NT) and sustained hypertensive (SHT) groups. PWV, pulse wave velocity; UACR, urine albumin–creatinine ratio.
Table 3.
Biochemical characteristics in groups with normotension, masked hypertension, and sustained hypertension
Covariables of masked hypertension
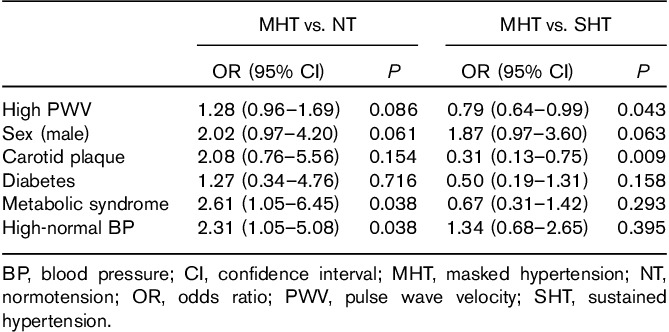
In univariable logistic regression analyses including NT and MHT patients, male sex, presence of metabolic syndrome, high-normal office BP, and high PWV were associated with a higher risk for the presence of MHT (all P<0.05) (Table 4). In univariable logistic regression analyses including MHT and SHT patients, MHT was associated with younger age, lower prevalence of metabolic syndrome and diabetes, and lower serum hemoglobin A1c and fasting glucose, as well as lower prevalence of established arterial damage measured by high PWV and carotid plaque (all P<0.05) (Table 4). In multinomial logistic regression analysis, MHT was associated with the presence of metabolic syndrome and high-normal office BP (both P<0.05) compared with NT, independent of sex, diabetes, PWV, and carotid plaque (Table 5). Compared with patients with SHT, MHT was associated with lower PWV and lower prevalence of carotid plaque (both P<0.05) when adjusted for sex, diabetes, metabolic syndrome, and presence of high-normal office BP (Table 5).
Table 4.
Significant covariates of masked hypertension compared with normotension and sustained hypertension patients in univariable logistic regression analyses
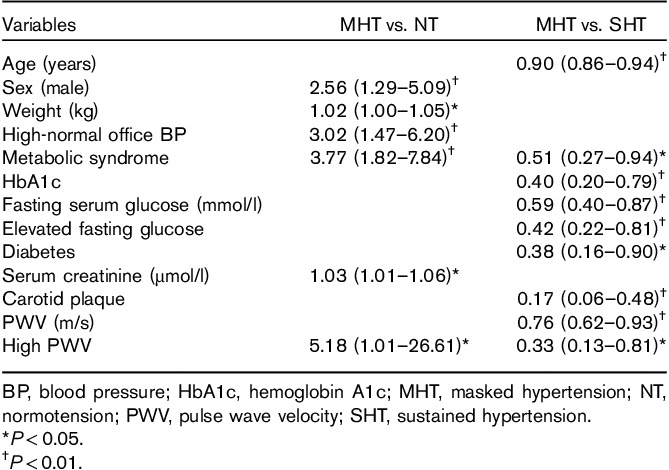
Table 5.
Covariates of masked hypertension compared with normotension and sustained hypertension patients in multivariable multinomial logistic regression analysis
Discussion
The present study in overweight and obese patients without known cardiovascular disease shows that MHT was common, found in 17.1% in the overall study population and in 39.2% among patients with normal office BP. Compared with NT patients, MHT was particularly associated with the presence of the metabolic syndrome and high-normal office BP. Compared with SHT patients, MHT patients had less established atherosclerosis identified from high aortic stiffness (PWV>10 m/s) or the presence of carotid plaque.
Previous studies have reported varying prevalences of MHT: from 8.5 to 17% in general populations 1,22. The present study found a prevalence of MHT comparable to the upper range of that reported from general populations 3. Treatment of hypertension is documented to effectively reduce subclinical target organ damage and associated cardiovascular event risk 23. The higher prevalence of subclinical arterial damage reported previously in MHT patients in some studies is probably related to the delayed diagnosis of hypertension in these patients 24. Other studies have also shown the association of high-normal office BP with MHT 3,25, suggesting that MHT may represent a missed diagnosis of hypertension because of normal fluctuations in office BP. A longitudinal study of Norwegian men with high-normal office BP at screening for military service showed increased incidence of MHT in these patients when re-examined 17 years later 26, in line with our findings.
