Abstract
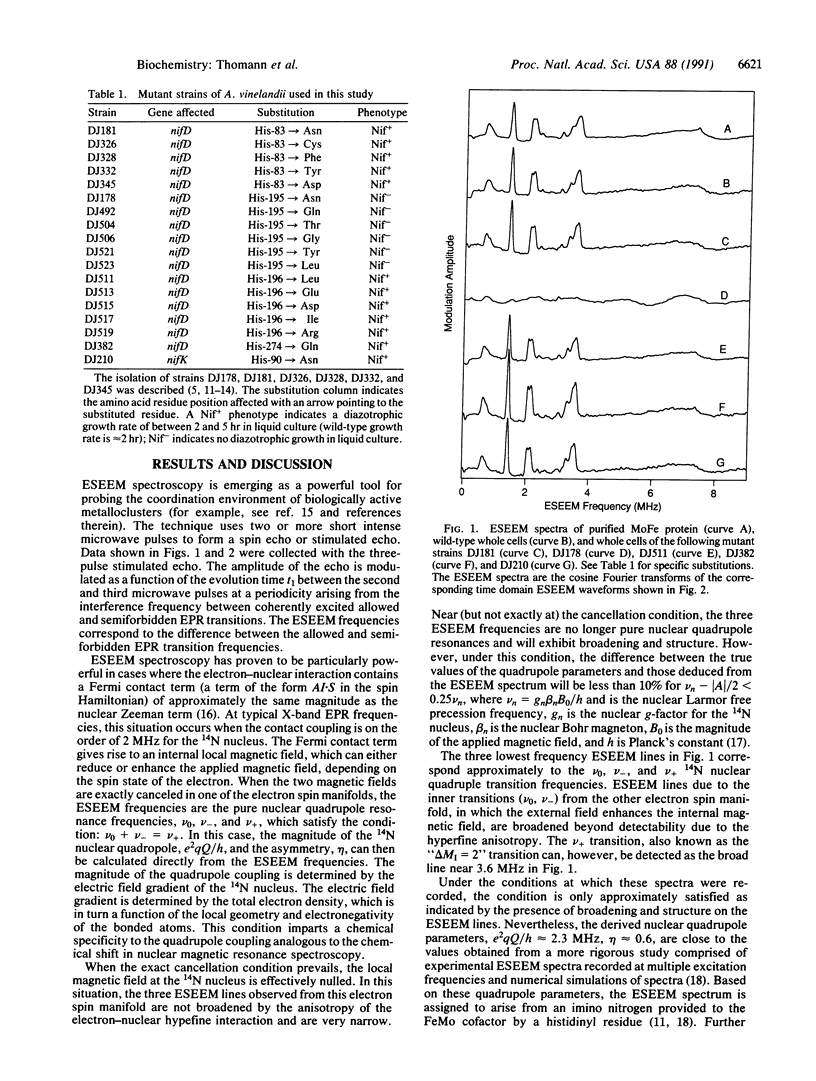
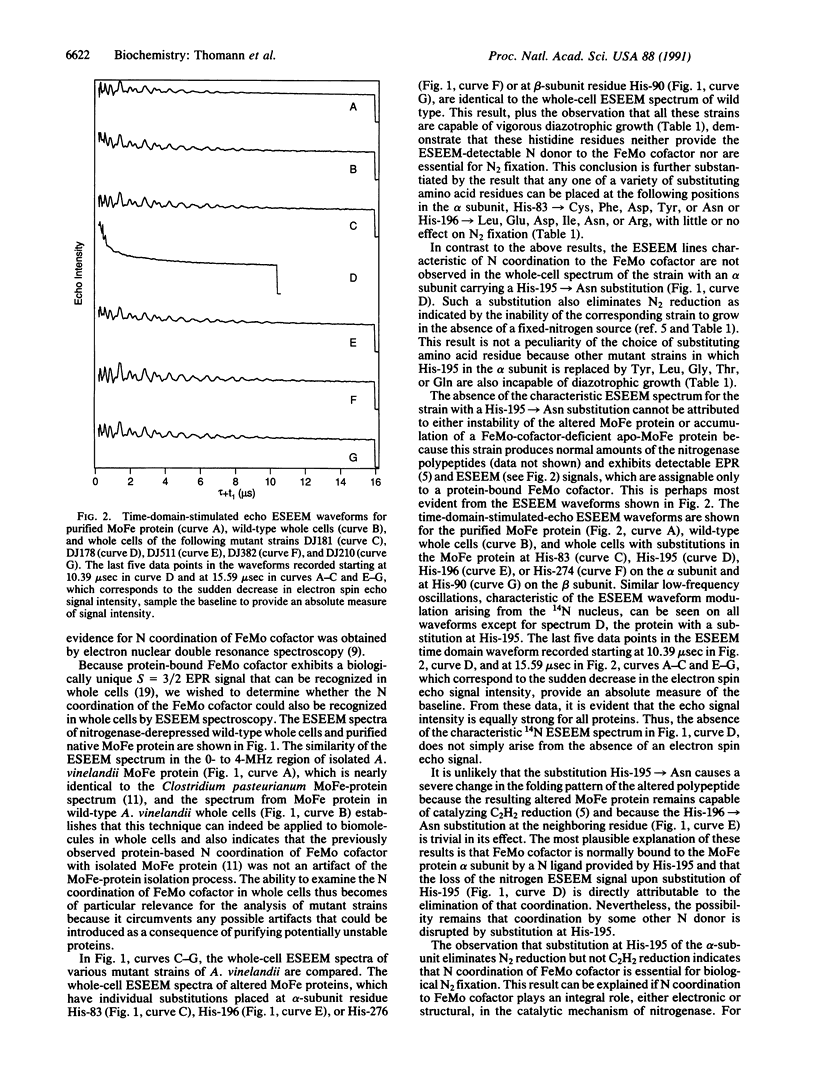
Electron spin echo envelope modulation (ESEEM) spectroscopy, a pulsed electron spin resonance technique, was used to analyze the N coordination of the iron-molybdenum (FeMo) cofactor contained within the nitrogenase MoFe protein. Comparison of spectra obtained from whole cells and purified MoFe protein established that the N coordination of the FeMo cofactor provided by the MoFe-protein polypeptide matrix can be unambiguously recognized in whole cells. ESEEM spectra of altered MoFe proteins, which were produced in certain mutant strains of Azotobacter vinelandii, showed that the N coordination to FeMo cofactor requires His-195 of the MoFe protein alpha subunit. Moreover, this requirement for His-195 was shown to be essential for biological nitrogen fixation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brigle K. E., Newton W. E., Dean D. R. Complete nucleotide sequence of the Azotobacter vinelandii nitrogenase structural gene cluster. Gene. 1985;37(1-3):37–44. doi: 10.1016/0378-1119(85)90255-0. [DOI] [PubMed] [Google Scholar]
- Brigle K. E., Setterquist R. A., Dean D. R., Cantwell J. S., Weiss M. C., Newton W. E. Site-directed mutagenesis of the nitrogenase MoFe protein of Azotobacter vinelandii. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7066–7069. doi: 10.1073/pnas.84.20.7066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess B. K., Stiefel E. I., Newton W. E. Oxidation-reduction properties and complexation reactions of the iron-molybdenum cofactor of nitrogenase. J Biol Chem. 1980 Jan 25;255(2):353–356. [PubMed] [Google Scholar]
