Abstract
Purpose
Our previous work has shown low serum 25-hydroxyvitamin D concentrations in association with aggressive breast cancer subtypes. Vitamin D receptor (VDR) is central for vitamin D-mediated transcription regulation. Few studies have examined breast VDR expression with tumor characteristics or patient survival.
Experimental Design
VDR expression in breast tumor tissue microarrays was determined by immunohistochemistry in 1,114 female patients as low, moderate and strong expression based on an immunoreactive score, and examined with histopathological tumor characteristics and survival outcomes including progression free survival, breast cancer specific survival, and overall survival.
Results
A majority (58%) of breast tumors showed moderate or strong VDR expression. VDR expression was inversely related to aggressive tumor characteristics, including large tumor size, hormonal receptor (HR) negativity, and triple-negative subtype (p<0.05). In addition, VDR expression was also inversely related to Ki-67 expression among patients older than 50 years. Nevertheless, VDR expression was not associated with any patient survival outcomes examined.
Conclusions
In a large patient population, VDR expression is inversely associated with more aggressive breast cancer, but not with breast cancer survival outcomes. The present findings of VDR expression are consistent with our previous results of circulating vitamin D biomarkers, which provide two converging lines of evidence supporting the putative benefits of vitamin D against aggressive breast cancer. Because of the observational nature of our analyses, future studies are warranted to establish the causality of the reported associations.
INTRODUCTION
Vitamin D receptor (VDR) is a ligand-dependent transcription factor in a nuclear receptor superfamily (1). When it binds to its ligand, calcitriol (1α, 25(OH)2-dihydroxyvitamin D), VDR translocates into the nucleus and binds to vitamin D response element (VDRE), activating the transcription of targeted genes. Activated VDR regulates numerous genes involved in a myriad of cellular functions and processes and impaired vitamin D activities have been widely implicated in human cancer (2, 3). In 1979, VDR was first identified in a breast cancer cell line (4, 5), and was later identified in many breast cancer cell lines and a majority of human breast tumor tissues examined (6-12). Experimental studies on VDR knockout mice showed higher rates of pre-neoplastic mammary lesions (13), and treatment with a vitamin D analogue prevented the development of carcinogen-induced mammary tumors (14), by mechanisms including inhibition of cellular proliferation, promoting differentiation and inducing apoptosis (15).
A growing body of epidemiological evidence suggests an inverse association between vitamin D levels and breast cancer risk, yet the results are still mixed (16-18). Our previous study has shown that higher serum concentrations of 25-hydroxyvitamin D (25OHD) were associated with reduced risk of breast cancer subtypes of poor prognosis, including estrogen receptor (ER) negative and triple-negative breast cancer (TNBC), in premenopausal women (19), highlighting the importance of considering tumor heterogeneity in epidemiological studies. In contrast to numerous studies on circulating vitamin D biomarkers and breast cancer, there are few studies on VDR expression in breast tumor tissues. In an earlier study of 228 breast cancer patients, VDR expression was not associated with any histopathological indicators, such as ER and Ki-67 status (20). Moreover, previous studies have shown VDR expression as a prognostic marker for prostate and lung cancer (21, 22), which has remained understudied in breast cancer. Recently, a small study based on 82 breast cancer patients found that VDR expression was strongly associated with better prognosis (23). Due to the small sample size of this study, further investigation of the clinical significance of VDR expression in breast cancer is warranted.
The present study aims to perform a comprehensive investigation of the association of tumor VDR expression with cancer clinical characteristics and survival outcomes based on a large breast cancer patient population.
PATIENTS AND METHODS
Patient population
Women included in the study were those who were diagnosed with invasive breast cancer at Roswell Park Cancer Institute (RPCI) from 1989-2011 and had breast tumor tissues collected by RPCI Pathology Research Network (PRN). From these tumors, tissue microarrays (TMAs) were constructed, with at least three 0.6 mm cores from each donor tumor block taken randomly from areas pre-selected by a pathologist after reviewing hematoxylin and eosin slides sectioned from the same block. The cores were then transferred to a recipient block typically holding cores from 40 patients. Initially, 32 TMAs encompassing 4,576 cores from 1,149 patients were included in the analysis. This study was approved by RPCI Institutional Review Board for human subject protection.
Immunohistochemistry
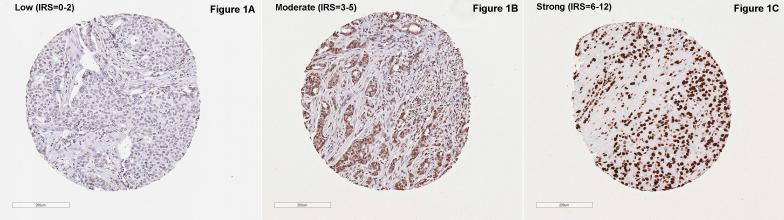
From each TMA block, a 5-micron section was cut and used for immunohistochemical (IHC) staining for VDR with a validated monoclonal antibody 9A7 (ThermoFisher catalog # MA1-710, Waltham, MA) and a Dako automated slide stainer following standardized protocols established by PRN. The antibody showed an exclusive nuclear staining without cytoplasmic reactivity in breast tissues (Figure 1), which is consistent with the literature (10, 24). After staining, whole-slide digital images were captured by the Aperio ScanScope CS Slide Scanner, and a computer-assisted image analysis algorithm optimized for the VDR antibody was used for automated quantitative assessment of staining intensity and percent of positive staining area. Any tumor with a total nuclei count less than 15 was excluded (n=18) from the analysis. An immunoreactive score (IRS) was computed as the product of intensity score (0-3) and percent of positive nuclei score (0-4) for each core and scores across multiple cores of each tumor block were compiled into a final score by the average score weighted by the total count of nuclei of each core. The resultant score thus ranged from 0-12. Based on the distribution of the IRS, VDR expression was classified into three levels with largely similar number of cases in each level: low (0-2), moderate (3-5), and strong (6-12) (see Figure 1 for representative stains).