MHT has been associated with clustering of cardiovascular risk factors and abnormal carbohydrate metabolism in previous population-based studies 5,6,27–32. Our findings in an overweight and obese cohort add to this knowledge by showing that specific clinical characteristics, including the presence of metabolic syndrome and high-normal office BP, may help to identify patients with MHT at a time when clinical atherosclerosis is still not detectable by cIMT and PWV.
In the present study, MHT patients had higher fasting C-peptide compared with NT, reflecting higher endogenous insulin production 33, whereas the prevalence of diabetes did not differ between MHT and NT groups. Some previous studies have shown a gradual increase in HOMA-IR from NT to MHT and SHT 29,31, but HOMA-IR did not differ between the BP categories in the present study. In the prospective Pressione Arteriose Monitorate E Loro Associazioni study, a higher incidence of diabetes was found in MHT patients compared with NT patients during 10 years of follow-up 5. Subclinical arterial damage has been reported to be equally common in MHT and SHT patients 34. As shown in multivariable analysis, MHT was associated with nominally higher PWV when adjusted for the presence of diabetes and metabolic syndrome, possibly reflecting early arterial dysfunction. In contrast, we found established atherosclerosis to be significantly less common in the MHT than the SHT group, whether measured as increased aortic stiffness by carotid–femoral PWV or by carotid plaques from ultrasound. MHT has been suggested as a precursor of SHT 6, and hence may represent an earlier stage of a progressive disease, which in turn may explain the lower prevalence of atherosclerosis found in patients with MHT in the present study.
Study limitations
Our study has several potential limitations. Although the standardized questionnaire to gather self-reported health information was quality assured by the general practitioner, health problems may have been under-reported. There is a possibility of volunteer bias as the patients included in the FATCOR study were recruited at a medical center specialized in the management of overweight and obese patients, probably recruiting patients more concerned about their health than unselected overweight and obese patients. However, more than 50% of SHT was diagnosed through study participation, as was more than 20% of diabetes in the total study population, pointing to the value of thorough cardiovascular risk factor assessment in obese patients. The FATCOR study included overweight and obese patients aged 30–65 years without known cardiovascular disease and generalization of results to other populations should be done with caution. Finally, because of the cross-sectional design, it is not possible to infer causality of the associations discovered in this study.
Conclusion
MHT was found in 17.1% of overweight and obese patients without cardiovascular disease participating in the FATCOR study, and in 39.2% of patients with normal office BP, pointing to the vast potential for a more timely diagnosis of hypertension in such patients if ambulatory BP recording is used more widely. Compared with NT patients, MHT was particularly common in patients with high-normal office BP or metabolic syndrome. Compared with SHT patients, MHT had significantly lower prevalence of subclinical arterial damage.
Acknowledgements
The authors thank the West-Norwegian Regional Health Authority for the financial support and Simon Fougner Hartmanns Family Fund, Oslo, Norway, for donating the SphygmoCor device. They also thank Marina V. Kokorina, MD, and study nurses Synnøve Ygre Hauge, RN, and Liv Himle, RN, for technical assistance with data collection, registration, and patient management.
The study was funded by grants from the Western Norway Regional Health Authorities.
Clinical trial registration NCT02805478 (http://www.clinicaltrials.gov).
Conflicts of interest
There are no conflicts of interest.
