Abstract
Background
It would be helpful for the decision-making process of patients with metastatic bone disease to understand which patients are at risk for worse quality of life (QOL), pain, anxiety, and depression. Normative data, and where these stand compared with general population scores, can be useful to compare and interpret results of similar patients or patient groups, but to our knowledge, there are no such robust data.
Questions/Purposes
We wished (1) to assess what factors are independently associated with QOL, pain interference, anxiety, and depression in patients with metastatic bone disease, and (2) to compare these outcomes with general US population values.
Methods
Between November 2011 and February 2015, 859 patients with metastatic bone disease presented to our orthopaedic oncology clinic; 202 (24%) were included as they completed the EuroQOL-5 Dimension (EQ-5DTM), PROMIS® Pain Interference, PROMIS® Anxiety, and PROMIS® Depression questionnaires as part of a quality improvement program. We did not record reasons for not responding and found no differences between survey respondents and nonrespondents in terms of age (63 versus 64 years; p = 0.916), gender (51% men versus 47% men; p = 0.228), and race (91% white versus 88% white; p = 0.306), but survey responders were more likely to be married or living with a partner (72%, versus 62%; p = 0.001). We assessed risk factors for QOL, pain interference, anxiety, and depression using multivariable linear regression analysis. We used the one-sample signed rank test to assess whether scores differed from US population averages drawn from earlier large epidemiologic studies.
Results
Younger age (β regression coefficient [β], < 0.01; 95% CI, 0.00–0.01; p = 0.041), smoking (β, −0.12; 95% CI, −0.22 to −0.01; p = 0.026), pathologic fracture (β, −0.10; 95% CI, −0.18 to −0.02; p = 0.012), and being unemployed (β, −0.09; 95% CI, −0.17 to −0.02; p = 0.017) were associated with worse QOL. Current smoking status was associated with more pain interference (β, 6.0; 95% CI, 1.6–11; p = 0.008). Poor-prognosis cancers (β, 3.8; 95% CI, 0.37–7.2; p = 0.030), and pathologic fracture (β, 6.3; 95% CI, 2.5–7.2; p = 0.001) were associated with more anxiety. Being single (β, 5.9; 95% CI, 0.83–11; p = 0.023), and pathologic fracture (β, 4.4; 95% CI, 0.8–8.0; p = 0.017) were associated with depression. QOL scores (0.68 versus 0.85; p < 0.001), pain interference scores (65 versus 50; p < 0.001), and anxiety scores (53 versus 50; p = 0.011) were worse for patients with bone metastases compared with general US population values, whereas depression scores were comparable (48 versus 50; p = 0.171).
Conclusions
Impending pathologic fractures should be treated promptly to prevent deterioration in QOL, anxiety, and depression. Our normative data can be used to compare and interpret results of similar patients or patient groups. Future studies could focus on specific cancers metastasizing to the bone, to further understand which patients are at risk for worse patient-reported outcomes.
Level of evidence
Level III, prognostic study.
Electronic supplementary material
The online version of this article (doi:10.1007/s11999-016-5118-3) contains supplementary material, which is available to authorized users.
Introduction
The main goal when treating patients with metastatic bone disease is to preserve or even improve quality of life (QOL) [7, 29]. Questionnaires that measure QOL can quantify treatment effectiveness and guide clinical decision-making [7, 18, 30]. Several studies identified risk factors for QOL in patients with metastatic bone disease; [8, 25, 28, 29] however, these often were measured after specific treatment(s), and therefore do not represent QOL for all patients with metastatic bone disease.
It would be useful to more fully understand which patients are at risk for worse QOL, pain, anxiety, and depression, so that deterioration for these patients can be anticipated and potentially overcome with medical treatment. In addition, it is helpful to have normative data for patients with metastatic bone disease to be able to compare and interpret results of similar patients or patient groups. To our knowledge, there are no rigorous sources to date that provide such information.
Therefore, we sought to identify factors independently associated with QOL, pain interference, anxiety, and depression. Secondly, we compared these patient-reported outcome scores with general US population scores.
Patients and Methods
Study Design, Setting, and Subjects
After approval by the institutional review board of Massachusetts General Hospital, we analyzed prospectively gathered patient-reported outcome data of patients with bone metastases from solid tumors, myeloma, or lymphoma, who presented to our clinic between November 1, 2011 and February 1, 2015. As part of a quality improvement program, patients completed the following questionnaires before visiting the surgeon at our orthopaedic oncology service since November 2011: the EuroQOL 5 Dimension Questionnaire (EQ-5DTM), Patient-Reported Outcomes Measurement Information System (PROMIS®) Pain Interference, PROMIS® Anxiety, and PROMIS® Depression.