Figure 1.
Representative immages of vitamin D receptor (VDR) protein expression in selected malignant breast tissue microarray cores, classified by immunoreactive score (IRS).
Clinical data
Patient demographic data, including age at diagnosis, race/ethnicity and family history of breast cancer, as well as tumor pathological data, including tumor size (T), lymph nodal metastasis (N), ER, PR, and Her2/neu status were obtained from RPCI Clinical Data Network (CDN). Because tumor staging criteria underwent several updates during the time span of tumor tissue procurement, we elected not to include tumor stage in the analysis, to avoid misclassification, but instead focus on tumor size and nodal status separately. Clinical subtypes were defined based on ER, PR, and Her2/neu status as the following: luminal A (ER+ and/or PR+, and Her2−); luminal B (ER+ and/or PR+, and Her2+); Her2-enriched (ER−, PR−, and Her2+); and triple-negative (ER−, PR−, and Her2−). Patients without clinical data from CDN were excluded from the analysis (n=17), resulting in a total of 1,114 patients included in the final analysis. In addition, as a part of two previous studies, data on tumor Ki-67 expression and serum 25OHD concentrations at the time of breast cancer diagnosis were available from 699 and 247 patients in this study, respectively. Data for patient survival outcomes, including recurrence, second primary cancer, all-cause mortality, and breast cancer-specific mortality, were obtained from cancer registry and NCCN database maintained by the RPCI Breast Program conducting regular patient follow-up.
Statistical analysis
Patient population characteristics were summarized using descriptive statistics. For associations of VDR expression (low, moderate and high) with demographic and tumor characteristics, univariate analysis was first conducted using chi-square test, followed by logistic regression, modeling the probability of high aggressive vs. low aggressive characteristic (e.g., larger tumor vs. small tumor; TNBC vs. luminal A), with adjustment for age at diagnosis. The analyses were further stratified by age group (≤50 years and >50 years) as a proxy of menopausal status. For survival analysis, three endpoints were assessed, including overall survival (OS), progression-free survival (PFS) (recurrence, second primary cancer, or death due to any cause), and breast cancer-specific survival (BCSS). For each endpoint, follow-up time began at the time of diagnosis and ended at the time of an event of interest or the date of last contact (censored). Kaplan-Meier survival curves by VDR expression levels were plotted with p-values derived from log-rank test. Cox proportional hazards models were used to derive hazards ratios (HRs) and 95% confidence intervals (CIs) associated with VDR expression, controlling for age at diagnosis. Subgroup analyses were performed by stratification on selected demographic and clinical characteristics. All statistical analyses were performed in SAS 9.4 with two-sided type I error rate of 0.05.
RESULTS
The demographic and clinical characteristics of the 1,114 breast cancer patients included in the final analysis are summarized in Table 1. The majority of patients were older than 50 years (65%) and Caucasian (86%). More than half of the tumors (55%) were T1 and node negative (54%). For clinical subtypes, 56% of the tumors were classified as luminal A, 16% luminal B, 7% Her2-enriched, and 21% TNBC. Among a subset of the patients with Ki-67 data (n=699), most (67%) had low expression.
Table 1.
Descriptive characteristics of the patient population (n=1,114)
| Variable | N | Percent (%) |
|---|---|---|
| Age at diagnosis | ||
| ≤ 50 years | 388 | 34.8 |
| >50 years | 726 | 65.2 |
| Race | ||
| Caucasian | 962 | 86.4 |
| African American | 135 | 12.1 |
| Other | 17 | 1.5 |
| Tumor size | ||
| T1 (≤2 cm) | 609 | 55.7 |
| T2 (2-5 cm) | 411 | 37.6 |
| T3 (>5 cm) | 73 | 6.7 |
| Lymph node | ||
| N0 (0 positive nodes) | 570 | 54 |
| N1 (1-3 positive nodes) | 293 | 27.7 |
| N2 (4-9 positive nodes) | 118 | 11.2 |
| N3 (≥10 positive nodes) | 75 | 7.1 |
| ER | ||
| Negative | 321 | 28.9 |
| Positive | 790 | 71.1 |
| PR | ||
| Negative | 480 | 43.2 |
| Positive | 631 | 56.8 |
| Her2/neu | ||
| Negative | 842 | 76.3 |
| Strong | 217 | 19.7 |
| Weak | 44 | 4 |
| Clinical subtype | ||
| Luminal A | 615 | 55.9 |
| Luminal B | 180 | 16.3 |
| Her2 enriched | 80 | 7.3 |
| Triple negative | 226 | 20.5 |
| Ki-67 | ||
| Low (<15%) | 467 | 66.8 |
| Moderate (16-30%) | 121 | 17.3 |
| High (>30%) | 111 | 15.9 |
| VDR expression by IHC | ||
| Low (IRS=0-2) | 477 | 42.8 |
| Moderate (IRS=3-5) | 354 | 31.8 |
| Strong (IRS=6-12) | 283 | 25.4 |
Footnote: For some variables, the total number does not add up to the total of 1,114 due to missing data.