References
- 1.Fagard RH, Cornelissen VA. Incidence of cardiovascular events in white-coat, masked and sustained hypertension versus true normotension: a meta-analysis. J Hypertens 2007; 25:2193–2198. [DOI] [PubMed] [Google Scholar]
- 2.Pierdomenico SD, Cuccurullo F. Prognostic value of white-coat and masked hypertension diagnosed by ambulatory monitoring in initially untreated subjects: an updated meta analysis. Am J Hypertens 2011; 24:52–58. [DOI] [PubMed] [Google Scholar]
- 3.Bobrie G, Clerson P, Menard J, Postel-Vinay N, Chatellier G, Plouin PF. Masked hypertension: a systematic review. J Hypertens 2008; 26:1715–1725. [DOI] [PubMed] [Google Scholar]
- 4.Sega R, Trocino G, Lanzarotti A, Carugo S, Cesana G, Schiavina R, et al. Alterations of cardiac structure in patients with isolated office, ambulatory, or home hypertension: data from the general population (Pressione Arteriose Monitorate E Loro Associazioni [PAMELA] Study). Circulation 2001; 104:1385–1392. [DOI] [PubMed] [Google Scholar]
- 5.Mancia G, Bombelli M, Facchetti R, Madotto F, Quarti-Trevano F, Grassi G, Sega R. Increased long-term risk of new-onset diabetes mellitus in white-coat and masked hypertension. J Hypertens 2009; 27:1672–1678. [DOI] [PubMed] [Google Scholar]
- 6.Mancia G, Bombelli M, Facchetti R, Madotto F, Quarti-Trevano F, Polo Friz H, et al. Long-term risk of sustained hypertension in white-coat or masked hypertension. Hypertension 2009; 54:226–232. [DOI] [PubMed] [Google Scholar]
- 7.Lurbe E, Torro I, Alvarez V, Nawrot T, Paya R, Redon J, et al. Prevalence, persistence, and clinical significance of masked hypertension in youth. Hypertension 2005; 45:493–498. [DOI] [PubMed] [Google Scholar]
- 8.Ogedegbe G, Agyemang C, Ravenell JE. Masked hypertension: evidence of the need to treat. Curr Hypertens Rep 2010; 12:349–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saeed S, Waje-Andreassen U, Fromm A, Øygarden H, Naess H, Gerdts E. Prevalence and covariates of masked hypertension in ischemic stroke survivors: the Norwegian Stroke in the Young Study. Blood Press Monit 2016; 21:244–250. [DOI] [PubMed] [Google Scholar]
- 10.Egan B, Grassi G. Sympathetic activation and endothelial dysfunction as therapeutic targets in obesity-related hypertension. J Hypertens 2013; 31:259–260. [DOI] [PubMed] [Google Scholar]
- 11.Vykoukal D, Davies MG. Vascular biology of metabolic syndrome. J Vasc Surg 2011; 54:819–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Strandheim A, Halland H, Saeed S, Cramariuc D, Hetland T, Lønnebakken MT, et al. Obesity-associated metabolic changes influence resting and peak heart rate in women and men. Scand Cardiovasc J 2015; 49:337–343. [DOI] [PubMed] [Google Scholar]
- 13.Bratberg JA, Bulut E, Rieck AE, Lønnebakken MT, Hetland T, Gerdts E. Determinants of systolic blood pressure response during exercise in overweight subjects. Blood Press 2014; 23:200–205. [DOI] [PubMed] [Google Scholar]
- 14.Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Böhm M, et al. 2013ESH/ESC guidelines for the management of arterial hypertension. Blood Press 2013; 22:193–278. [DOI] [PubMed] [Google Scholar]
- 15.World Health Oraganization. Obesity and overweight, fact sheet N°311; 2016. Available at: http://www.who.int/mediacentre/factsheets/fs311/en. [Accessed June 2016].