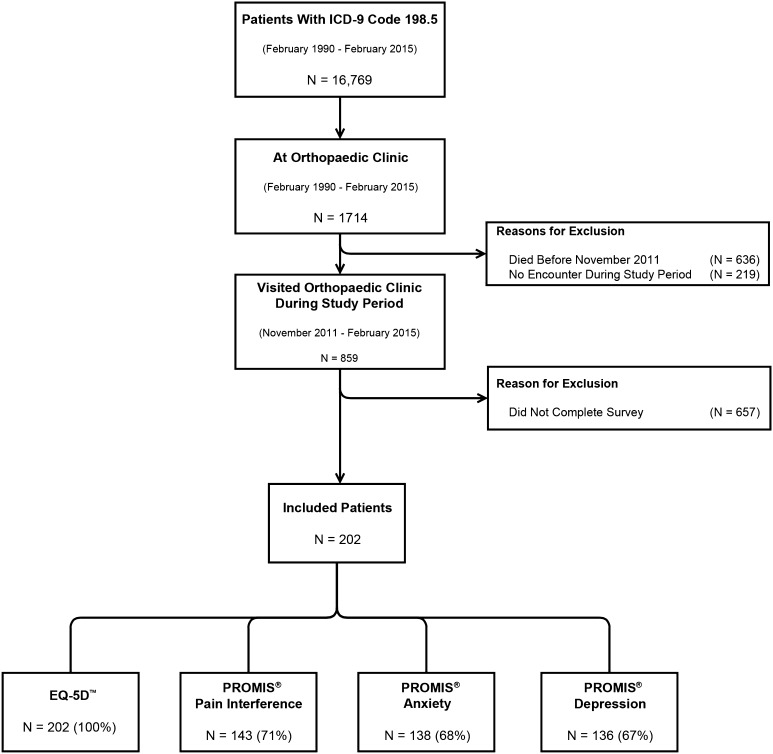
Through the information system (Research Patient Data Registry) from Massachusetts General Hospital, we identified 16,769 unique patients who received the ICD-9 code for “Secondary malignant neoplasm of bone and bone marrow” between February 1990 and February 2015; 1714 of those patients were seen at our orthopaedic oncology clinic, of whom 859 were seen during the period of the quality improvement program (November 2011 to February 2015). Of the 859 potentially eligible patients, 202 (24%) completed the survey and 657 (76%) did not (Fig. 1). We were unable to track reasons for nonparticipation, but we did analyze differences in baseline characteristics between the surveyed and nonsurveyed patients. There were no differences in age (63 versus 64 years; p = 0.916), sex (51% men versus 47% men; p = 0.228), and race (91% white versus 88% white; p = 0.306), but survey responders were more likely to be married or living with a partner (72% versus 62%; p = 0.001) (Appendix 1. Supplemental material is available with the online version of CORR ®). We therefore believe that our results can be generalized to the overall population of patients with bone metastasis presenting to an orthopaedic oncology clinic.
Fig. 1.
This flow chart shows the selection procedure of the included cohort. The ICD-9 code 198.5 corresponds to “Secondary malignant neoplasm of bone and bone marrow”
Questionnaires were completed through the REDCap (Research Electronic Data Capture) data capture tool on a tablet computer [14]. We included the first completed survey in the case patients had completed surveys multiple times, as not to violate the statistical assumption of independence.
Outcome Measures and Explanatory Variables
Our outcome measures were (1) QOL, as measured with the EQ-5DTM questionnaire (completed by all patients), (2) how pain interfered with physical function as measured with the PROMIS® Pain Interference questionnaire (completed by 143; 71%), (3) anxiety as measured with the PROMIS® Anxiety questionnaire (completed by 138; 68%), and (4) depression as measured with the PROMIS® Depression questionnaire (completed by 136; 67%). The latter three questionnaires were not completed by all patients as these were introduced to the quality improvement program at a later time (August 1, 2012). We assessed baseline differences between patients who did not complete a PROMIS® questionnaire (n = 56), and those who did (n = 146). There were no differences in median age (62 versus 64 years; p = 0.972), sex (46% male versus 53% male; p = 0.432), marital status (73% married versus 72% married; p = 0.271), median Charlson Comorbidity Index (6 versus 6; p = 0.806), pathologic fracture (27% versus 29%; p = 0.862), or previous surgery (14% versus 8.9%; p = 0.304).
The EQ-5DTM-3L questionnaire covers five dimensions (mobility, self-care, usual activities, pain/discomfort, and anxiety/depression), and each dimension is divided into three levels (no problems, moderate problems, or extreme problems). The EQ-5DTM describes 245 unique health states [24]. Fryback et al. [10] obtained age-by-gender EQ-5DTM norms for adults in the US; we compared the EQ-5DTM scores to the value of 0.85, based on the age of our study participants.