Abbreviation: IRS, immunoereactive score; ER, estrogen receptor; PR, progesterone receptor; Her2, human epidermal growth factor 2.
Based on the IRS classification, VDR was not or expressed at low level in 42% of tumors (n=477), moderately expressed in 32% (n=354), and strongly expressed in the other 25% (n=283). There was no difference in VDR expression between Caucasian American and African American patients (p=0.80); however, older patients tended to have stronger VDR expression than younger patients (p=0.05). In a subset of patients (n=247) with serum 25OHD concentrations at the time of diagnosis, no association between 25OHD concentrations and VDR expression was found (p=0.97).
Table 2 summaries VDR expression by tumor characteristics. Except for nodal status and Her2/neu status, significant differences in VDR expression were found. VDR expression was significantly lower in tumors with more aggressive characteristics (larger tumors, ER-negative, PR-negative and TNBC) than in tumors with better prognosis characteristics. In addition, VDR expression appeared to be inversely related to Ki-67 expression (p=0.02). In logistic regression analyses these associations remained after adjustment for age (Supplementary Table 1). For the most part, associations were similar between younger (≤50 years) and older (>50 years) patients after stratification by age, except that Ki-67 were inversely related to VDR expression only among older patients (p<0.05).
Table 2.
Vitamin D receptor (VDR) expression in breast tumors by histopathological characterstics
| Prognostic characteristics | VDR expression |
P-value | ||
|---|---|---|---|---|
| Low (IRS=0-2) | Moderate (IRS=3-5) | Strong (IRS=6-12) | ||
| Tumor size | 0.001 | |||
| ≤2 cm | 233 (38) | 201 (33) | 175 (29) | |
| > 2 cm | 237 (49) | 147 (30) | 100 (21) | |
| # positive lymph node | 0.59 | |||
| 0 | 240 (42) | 192 (34) | 138 (24) | |
| 1+ | 217 (45) | 150 (31) | 119 (24) | |
| ER status | <0.0001 | |||
| Negative | 184 (57) | 88 (27) | 49 (15) | |
| Positive | 292 (37) | 265 (34) | 233 (29) | |
| PR status | <0.0001 | |||
| Negative | 243 (51) | 138 (29) | 99 (21) | |
| Positive | 233 (37) | 215 (34) | 183 (29) | |
| Her2 status | 0.66 | |||
| Negative | 369(44) | 267(32) | 206(24) | |
| Positive | 106(41) | 87(33) | 68(26) | |
| Tumor subtype | <0.0001 | |||
| Luminal A | 43 (53.75) | 20 (25) | 17 (21.25) | |
| Luminal B | 236 (38.37) | 202 (32.85) | 177 (28.78) | |
| Her2 expressing | 63 (35) | 66 (36.67) | 51 (28.33) | |
| Triple negative | 132 (58.41) | 65 (28.76) | 29 (12.83) | |
| Ki-67 status | 0.02 | |||
| ≤15% | 187 (40) | 130 (28) | 150 (32) | |
| 16%-30% | 57 (47) | 35 (29) | 29 (24) | |
| >30% | 50 (45) | 41 (37) | 20 (18) | |
Footnote: Count (row percentage) is presented for VDR expression by tumor histopathological characteristics.
Abbreviation: IRS, immunoereactive score; ER, estrogen receptor; PR, progesterone receptor; Her2, human epidermal growth factor 2.
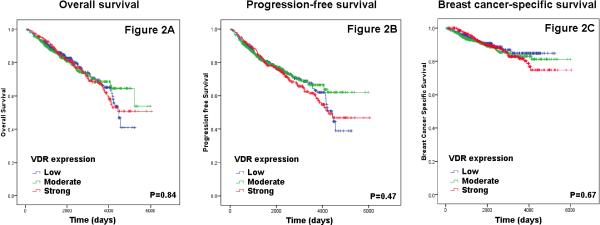
The median follow-up time was 72 months (range 3-201 months), during which time 271 death, including 130 breast cancer-specific death, and 311 disease-free events occurred. There were no differences in patients’ survival outcomes, including OS, PFS, or BCSS, by tumor VDR expression as shown by Kaplan-Meier curves in Figure 2. In Cox proportional hazards model with adjustment for age, no significant associations were found (Table 3). In further subgroup analyses by age, tumor size, nodal status, ER, PR, Her2/neu, clinical subtype, and Ki-67 expression, no associations were found (data not shown).
Figure 2.
Kaplan-Meier survival curves by vitamin D receptor (VDR) expression levels
Table 3.