- 16.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005; 112:2735–2752. [DOI] [PubMed] [Google Scholar]
- 17.Genuth S, Alberti KG, Bennett P, Buse J, Defronzo R, Kahn R, et al. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care 2003; 26:3160–3167. [DOI] [PubMed] [Google Scholar]
- 18.Touboul PJ, Hennerici MG, Meairs S, Adams H, Amarenco P, Desvarieux M, et al. Mannheim intima–media thickness consensus. Cerebrovasc Dis 2004; 18:346–349. [DOI] [PubMed] [Google Scholar]
- 19.Van Bortel LM, Duprez D, Starsmans-Kool MJ, Safar ME, Giannattasio C, Cockcroft J, et al. Clinical applications of arterial stiffness, task force III: recommendations for user procedures. Am J Hypertens 2002; 15:445–452. [DOI] [PubMed] [Google Scholar]
- 20.Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J 2006; 27:2588–2605. [DOI] [PubMed] [Google Scholar]
- 21.Van Bortel LM, Laurent S, Boutouyrie P, Chowienczyk P, Cruickshank JK, De Backer T, et al. Expert consensus document on the measurement of aortic stiffness in daily practice using carotid-femoral pulse wave velocity. J Hypertens 2012; 30:445–448. [DOI] [PubMed] [Google Scholar]
- 22.Peacock J, Diaz KM, Viera AJ, Schwartz JE, Shimbo D. Unmasking masked hypertension: prevalence, clinical implications, diagnosis, correlates and future directions. J Hum Hypertens 2014; 28:521–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Devereux RB, Wachtell K, Gerdts E, Boman K, Nieminen MS, Papademetriou V, et al. Prognostic significance of left ventricular mass change during treatment of hypertension. JAMA 2004; 292:2350–2356. [DOI] [PubMed] [Google Scholar]
- 24.Lovibond K, Jowett S, Barton P, Caulfield M, Heneghan C, Hobbs FD, et al. Cost-effectiveness of options for the diagnosis of high blood pressure in primary care: a modelling study. Lancet 2011; 378:1219–1230. [DOI] [PubMed] [Google Scholar]
- 25.Viera AJ, Hinderliter AL, Kshirsagar AV, Fine J, Dominik R. Reproducibility of masked hypertension in adults with untreated borderline office blood pressure: comparison of ambulatory and home monitoring. Am J Hypertens 2010; 23:1190–1197. [DOI] [PubMed] [Google Scholar]
- 26.Skårn SN, Flaa A, Kjeldsen SE, Rostrup M, Brunborg C, Reims HM, et al. High screening blood pressure at young age predicts future masked hypertension: a 17 year follow-up study. Blood Press 2015; 24:131–138. [DOI] [PubMed] [Google Scholar]
- 27.Hänninen MR, Niiranen TJ, Puukka PJ, Jula AM. Comparison of home and ambulatory blood pressure measurement in the diagnosis of masked hypertension. J Hypertens 2010; 28:709–714. [DOI] [PubMed] [Google Scholar]
- 28.Hänninen MR, Niiranen TJ, Puukka PJ, Mattila AK, Jula AM. Determinants of masked hypertension in the general population: the Finn-Home study. J Hypertens 2011; 29:1880–1888. [DOI] [PubMed] [Google Scholar]
- 29.Bjorklund K, Lind L, Zethelius B, Andren B, Lithell H. Isolated ambulatory hypertension predicts cardiovascular morbidity in elderly men. Circulation 2003; 107:1297–1302. [DOI] [PubMed] [Google Scholar]
- 30.Hansen TW, Jeppesen J, Rasmussen S, Ibsen H, Torp-Pedersen C. Ambulatory blood pressure monitoring and risk of cardiovascular disease: a population based study. Am J Hypertens 2006; 19:243–250. [DOI] [PubMed] [Google Scholar]
- 31.Hänninen MR, Niiranen TJ, Puukka PJ, Jula AM. Metabolic risk factors and masked hypertension in the general population: the Finn-Home study. J Hum Hypertens 2014; 28:421–426. [DOI] [PubMed] [Google Scholar]
- 32.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002; 106:3143–3421. [PubMed] [Google Scholar]
- 33.Hirai FE, Moss SE, Klein BE, Klein R. Relationship of glycemic control, exogenous insulin, and C-peptide levels to ischemic heart disease mortality over a 16-year period in people with older-onset diabetes: the Wisconsin Epidemiologic Study of Diabetic Retinopathy (WESDR). Diabetes Care 2008; 31:493–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Angeli F, Reboldi G, Verdecchia P. Masked hypertension: evaluation, prognosis, and treatment. Am J Hypertens 2010; 23:941–948. [DOI] [PubMed] [Google Scholar]