The PROMIS® was established by the National Institutes of Health (NIH) to develop standardized item banks to assess physical, mental, and social well-being in the medical field. The PROMIS® Pain Interference item bank (six items) assesses the extent to which pain interferes with functioning. The PROMIS® Anxiety questionnaire (six items) includes questions regarding fear, anxious misery, hyperarousal, and somatic symptoms related to arousal. The PROMIS® Depression questionnaire (six items) focuses on negative mood, decrease in positive affect, information-processing deficits, negative views of self, and negative social cognition. All PROMIS® scores have a general US population-based mean T-score of 50; these scores are not adjusted by age or gender [5].
Two researchers (QvdV, SJJ) extracted the following explanatory variables from medical records in a retrospective manner, as these were known or suggested to be associated with patient-reported outcomes [8, 25, 28, 29]: age, sex, race, employment status, marital status, smoking status, comorbidity status, BMI in kg/m2, primary tumor type, time between diagnosis of primary tumor and survey, time between diagnosis of metastatic disease and survey, location of the bone metastasis leading to the consultation (spine, lower extremity, upper extremity, pelvis, multiple, and other locations), current radiotherapy, current systemic therapy, prior surgery for bone metastasis, pathologic fracture (an impending pathologic fracture was not considered a fracture), presence of other bone metastases, and presence of visceral or brain metastases.
We assessed the comorbidity status using a modified Charlson Comorbidity Index; this is an index weighting 12 comorbidities known to be associated with 10-year survival [6]. We used a modified Charlson Comorbidity Index calculated by a previously described algorithm based on ICD-9 codes [23]. The presence of additional comorbidities was based on the modified Charlson Comorbidity Index and defined as the presence of conditions other than metastatic bone disease, multiple myeloma, or lymphoma. We extracted BMI closest to completion of the survey and omitted values recorded longer than 90 days before the survey. “Former smoker” was defined as stopped smoking at least 1 year before the survey. Based on a study by Katagiri et al. [17], we categorized primary tumors as those with a relatively good prognosis (breast, kidney, prostate, thyroid, myeloma, and lymphoma), and those with a relatively poor prognosis (all other tumor types). Systemic therapy was defined as any type of hormonal therapy, chemotherapy, or immunotherapy for the primary cancer. The fracture status, presence of other bone metastases, and visceral metastases were derived from radiology reports.
An independent researcher (NRPP) crosschecked a random 10% sample of the retrospectively collected data to ensure a robust database; there were less than 5% inconsistencies overall, and there were no repeated inconsistencies within one variable.
Statistical Analysis
Continuous variables were presented as medians with interquartile ranges (IQR), and categorical variables as frequencies with percentages. Data were analyzed using nonparametric tests, as histograms suggested nonnormal distribution of continuous variables.
We did an exploratory bivariate analysis to assess differences in outcome measures using the Mann-Whitney U test for dichotomous explanatory variables, Kruskal-Wallis test for categorical explanatory variables, and Spearman’s rank correlation coefficient for continuous explanatory variables. We used stepwise backward multivariable linear regression analysis—retaining variables with a p value less than 0.10—to assess if explanatory variables (as identified by the bivariate analyses) were independently associated with the outcome measures.
We used the one-sample signed rank test to assess whether scores differed from general US population averages.
All statistical analyses were performed with STATA® 13.0 (StataCorp LP, College Station, TX, USA), and two-tailed p values less than 0.05 were considered significant.
Patient Demographics
The study population consisted of 104 (51%) men and 98 (49%) women, with a median age of 63 years (range, 27–89 years) (Table 1). Lung cancer was the most common primary cancer (35; 17%), followed by breast cancer (33; 16%), and multiple myeloma (33; 16%). The most common location for a bone lesion was the spine (72; 36%), and 57 (28%) patients presented with a pathologic fracture. Twenty-one (10%) patients had undergone prior surgery for their metastatic bone lesion(s), with a median of 132 days (IQR, 23–520 days; range, 2–1617 days) between the surgery and survey completion. Seventy-nine (39%) were being treated with systemic therapy, and nine (4%) were receiving radiotherapy for their bone lesion(s) at the time of completing the survey.
Table 1.