Cox proportional hazards regression analysis of patient survival outcomes by tumor vitamin D receptor (VDR) expression
| VDR expression | #event/total | Unadjusted model |
Age-adjusted model |
||
|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | ||
| Overall survival | |||||
| Low | 104/474 | 1.00 | 0.8 | 1.00 | 0.9 |
| Moderate | 84/353 | 1.05 (0.79-1.41) | 1.02 (0.77-1.36) | ||
| Strong | 82/282 | 1.09 (0.82-1.46) | 1.04 (0.78-1.39) | ||
|
Progression-free survival | |||||
| Low | 117/474 | 1.00 | 0.5 | 1.00 | 0.6 |
| Moderate | 99/353 | 1.12 (0.85-1.46) | 1.09 (0.84-1.43) | ||
| Strong | 95/282 | 1.18 (0.90-1.55) | 1.14 (0.87-1.50) | ||
|
Breast cancer specific survival | |||||
| Low | 53/474 | 1.00 | 0.7 | 1.00 | 0.7 |
| Moderate | 35/353 | 0.85 (0.56-1.31) | 0.87 (0.57-1.33) | ||
| Strong | 39/282 | 1.04 (0.69-1.57) | 1.06 (0.70-1.61) | ||
Abbreviation: HR hazards ratio; CI, confidence interval.
DISCUSSION
In this large sample of 1,114 breast cancer patients, we found that low VDR expression in tumor tissues was associated with more aggressive characteristics, specifically large tumor size, ER and PR negativity, TNBC, and high Ki-67 expression. No associations of VDR expression with lymph node involvement or Her2 status was found. Despite the strong associations with tumor histopathological features that are known to be prognostic, breast tumor VDR expression was not associated with patient survival outcomes after a median of 6 years follow up in our patient population.
The finding of an inverse association between VDR expression and tumor aggressiveness suggests that VDR may be a target subjected to down-regulation or ablation along the breast cancer progression cascade into more aggressive stages. In an earlier study by Lopes et al, the IHC expression of VDR and two metabolizing enzymes, CYP27B1 and CYP24A1, were examined in a full spectrum of breast tissues ranging from normal tissue, to benign lesions, ductal carcinoma in situ (DCIS) and invasive tumors (10). It was found that VDR expression was significantly lower in DCIS and invasive tumor than in normal or benign tissues, and further, VDR expression was correlated with tumor ER expression, which is consistent with our findings. In addition to decreased expression of VDR in tumor tissue, VDR activity may also be subjected to negative regulation. In the same study, it was also found that in contrast to a decreasing trend of VDR expression, vitamin D catabolizing enzyme CYP24A1 was increased in tumor tissues. Similar findings of a deregulated vitamin D signaling pathway favoring lower VDR activity were also reported in other studies (25, 26). The degraded VDR expression and activity may be a common molecular alteration that has been found in multiple tumor types, such as breast, prostate and colon cancer (27-29). For example, in colon cancer cells, VDR gene expression was suppressed by elevated expression of SNAIL transcription factor, which blocked cancer cell response to vitamin D treatment (30).
However, caution should be taken when interpreting the loss of VDR as a driving mechanism responsible for the more aggressive breast cancer phenotypes. It is possible that the alteration in VDR expression or activity may only be a “passenger” event occurring along with the loss of other cell differentiation markers with no obviously meaningful consequences. Interestingly in a 2014 study by Santagata et al, it was found that a new breast cancer subtyping schema based on ER, androgen receptor (AR) and VDR provided a much stronger prognostication than the commonly used schema by ER, PR and Her2 (31). This suggests that VDR may not simply be tracking with ER in tumor tissues as a differentiation marker, but complex interplays may exist among these hormonal receptors and influence the development and progression of breast cancer cells. Estrogen induces ERK 1/2 activation and transcriptional activity, which results in up-regulation of VDR gene expression (32). In the study by Santagata et al, the majority (93%) of ER+ tumors were also VDR+ (31). Nevertheless, in ER+ breast cancer cells, calcitriol significantly reduced ER expression and inhibited estrogen stimulation of cell proliferation (33, 34). Thus, in breast cancer cells, ER tends to co-express with VDR, the latter, upon activation by its ligand, suppresses ER in a negative feedback loop. This suggests that active vitamin D signaling activity may synergize with anti-estrogen agents in treating ER+ breast cancer. Indeed, it has been reported that vitamin D enhances the apoptotic effect of Tamoxifen in MCF-7 breast cancer cells (35) and in mice models (36). Apparently, further studies are warranted to elucidate the molecular mechanisms underlying the deregulation of VDR in breast tumors and to establish the causality between VDR loss and tumor progression in a prospective manner.
We previously reported inverse associations of serum 25OHD concentrations with risk of aggressive breast cancer, including higher stage, ER-negative and TNBC (19). The present study of tumor VDR expression now corroborates with the findings from our previous study of serum 25OHD. Although both studies are of observatory nature and we cannot exclude the possibility of a reverse causality, these two converging lines of evidence from circulating biomarker and tumor tissue may not simply be coincidence, as they are endorsed by a variety of anti-cancer activities of vitamin D demonstrated in a rich body of literature from experimental studies. For example, cell culture and animal studies have well established that vitamin D has anti-proliferation and pro-differentiation properties (2, 3), which may well explain our findings of low VDR expression in tumors of large size and high Ki-67 expression. In a randomized clinical trial, vitamin D3 supplementation raised calcitriol concentrations in prostate tissue, which was correlated with lower levels of Ki-67 expression in prostate cancer tissues (37). Although similar data from breast cancer randomized trials are currently lacking, a recent study examining hormonal receptor markers at the single cell level showed that most proliferating breast cancer cells positive for Ki-67 were VDR negative (31). In addition to anti-proliferation effects, vitamin D has also been shown to have anti-oxidative stress, anti-invasion and anti-angiogenesis activities, and most recently also anti-metastasis by targeting the tumor progression gene inhibition of differentiation 1 (ID1) (38). These versatile anti-tumor activities of vitamin D support the findings from our and others’ observational studies and argue against the possibility of reverse causality. Nevertheless, a definitive conclusion awaits from future prospective intervention trials.