- Davis L. C., Shah V. K., Brill W. J., Orme-Johnson W. H. Nitrogenase. II. Changes in the EPR signal of component I (iron-molybdenum protein) of Azotobacter vinelandii nitrogenase during repression and derepression. Biochim Biophys Acta. 1972 Feb 28;256(2):512–523. doi: 10.1016/0005-2728(72)90079-5. [DOI] [PubMed] [Google Scholar]
- Dean D. R., Setterquist R. A., Brigle K. E., Scott D. J., Laird N. F., Newton W. E. Evidence that conserved residues Cys-62 and Cys-154 within the Azotobacter vinelandii nitrogenase MoFe protein alpha-subunit are essential for nitrogenase activity but conserved residues His-83 and Cys-88 are not. Mol Microbiol. 1990 Sep;4(9):1505–1512. [PubMed] [Google Scholar]
- Hawkes T. R., McLean P. A., Smith B. E. Nitrogenase from nifV mutants of Klebsiella pneumoniae contains an altered form of the iron-molybdenum cofactor. Biochem J. 1984 Jan 1;217(1):317–321. doi: 10.1042/bj2170317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlings J., Shah V. K., Chisnell J. R., Brill W. J., Zimmermann R., Münck E., Orme-Johnson W. H. Novel metal cluster in the iron-molybdenum cofactor of nitrogenase. Spectroscopic evidence. J Biol Chem. 1978 Feb 25;253(4):1001–1004. [PubMed] [Google Scholar]
- Scott D. J., May H. D., Newton W. E., Brigle K. E., Dean D. R. Role for the nitrogenase MoFe protein alpha-subunit in FeMo-cofactor binding and catalysis. Nature. 1990 Jan 11;343(6254):188–190. doi: 10.1038/343188a0. [DOI] [PubMed] [Google Scholar]
- Shah V. K., Brill W. J. Isolation of an iron-molybdenum cofactor from nitrogenase. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3249–3253. doi: 10.1073/pnas.74.8.3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S. S., Pan W. H., Friesen G. D., Burgess B. K., Corbin J. L., Stiefel E. I., Newton W. E. Iron-molybdenum cofactor from nitrogenase. Modified extraction methods as probes for composition. J Biol Chem. 1982 Jul 25;257(14):8042–8048. [PubMed] [Google Scholar]