Baseline characteristics of 202 patients
| Characteristic | Median | Interquartile range |
|---|---|---|
| Age (years) | 63 | 54–72 |
| Modified Charlson Comorbidity Index* | 6.0 | 6.0–8.0 |
| BMI (kg/m2)* | 27 | 24–31 |
| Time between diagnosis of primary tumor and survey (months)* | 26 | 4.1–78 |
| Time between diagnosis of metastatic disease and survey (months)* | 7.4 | 0.4–34 |
| Number | % | |
| Men | 104 | 51 |
| White race* | 174 | 91 |
| Additional comorbidities*† | 94 | 47 |
| Employment status* | ||
| Employed | 79 | 40 |
| Unemployed | 118 | 60 |
| Marital status* | ||
| Married/living with partner | 144 | 72 |
| Separated/divorced/widowed | 35 | 18 |
| Single | 21 | 11 |
| Smoking status* | ||
| Never smoked | 79 | 41 |
| Former smoker‡ | 81 | 42 |
| Current smoker | 35 | 18 |
| Primary tumor type | ||
| Lung | 35 | 17 |
| Breast | 33 | 16 |
| Multiple myeloma | 33 | 16 |
| Kidney | 28 | 14 |
| Prostate | 13 | 6 |
| Other/unknown§ | 60 | 30 |
| Location of presenting metastasis | ||
| Spine | 72 | 36 |
| Lower extremity | 40 | 20 |
| Upper extremity | 31 | 15 |
| Pelvis | 27 | 13 |
| Multiple | 30 | 15 |
| Other|| | 2 | 1 |
| Current radiotherapy | 9 | 4 |
| Current systemic therapy | 79 | 39 |
| Prior surgery for bone metastasis | 21 | 10 |
| Pathologic fracture | 57 | 28 |
| Multiple bone metastases | 127 | 63 |
| Lung/liver/adrenal/brain metastases | 81 | 40 |
*Modified Charlson Comorbidity Index was available for 200 patients (99%), BMI was available for 198 patients (98%), race was available for 191 patients (95%), additional comorbidities were available for 200 patients (99%), employment status was available for 197 patients (98%), marital status was available for 200 patients (99%), smoking status was available for 195 patients (97%), time between diagnosis of primary tumor and survey was available for 193 patients (96%), and time between diagnosis of metastatic disease and survey was available for 196 patients (97%); †based on any additional comorbidity in addition to the metastatic disease score following the Charlson Comorbidity Index; ‡quit at least 1 year before survey; §thyroid (n = 8), lymphoma (n = 6), colorectal (n = 6), adenocarcinoma (n = 6), neuroendocrine (n = 5), esophageal (n = 4), melanoma (n = 3), liver (n = 3), bladder (n = 2), endometrial (n = 2), ovarian (n = 1), and pancreatic (n = 1); ||rib (n =1), occipital condyle (n = 1).
Results
Factors Associated With Worse QOL
After controlling for relevant confounding variables, we found that younger age (β regression coefficient [β], < 0.01; 95% CI, 0.00–0.01; p = 0.041), current smoking status (β, −0.12; 95% CI, −0.22 to −0.01; p = 0.026), pathologic fracture (β, −0.10; 95% CI, −0.18 to −0.02; p = 0.012), and being unemployed (β, −0.09; 95% CI, −0.17 to −0.02; p = 0.017) were independently associated with worse QOL (Table 2).
Table 2.
Multivariable linear regression analysis assessing factors independently associated with patient-reported outcomes
| Explanatory variables per questionnaire | β regression coefficient (95% CI) | Standard error | p Value |
|---|---|---|---|
| EQ-5DTM index | |||
| Age (in years) | < 0.01 (0.00–0.01) | 0.001 | 0.041¶ |
| Smoking status* | |||
| Never smoked | Reference | ||
| Former smoker† | −0.05 (−0.13 to 0.03) | 0.04 | 0.252 |
| Current smoker | −0.12 (−0.22 to −0.01) | 0.05 | 0.026¶ |
| Pathologic fracture | |||
| No | Reference | ||
| Yes | −0.10 (−0.18 to −0.02) | 0.04 | 0.012¶ |
| Employment status* | |||
| Employed | Reference | ||
| Unemployed | −0.09 (−0.17 to −0.02) | 0.04 | 0.017¶ |
| PROMIS® Pain Interference | |||
| Smoking status* | |||
| Never smoked | Reference | ||
| Former smoker† | 3.1 (−0.37 to 6.7) | 1.72 | 0.080 |
| Current smoker | 6.0 (1.6–11) | 2.25 | 0.008¶ |
| PROMIS® Anxiety | |||
| Primary tumor type‡ | |||
| Good prognosis | Reference | ||
| Poor prognosis | 3.8 (0.37–7.2) | 1.73 | 0.030¶ |
| Pathologic fracture | |||
| No | Reference | ||
| Yes | 6.3 (2.5–7.2) | 1.90 | 0.001¶ |
| PROMIS® Depression | |||
| Marital status* | |||
| Married/living with partner | Reference | ||
| Separated/divorced/widowed | 0.53 (−4.0 to 5.0) | 2.27 | 0.817 |
| Single | 5.9 (0.83–11) | 2.54 | 0.023¶ |
| Pathologic fracture | |||
| No | Reference | ||
| Yes | 4.4 (0.8–8.0) | 1.82 | 0.017¶ |
¶Two-tailed p value less than 0.05; adjusted R squared values for the multivariate analyses were 0.0586 for the EQ-5DTM, 0.0407 for the PROMIS® Pain Interference, 0.0902 for the PROMIS® Anxiety, and 0.0632 for the PROMIS® Depression; *marital status was available for 200 patients (99%); †quit at least one year before survey; ‡primary tumor type with good prognosis includes breast, kidney, prostate, thyroid, myeloma and lymphoma, and with poor prognosis includes all other tumor types; EQ-5DTM = EuroQol 5 Dimension Questionnaire; PROMIS® = Patient-Reported Outcomes Measurement Information System.