Our data also showed significantly lower VDR expression in TNBC compared to luminal A subtype. We know of no other studies in the literature examining VDR expression with the TNBC phenotype. The finding is consistent with our data from serum vitamin D biomarker that 25OHD concentrations were the lowest in premenopausal patients with TNBC compared with other subtypes (19). Experimental studies suggest that vitamin D may modulate tumor microenvironment by regulating genes involved in extracellular remodeling and epithelialmesenchymal transition (EMT) and thus alter breast tumor phenotypes, including TNBC (39, 40). Intriguingly, in a study of mammospheres which were enriched with mammary cancer stem cells (MCSCs), VDR expression was significantly down-regulated in mammospheres, MCSCs, and triple-negative cancer cells (41). When VDR was over expressed, the ability of cells to form mammospheres was compromised. TNBC is currently the most difficult to treat breast cancer subgroup. Our findings imply that vitamin D might have cancer preventive benefits against the occurrence of TNBC and VDR may be a therapeutic target for treatment. A recent study in TNBC cell lines provides support for the latter hypothesis (42).
Because VDR expression was inversely related to poor breast cancer aggressive characteristics in our study, it is unexpected to find no associations of VDR expression with patient survival outcomes. To understand this discordance, we first examined tumor aggressive characteristics, including tumor size, lymph node, ER, PR, Her2, IHC subtype, and Ki-67, with patient survival outcomes, and all the results except for Ki-67 were significant and the hazards ratios were in the expected directions. This affirms the validity of the survival outcome data. We then included VDR variable as an additional variable in the Cox models already containing each of the tumor aggressive characteristics. The associations with these characteristics were not changed, while there was still no significant association with VDR levels in any of the models. This suggests that, although VDR levels were correlated with many of the tumor aggressive characteristics, the associations may not be strong enough to subsequently impact patient survival. In fact, VDR levels were not associated with all of the aggressive characteristics, particularly not with the number of positive lymph node, which is known to be a very strong prognostic factor. To test whether VDR levels were associated with survival outcomes only within certain cancer subgroups, we performed a number of stratified analyses by age group and tumor characteristics. Although the sample size became rather limited in some strata, we still did not see any trends of associations. Alternatively, it is possible that the associations of VDR levels with survival outcomes were modified by circulating 25D levels. We thus did some exploratory analyses using the small number of patients (n=247) in this study who had both VDR staining and 25D levels data. There was no correlation between VDR levels and 25D levels. There were also no significant associations of 25D tertiles with survival; but when stratified by a binary 25D levels (high and low by the median), there was a trend of better survival in patients with strong VDR levels and 25D levels above the median. However, the confidence intervals are very wide due to small sample sizes. It is also possible that tumor VDR levels might change along the course of disease progression and be subject to regulations by vitamin D, estrogen and other factors. Thus the levels at the time of diagnosis might not necessarily represent the tumor cells progress to a tumor mass at relapse. Because we do not have VDR data from recurrent tumor tissues, which are usually rare to get access to, we cannot test this possibility.
Three earlier studies based on small patient populations found high VDR expression related to longer survival (23, 43, 44). The study by Santagata et al also found patients with triple-positive breast cancer subtype (ER+, AR+ and VDR+) had the best prognosis compared with those with other subtypes (31). However, two other small studies in the literature reported null associations of VDR expression with survival in breast cancer patients (11, 12). Given the scarcity of data from large prospective studies on tumor VDR expression and breast cancer outcomes, future studies are needed. It will be particularly interesting to investigate VDR expression in tumor tissues together with serum 25OHD levels and common genetic variations in the VDR locus. Several single nucleotide polymorphisms (SNPs) with potential functional impact have been repeatedly studied with breast cancer risk (45), but much fewer studies have been conducted with survival outcomes (17, 46). These genetic variations, as well as in vivo vitamin D availability indicated by circulating 25OHD levels, may modify the associations of VDR expression and breast cancer prognosis.
Our study has some limitations. First, we did not control for treatment patients received in the survival analysis. Because breast cancer therapy regimens depend heavily on tumor histopathological characteristics, the two are closely correlated. We thus adjusted for tumor characteristics in the models, which reduced potential bias due to different treatment patients received. However, we cannot completely refute the possibility of residual confounding effects. Because our findings from the survival analyses were null, if residual confounding existed, it would have only biased the results towards null. Second, VDR expression was categorized into three levels based on the distribution of the IRS. This categorization is primarily data driven and could be considered arbitrary. However, this approach is a common practice in epidemiological analysis, especially when there is no alternative cutoff based on biological or clinical meanings. We further performed a trend test by using the midpoint value of each IRS category (1, 4 and 9 for low, moderate and strong VDR expression levels, respectively) as a continuous variable in the regression models for tumor aggressive characteristics and survival. The results are very similar to those by treating VDR expression levels as a categorical variable. Lastly, we did not have data on intake of vitamin D supplement and multivitamins before and after breast cancer diagnosis, which may affect in vivo vitamin D levels and VDR activity, and further on patient survival. A prospective study to collect supplement intake and measure 25OHD repeatedly at baseline and during follow up will have capacity to address this important question.
In conclusion, VDR expression measured by IHC staining is inversely associated with aggressive characteristics in breast cancer but not with patient survival outcomes. The down-regulation of VDR expression in more aggressive breast cancer suggests functional vitamin D activity may slow or block cancer progression. However, this should be taken with caution of reverse causality. Future studies based on large prospective cohorts are warranted to further investigate the prognostic significance of expression of VDR, particularly with joint consideration of circulating 25OHD levels and related genetic variations.
Supplementary Material
TRANSLATIONAL RELEVANCE.
There has been long-standing interest in using vitamin D as a natural compound for breast cancer prevention and therapy, largely supported by experimental data from cell culture and animal models. However, epidemiological and clinical studies provide inconclusive data. In a large breast cancer patient population, deficient VDR expression was associated with more aggressive disease but not with decreased survival. The findings corroborate data form our previous serum biomarker study and support the putative benefits of vitamin D against the aggressive forms of breast cancer; yet the effect seems not to extend to clinical benefit in terms of patient survival. Future studies are needed to investigate prognostic significance of VDR expression in the context of in vivo vitamin D levels and inherited genetic variations.
ACKNOWLEDGEMENTS
This study was funded by King Fahad Specialist Hospital, Dammam, Saudi Arabia and also supported in part by funding from the National Institutes of Health National Cancer Institute grant R03CA128035; the Pathology Resources Network and the Clinical Data Network are Roswell Park Cancer Center Support Grant shared resources, supported by P30CA016056-32 from the National Cancer Institute.
Footnotes
CONFLICT OF INTEREST STATEMENT:
There are no financial disclosures or conflicts of interest to report for any of the authors.
REFERENCES
- 1.Margolis RN, Christakos S. The nuclear receptor superfamily of steroid hormones and vitamin D gene regulation. An update. Ann N Y Acad Sci. 2010;1192:208–14. doi: 10.1111/j.1749-6632.2009.05227.x. [DOI] [PubMed] [Google Scholar]
- 2.Deeb KK, Trump DL, Johnson CS. Vitamin D signalling pathways in cancer: potential for anticancer therapeutics. Nat Rev Cancer. 2007;7:684–700. doi: 10.1038/nrc2196. [DOI] [PubMed] [Google Scholar]
- 3.Feldman D, Krishnan AV, Swami S, Giovannucci E, Feldman BJ. The role of vitamin D in reducing cancer risk and progression. Nat Rev Cancer. 2014;14:342–57. doi: 10.1038/nrc3691. [DOI] [PubMed] [Google Scholar]
- 4.Eisman JA, Martin TJ, Macintyre I, Moseley JM. 1,25-Dihydroxyvitamin-D Receptor in Breast-Cancer Cells. Lancet. 1979;2:1335–6. doi: 10.1016/s0140-6736(79)92816-2. [DOI] [PubMed] [Google Scholar]
- 5.Evans RM. The Steroid and Thyroid-Hormone Receptor Superfamily. Science. 1988;240:889–95. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eisman JA, Suva LJ, Sher E, Pearce PJ, Funder JW, Martin TJ. Frequency of 1,25-Dihydroxyvitamin-D3 Receptor in Human-Breast Cancer. Cancer research. 1981;41:5121–4. [PubMed] [Google Scholar]
- 7.Frampton RJ, Suva LJ, Eisman JA, Findlay DM, Moore GE, Moseley JM, et al. Presence of 1,25-Dihydroxyvitamin-D3 Receptors in Established Human Cancer Cell-Lines in Culture. Cancer research. 1982;42:1116–9. [PubMed] [Google Scholar]
- 8.Buras RR, Schumaker LM, Davoodi F, Brenner RV, Shabahang M, Nauta RJ, et al. Vitamin-D Receptors in Breast-Cancer Cells. Breast Cancer Res Tr. 1994;31:191–202. doi: 10.1007/BF00666153. [DOI] [PubMed] [Google Scholar]
- 9.Eisman JA, Macintyre I, Martin TJ, Frampton RJ, King RJB. Normal and Malignant Breast-Tissue Is a Target Organ for 1,25-(Oh)2 Vitamin-D3. Clin Endocrinol. 1980;13:267–72. doi: 10.1111/j.1365-2265.1980.tb01053.x. [DOI] [PubMed] [Google Scholar]
- 10.Lopes N, Sousa B, Martins D, Gomes M, Vieira D, Veronese LA, et al. Alterations in Vitamin D signalling and metabolic pathways in breast cancer progression: a study of VDR, CYP27B1 and CYP24A1 expression in benign and malignant breast lesions. BMC Cancer. 2010;10:483. doi: 10.1186/1471-2407-10-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eisman JA, Suva LJ, Martin TJ. Significance of 1,25-Dihydroxyvitamin-D3 Receptor in Primary Breast Cancers. Cancer research. 1986;46:5406–8. [PubMed] [Google Scholar]