Factors Associated With More Pain Interference
Current smoking status was the only factor associated with worse pain interference scores (β, 6.0; 95% CI, 1.6–11; p = 0.008) (Table 2).
Factors Associated With More Anxiety
After controlling for relevant confounding variables, we found that a primary tumor type with poor prognosis (β, 3.8; 95% CI, 0.37–7.2; p = 0.030), and pathologic fracture (β, 6.3; 95% CI, 2.5–7.2; p = 0.001) were independently associated with worse anxiety scores (Table 2).
Factors Associated With Depression
After controlling for relevant confounding variables, we found that pathologic fracture (β, 4.4; 95% CI, 0.8–8.0; p = 0.017), and being single (β, 5.9; 95% CI, 0.8–11; p = 0.023) were independently associated with worse depression scores (Table 2).
Comparison With General US Population Norms
Patients with metastatic bone disease had worse QOL scores than the US population norm of 0.85 (0.68; range, −0.04 to 1; p < 0.001), worse PROMIS® Pain Interference scores than the US population norm of 50 (T-score, 65; range, 41–76; p < 0.001), and worse PROMIS® Anxiety scores than the US population norm of 50 (T-score, 53; range, 39–79; p = 0.011). Patients with metastatic bone disease had PROMIS® Depression scores comparable to the US population norm of 50 (T-score, 48; range, 38–73; p = 0.171) (Table 3).
Table 3.
Patient-reported outcomes for patients with bone metastases, and comparison with general population values
| Questionnaire | Number (%) | Median (interquartile range) | General population value | p Value |
|---|---|---|---|---|
| EQ-5DTM index | 202 (100) | 0.68 (0.40–0.81) ‘ | 0.85 | < 0.001* |
| PROMIS® Pain Interference | 143 (71) | 65 (56–70) | 50 | < 0.001* |
| PROMIS® Anxiety | 138 (68) | 53 (39–61) | 50 | 0.011* |
| PROMIS® Depression | 136 (67) | 48 (38–57) | 50 | 0.171 |
*Significant; EQ-5DTM = EuroQol 5 Dimension Questionnaire; PROMIS® = Patient-Reported Outcomes Measurement Information System.
Discussion
QOL plays a crucial role in the treatment of metastatic bone disease [7, 29], and questionnaires that measure QOL can quantify treatment effectiveness and guide clinical decision-making [7, 18, 30]. Previous studies identified risk factors for QOL after medical treatments [8, 25, 28, 29], although it would be useful for clinical decision-making to understand which patients are more prone to have worse QOL regardless of any (prior) treatment. Furthermore, it would be helpful to have normative data for these patients to be able to compare and interpret results of similar patients or patient groups. We found that having a pathologic fracture was independently associated with worse QOL, increased anxiety, and more depression. In addition, younger age, current smoking status, and being unemployed were independently associated with worse QOL. Current smoking status was independently associated with more pain interference. A primary tumor type with poor prognosis was associated with more anxiety, and being single was associated with more depression. Patients with metastatic bone disease reported worse QOL, more pain interference, and more anxiety compared with general population values; their depression scores were not different with the numbers available.
This study has limitations. First, selection bias might have occurred, as we had patient-reported outcomes on only 24% [202/859] of the eligible patients who presented to our clinic. Nonresponders more often were single, and being single was associated with worse depression scores; if all 859 eligible patients had been included, this could have worsened overall patient-reported outcome scores (especially depression scores), giving larger differences between our sample and US population norms. Second, 56 (28%) of the 202 patients did not complete a PROMIS® questionnaire, resulting in reduced statistical power for PROMIS®- related outcomes. However, we found no baseline differences between the patients who did and did not complete the PROMIS® questionnaires. Third, questionnaires were completed at different stages of treatment. This cross-sectional survey study represents the breadth of patients with bone metastasis who present to an orthopaedic oncology clinic; some patients filled out the questionnaires during their preoperative visit, whereas others completed the survey after an extensive surgical procedure. The normative data for patient-reported outcomes that we present are not reflections of the effectiveness of certain medical treatments that we provide, and should not be interpreted as such. Fourth, two researchers collected several variables in a retrospective manner (except for the patient-reported outcomes); these variables might have information bias. Another researcher verified a random 10% sample (21 patients) of the database to detect inconsistencies in all retrospectively collected data; less than 5% of the data were discrepant, and discrepancies were not consistent within one variable. Therefore we considered this a minor limitation. Fifth, we included predominantly white patients from an orthopaedic clinic at a tertiary care hospital in an urban area, therefore our results may not be generalizable to all patients with metastatic bone disease or patients being treated in other settings. Patients who present to our clinic often have an acute or impending fracture or a painful bone lesion; this might result in a sicker patient population compared with patients who do not consult an orthopaedic surgeon [1]. Therefore, our results might show a slight underestimation of QOL