- 12.Freake HC, Abeyasekera G, Iwasaki J, Marcocci C, Macintyre I, Mcclelland RA, et al. Measurement of 1,25-Dihydroxyvitamin-D3 Receptors in Breast-Cancer and Their Relationship to Biochemical and Clinical Indexes. Cancer Res. 1984;44:1677–81. [PubMed] [Google Scholar]
- 13.Welsh J. Vitamin D and breast cancer: insights from animal models. Am J Clin Nutr. 2004;80:1721S–4S. doi: 10.1093/ajcn/80.6.1721S. [DOI] [PubMed] [Google Scholar]
- 14.Anzano MA, Smith JM, Uskokovic MR, Peer CW, Mullen LT, Letterio JJ, et al. 1-Alpha,25-Dihydroxy-16-Ene-23-Yne-26,27-Hexafluorocholecalciferol (Ro24-5531), a New Deltanoid (Vitamin-D Analog) for Prevention of Breast-Cancer in the Rat. Cancer Res. 1994;54:1653–6. [PubMed] [Google Scholar]
- 15.Crew KD, Gammon MD, Steck SE, Hershman DL, Cremers S, Dworakowski E, et al. Association between Plasma 25-Hydroxyvitamin D and Breast Cancer Risk. Cancer Prev Res. 2009;2:598–604. doi: 10.1158/1940-6207.CAPR-08-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giovannucci E. The epidemiology of vitamin D and cancer incidence and mortality: a review (United States). Cancer Causes Control. 2005;16:83–95. doi: 10.1007/s10552-004-1661-4. [DOI] [PubMed] [Google Scholar]
- 17.Chlebowski RT. Vitamin D and breast cancer: interpreting current evidence. Breast Cancer Res. 2011;13:217. doi: 10.1186/bcr2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keum N, Giovannucci E. Vitamin D supplements and cancer incidence and mortality: a meta-analysis. Br J Cancer. 2014;111:976–80. doi: 10.1038/bjc.2014.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yao S, Sucheston LE, Millen AE, Johnson CS, Trump DL, Nesline MK, et al. Pretreatment serum concentrations of 25-hydroxyvitamin D and breast cancer prognostic characteristics: a case-control and a case-series study. PLoS One. 2011;6:e17251. doi: 10.1371/journal.pone.0017251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Friedrich M, Axt-Fliedner R, Villena-Heinsen C, Tilgen W, Schmidt W, Reichrath J. Analysis of vitamin D-receptor (VDR) and retinoid X-receptor alpha in breast cancer. Histochem J. 2002;34:35–40. doi: 10.1023/a:1021343825552. [DOI] [PubMed] [Google Scholar]
- 21.Hendrickson WK, Flavin R, Kasperzyk JL, Fiorentino M, Fang F, Lis R, et al. Vitamin D Receptor Protein Expression in Tumor Tissue and Prostate Cancer Progression. J Clin Oncol. 2011;29:2378–84. doi: 10.1200/JCO.2010.30.9880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Srinivasan M, Parwani AV, Hershberger PA, Lenzner DE, Weissfeld JL. Nuclear vitamin D receptor expression is associated with improved survival in non-small cell lung cancer. J Steroid Biochem Mol Biol. 2011;123:30–6. doi: 10.1016/j.jsbmb.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ditsch N, Toth B, Mayr D, Lenhard M, Gallwas J, Weissenbacher T, et al. The Association between Vitamin D Receptor Expression and Prolonged Overall Survival in Breast Cancer. J Histochem Cytochem. 2012;60:121–9. doi: 10.1369/0022155411429155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suetani RJ, Ho K, Jindal S, Manavis J, Neilsen PM, Pishas KI, et al. A comparison of vitamin D activity in paired non-malignant and malignant human breast tissues. Mol Cell Endocrinol. 2012;362:202–10. doi: 10.1016/j.mce.2012.06.022. [DOI] [PubMed] [Google Scholar]
- 25.Banwell CM, MacCartney DP, Guy M, Miles AE, Uskokovic MR, Mansi J, et al. Altered nuclear receptor corepressor expression attenuates vitamin D receptor signaling in breast cancer cells. Clin Cancer Res. 2006;12:2004–13. doi: 10.1158/1078-0432.CCR-05-1218. [DOI] [PubMed] [Google Scholar]
- 26.Townsend K, Banwell CM, Guy M, Colston KW, Mansi JL, Stewart PM, et al. Autocrine metabolism of vitamin D in normal and malignant breast tissue. Clin Cancer Res. 2005;11:3579–86. doi: 10.1158/1078-0432.CCR-04-2359. [DOI] [PubMed] [Google Scholar]
- 27.Anderson MG, Nakane M, Ruan X, Kroeger PE, Wu-Wong JR. Expression of VDR and CYP24A1 mRNA in human tumors. Cancer Chemother Pharmacol. 2006;57:234–40. doi: 10.1007/s00280-005-0059-7. [DOI] [PubMed] [Google Scholar]
- 28.Matusiak D, Murillo G, Carroll RE, Mehta RG, Benya RV. Expression of vitamin D receptor and 25-hydroxyvitamin D3-1{alpha}-hydroxylase in normal and malignant human colon. Cancer Epidemiol Biomarkers Prev. 2005;14:2370–6. doi: 10.1158/1055-9965.EPI-05-0257. [DOI] [PubMed] [Google Scholar]
- 29.Chen TC, Sakaki T, Yamamoto K, Kittaka A. The roles of cytochrome P450 enzymes in prostate cancer development and treatment. Anticancer Res. 2012;32:291–8. [PubMed] [Google Scholar]