for patients with bone metastases. Sixth, we used ICD-9 codes to identify eligible patients and to extract the modified Charlson Comorbidity Index. Coding might have been inaccurate, although we believe this is limited and not of influence on our results. Seventh, outcome measures and explanatory variables were not available for all patients. However, missing data were relatively low (PROMIS® Pain Interference missing in 29%; PROMIS® Anxiety missing in 32%; PROMIS® Depression missing in 33%; range of percentages of missing data for explanatory variables 1% to 4%). Eighth, the Karnofsky Performance Score was found to be associated with QOL in previous studies [8, 28, 29]. Unfortunately, data on this score were available only for a very small number of patients in our cohort, therefore we could not assess the effect of the performance score on patient-reported outcomes. Ninth, one may advocate that it is inappropriate to include patients with hematologic cancers in this study because these cancers (often) have a better prognosis, which might modify the patients’ perception of the disease. We tried to account for this by including the variable “primary tumor type”, where hematologic malignancies accounted for 32% (39/121) of the “good prognosis” group. Finally, the normative data on PROMIS® scores we used are not adjusted by age or gender [5]. As we observed in our data, QOL scores may vary based on age: likely, this is also the case in the general population [15, 16, 19]. This limitation might have resulted in an unfair comparison with general population values for PROMIS® questionnaire outcomes. Future studies will need to perform such adjustments, as it seems nearly certain that age and gender would influence patients’ PROMIS® scores.
To our knowledge, our findings that patients who were younger, patients who were smokers, patients who were not employed, and patients with pathologic fractures all had poorer QOL, are novel. However, other studies have reported on other risk factors. Choi et al. [8] measured QOL by giving the EQ-5DTM questionnaire to 922 patients after surgery for metastatic spine disease; predictive factors for postoperative QOL were the preoperative EQ-5DTM score, the Frankel score, and the Karnofsky performance status. Westhoff et al. [28] collected QOL for 956 patients with breast, prostate, and lung cancers after irradiation for painful bone metastases by giving the Rotterdam Symptom Checklist, and found that patients with lung cancer had worse QOL scores than patients with prostate and breast cancers. In a study by Wong et al. [29], 396 patients with metastatic bone disease completed the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Bone Metastases module: a Karnofsky Performance scale greater than 80 (compared with less than 80) and breast or prostate cancer (compared with other cancer types) were associated with better QOL. Rustøen et al. [25] obtained QOL information from 157 oncology outpatients with pain from bone metastasis, using the Multidimensional Quality of Life Scale-Cancer. Depression, social functioning, and physical functioning were the factors associated with QOL.
Having a pathologic fracture was independently associated with worse QOL, more anxiety, and depression. We believe that patients with a pathologic fracture have worse QOL owing to increased pain and disability (as these are components of the EQ-5DTM) [24], and more anxiety and depression owing to overall deterioration in health and accompanying death anxiety. This stresses that it is of utmost importance to prevent pathologic fractures, and could serve as a basis for a lower threshold for surgery (such as intramedullary nailing) in patients with impending fractures from metastatic bone lesions.
Patients who were unemployed reported worse QOL, but it is unclear if poor QOL led to unemployment or vice versa. Wong et al. [29] studied 396 patients with bone metastases from various cultures and found that employment status was not associated with QOL. This mixture of cultures might have led to a different contribution of employment status, showing that the extrapolarity of our results to other countries is limited. Current smoking status was associated with worse QOL and more pain interference in our study patients. Although the psychological and physiologic reasons remain unclear, pain and tobacco addiction are theorized to interact in a bidirectional manner [22]; individuals with pain are more likely to be dependent on tobacco, and tobacco addiction is a risk factor for having chronic pain develop. Although the direction of causality is unclear, this interaction gives a positive feedback loop between tobacco abuse and pain, leading to worsening of both conditions [9].
Patients with relatively poor-prognosis tumor types reported more anxiety compared with patients with good-prognosis primary tumor types; this might be attributable to the awareness of a poorer prognosis and fear of death. We broke down this variable further per cancer type and ascertained that patients with lung cancer and adenocarcinoma reported more anxiety compared with patients with breast cancer, multiple myeloma, renal cancer, prostate cancer, or thyroid cancer (Table 4). We also stratified for pathologic fracture, as this also was a risk factor; these normative data can be used to compare and interpret results of similar patients or patient groups. Furthermore, awareness of anxiety and referral for psychological counseling in patients with poor-prognosis tumor types is recommended.
Table 4.
PROMIS® Anxiety t-scores with 95% CIs, stratified for most common primary tumor types, and pathologic fracture
| Patient group | Poor-prognosis tumors* | Good-prognosis tumors* | ||||||
|---|---|---|---|---|---|---|---|---|
| Lung carcinoma | Adenocarcinoma | Breast carcinoma | Multiple myeloma | Renal cell carcinoma | Prostate carcinoma | Thyroid carcinoma | All patients | |
| n = 26 | n = 5 | n = 23 | n = 22 | n = 19 | n = 9 | n = 5 | n = 138 | |
| All patients (n = 138) | 54 (49–61) | 57 (54–63) | 53 (46–63) | 51 (39–62) | 51 (39–63) | 49 (39–51) | 49 (39–51) | 53 (39–61) |
| Pathologic fracture | ||||||||
| No (n = 100) | 51 (39–57) | 56 (52–30) | 51 (39–61) | 54 (39–63) | 46 (39–57) | 49 (43–53) | 49 (39–51) | 51 (39–57) |
| Yes (n = 38) | 61 (53–63) | 74 (74–74) | 57 (51–63) | 50 (46–58) | 66 (63–69) | 39 (39–39) | – | 58 (49–63) |
The most common primary tumor types are included (n > 5); *based on study by Katagiri et al. [17], primary tumors were categorized as tumors with a relatively good prognosis (breast, kidney, prostate, thyroid, myeloma, and lymphoma), and tumors with a relatively poor prognosis (all other tumor types).
Studies show that in the general population older age is associated with worse QOL scores [15, 16, 19], whereas in our study population younger age was associated with worse QOL. We speculate that younger patients may be more traumatized when receiving a diagnosis of metastatic disease (it comes more unexpectedly), have greater responsibilities to their family and community, and have more life goals compared with older patients [20, 27, 32]. Like in our study, previous studies showed an association between being married and better QOL [3, 11, 29]. Goodwin et al. [13] suggested that married patients with cancer may have a better QOL because they are diagnosed at an earlier stage, they may show better response to treatment, and because they benefit from the social support and care-taking skills of the spouse.
To our knowledge, this is the first study comparing QOL, pain interference, anxiety, and depression scores for patients with bone metastases with general population values. Previously, patients with any type of cancer were found to have similar QOL compared with a general US adult population sample [4]. In light of this, we questioned why patients with bone metastases from our cohort reported worse QOL. Patients with bone metastases might have more pain, reduced mobility, impaired role functioning, and reduced physical performance [2]. Pain interference scores were higher compared with US population values; this can be explained by the painful conditions (pathologic fractures, painful bone lesions) that are the most common reasons for presenting to an orthopaedic surgeon [1]. Furthermore, we believe that patients had higher anxiety scores owing to fear of pain or to death anxiety [26]. Death anxiety is common in patients with advanced cancer and has been associated with generalized anxiety [12, 21, 26]. Surprisingly, depression scores were comparable to general population values. Previous studies explained this phenomenon as emotional and spiritual adaptation, and taking the cancer diagnosis in consideration when evaluating health status [21, 31].
Physicians can use the factors we associated with poorer scores for QOL, pain interference, anxiety, and depression to anticipate which patients might need additional psychosocial support during treatment for metastatic bone disease. Furthermore, our normative data can be used to compare and interpret results of other patients or patient groups for future studies. Our study results support that impending pathologic fractures should be treated promptly (perhaps by intramedullary nailing) to prevent further deterioration in QOL, anxiety, and depression. Every type of cancer that metastasizes to the bone has a different disease course, treatment, and prognosis; therefore, it would merit further study to reproduce our study in cancer-specific groups, to identify more accurate factors accounting for variation in patient-reported outcomes.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Footnotes
One of the authors certifies that he (SJJ), or a member of his or her immediate family, has or may receive payments or benefits, during the study period, an amount of less than USD 10,000 from the Anna Foundation (Oegstgeest, The Netherlands), an amount of less than USD 10,000 from the De Drie Lichten Foundation (Hilversum, The Netherlands), an amount of less than USD 10,000 from the KWF Kankerbestrijding (Amsterdam, The Netherlands), and an amount of less than USD 10,000 from the Michael van Vloten Foundation (Rotterdam, The Netherlands).
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research ® editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This study was performed at Massachusetts General Hospital & Brigham and Women’s Hospital, Boston, MA, USA.
Electronic supplementary material
The online version of this article (doi:10.1007/s11999-016-5118-3) contains supplementary material, which is available to authorized users.
A comment to this article is available at http://dx.doi.org/10.1007/s11999-016-5185-5.