- 30.Palmer HG, Larriba MJ, Garcia JM, Ordonez-Moran P, Pena C, Peiro S, et al. The transcription factor SNAIL represses vitamin D receptor expression and responsiveness in human colon cancer. Nat Med. 2004;10:917–9. doi: 10.1038/nm1095. [DOI] [PubMed] [Google Scholar]
- 31.Santagata S, Thakkar A, Ergonul A, Wang B, Woo T, Hu R, et al. Taxonomy of breast cancer based on normal cell phenotype predicts outcome. J Clin Invest. 2014;124:859–70. doi: 10.1172/JCI70941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gilad LA, Bresler T, Gnainsky J, Smirnoff P, Schwartz B. Regulation of vitamin D receptor expression via estrogen-induced activation of the ERK 1/2 signaling pathway in colon and breast cancer cells. J Endocrinol. 2005;185:577–92. doi: 10.1677/joe.1.05770. [DOI] [PubMed] [Google Scholar]
- 33.James SY, Mackay AG, Binderup L, Colston KW. Effects of a New Synthetic Vitamin-D Analog, Eb1089, on the Estrogen-Responsive Growth of Human Breast-Cancer Cells. J Endocrinol. 1994;141:555–63. doi: 10.1677/joe.0.1410555. [DOI] [PubMed] [Google Scholar]
- 34.Stoica A, Saceda M, Fakhro A, Solomon HB, Fenster BD, Martin MB. Regulation of estrogen receptor-alpha gene expression by 1, 25-dihydroxyvitamin D in MCF-7 cells. J Cell Biochem. 1999;75:640–51. [PubMed] [Google Scholar]
- 35.Welsh J. Induction of apoptosis in breast cancer cells in response to vitamin D and antiestrogens. Biochem Cell Biol. 1994;72:537–45. doi: 10.1139/o94-072. [DOI] [PubMed] [Google Scholar]
- 36.Abehashimoto J, Kikuchi T, Matsumoto T, Nishii Y, Ogata E, Ikeda K. Antitumor Effect of 22-Oxa-Calcitriol, a Noncalcemic Analog of Calcitriol, in Athymic Mice Implanted with Human Breast-Carcinoma and Its Synergism with Tamoxifen. Cancer Res. 1993;53:2534–7. [PubMed] [Google Scholar]
- 37.Wagner D, Trudel D, Van der Kwast T, Nonn L, Giangreco AA, Li D, et al. Randomized clinical trial of vitamin D3 doses on prostatic vitamin D metabolite levels and ki67 labeling in prostate cancer patients. J Clin Endocrin Metab. 2013;98:1498–507. doi: 10.1210/jc.2012-4019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Williams JD, Aggarwal A, Swami S, Krishnan AV, Ji L, Albertelli MA, et al. Tumor Autonomous Effects of Vitamin D Deficiency Promote Breast Cancer Metastasis. Endocrinology. 2016;157:1341–7. doi: 10.1210/en.2015-2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pendas-Franco N, Gonzalez-Sancho JM, Suarez Y, Aguilera O, Steinmeyer A, Gamallo C, et al. Vitamin D regulates the phenotype of human breast cancer cells. Differentiation. 2007;75:193–207. doi: 10.1111/j.1432-0436.2006.00131.x. [DOI] [PubMed] [Google Scholar]
- 40.Fischer KD, Agrawal DK. Vitamin D regulating TGF-ss induced epithelial-mesenchymal transition. Respiratory Res. 2014;15:146. doi: 10.1186/s12931-014-0146-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pervin S, Hewison M, Braga M, Tran L, Chun R, Karam A, et al. Down-regulation of vitamin D receptor in mammospheres: implications for vitamin D resistance in breast cancer and potential for combination therapy. PLoS One. 2013;8:e53287. doi: 10.1371/journal.pone.0053287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thakkar A, Wang B, Picon-Ruiz M, Buchwald P, Ince TA. Vitamin D and androgen receptor-targeted therapy for triple-negative breast cancer. Breast Cancer Res Treat. 2016;157:77–90. doi: 10.1007/s10549-016-3807-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Berger U, Mcclelland RA, Wilson P, Greene GL, Haussler MR, Pike JW, et al. Immunocytochemical Determination of Estrogen-Receptor, Progesterone-Receptor, and 1,25-Dihydroxyvitamin-D3 Receptor in Breast-Cancer and Relationship to Prognosis. Cancer Res. 1991;51:239–44. [PubMed] [Google Scholar]
- 44.Colston KW, Berger U, Coombes RC. Possible Role for Vitamin-D in Controlling Breast-Cancer Cell-Proliferation. Lancet. 1989;1:188–92. doi: 10.1016/s0140-6736(89)91204-x. [DOI] [PubMed] [Google Scholar]
- 45.Mun MJ, Kim TH, Hwang JY, Jang WC. Vitamin D receptor gene polymorphisms and the risk for female reproductive cancers: A meta-analysis. Maturitas. 2015;81:256–65. doi: 10.1016/j.maturitas.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 46.Pande M, Thompson PA, Do KA, Sahin AA, Amos CI, Frazier ML, et al. Genetic variants in the vitamin D pathway and breast cancer disease-free survival. Carcinogenesis. 2013;34:587–94. doi: 10.1093/carcin/bgs369. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.