References
- 1.Agarwal MG, Nayak P. Management of skeletal metastases: an orthopaedic surgeon’s guide. Indian J Orthop. 2015;49:83–100. doi: 10.4103/0019-5413.143915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barton MB, Dawson R, Jacob S, Currow D, Stevens G, Morgan G. Palliative radiotherapy of bone metastases: an evaluation of outcome measures. J Eval Clin Pract. 2001;7:47–64. doi: 10.1046/j.1365-2753.2001.00262.x. [DOI] [PubMed] [Google Scholar]
- 3.Broeckel JA, Jacobsen PB, Balducci L, Horton J, Lyman GH. Quality of life after adjuvant chemotherapy for breast cancer. Breast Cancer Res Treat. 2000;62:141–150. doi: 10.1023/A:1006401914682. [DOI] [PubMed] [Google Scholar]
- 4.Brucker PS, Yost K, Cashy J, Webster K, Cella D. General population and cancer patient norms for the Functional Assessment of Cancer Therapy-General (FACT-G) Eval Health Prof. 2005;28:192–211. doi: 10.1177/0163278705275341. [DOI] [PubMed] [Google Scholar]
- 5.Cella D, Riley W, Stone A, Rothrock N, Reeve B, Yount S, Amtmann D, Bode R, Buysse D, Choi S, Cook K, Devellis R, DeWalt D, Fries JF, Gershon R, Hahn EA, Lai JS, Pilkonis P, Revicki D, Rose M, Weinfurt K. Hays R; PROMIS Cooperative Group. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J Clin Epidemiol. 2010;63:1179–1194. doi: 10.1016/j.jclinepi.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 7.Cheng EY. Prospective quality of life research in bony metastatic disease. Clin Orthopaed Relat Res. 2003;415(suppl):S289–297. doi: 10.1097/01.blo.0000093054.96273.20. [DOI] [PubMed] [Google Scholar]
- 8.Choi D, Fox Z, Albert T, Arts M, Balabaud L, Bunger C, Buchowski JM, Coppes MH, Depreitere B, Fehlings MG, Harrop J, Kawahara N, Martin-Benlloch JA, Massicotte EM, Mazel C, Oner FC, Peul W, Quraishi N, Tokuhashi Y, Tomita K, Verlaan JJ, Wang M, Crockard HA. Prediction of quality of life and survival after surgery for symptomatic spinal metastases: a multicenter cohort study to determine suitability for surgical treatment. Neurosurgery. 2015;77:698–708; discussion 708. [DOI] [PubMed]
- 9.Ditre JW, Brandon TH, Zale EL, Meagher MM. Pain, nicotine, and smoking: research findings and mechanistic considerations. Psychol Bull. 2011;137:1065–1093. doi: 10.1037/a0025544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fryback DG, Dunham NC, Palta M, Hanmer J, Buechner J, Cherepanov D, Herrington SA, Hays RD, Kaplan RM, Ganiats TG, Feeny D, Kind P. US norms for six generic health-related quality-of-life indexes from the National Health Measurement study. Med Care. 2007;45:1162–1170. doi: 10.1097/MLR.0b013e31814848f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ganz PA, Lee JJ, Siau J. Quality of life assessment: an independent prognostic variable for survival in lung cancer. Cancer. 1991;67:3131–3135. doi: 10.1002/1097-0142(19910615)67:12<3131::AID-CNCR2820671232>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 12.Gonen G, Kaymak SU, Cankurtaran ES, Karslioglu EH, Ozalp E, Soygur H. The factors contributing to death anxiety in cancer patients. J Psychosoc Oncol. 2012;30:347–358. doi: 10.1080/07347332.2012.664260. [DOI] [PubMed] [Google Scholar]
- 13.Goodwin JS, Hunt WC, Key CR, Samet JM. The effect of marital status on stage, treatment, and survival of cancer patients. JAMA. 1987;258:3125–3130. doi: 10.1001/jama.1987.03400210067027. [DOI] [PubMed] [Google Scholar]
- 14.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap): a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hjermstad MJ, Fayers PM, Bjordal K, Kaasa S. Using reference data on quality of life: the importance of adjusting for age and gender, exemplified by the EORTC QLQ-C30 (+3) Eur J Cancer. 1998;34:1381–1389. doi: 10.1016/S0959-8049(98)00136-1. [DOI] [PubMed] [Google Scholar]
- 16.Holzner B, Kemmler G, Cella D, De Paoli C, Meraner V, Kopp M, Greil R, Fleischhacker WW, Sperner-Unterweger B. Normative data for functional assessment of cancer therapy: general scale and its use for the interpretation of quality of life scores in cancer survivors. Acta Oncol. 2004;43:153–160. doi: 10.1080/02841860310023453. [DOI] [PubMed] [Google Scholar]
- 17.Katagiri H, Takahashi M, Wakai K, Sugiura H, Kataoka T, Nakanishi K. Prognostic factors and a scoring system for patients with skeletal metastasis. J Bone Joint SurgBr. 2005;87:698–703. doi: 10.1302/0301-620X.87B5.15185. [DOI] [PubMed] [Google Scholar]
- 18.Lipscomb J, Gotay CC, Snyder CF. Patient-reported outcomes in cancer: a review of recent research and policy initiatives. CA Cancer J Clin. 2007;57:278–300. doi: 10.3322/CA.57.5.278. [DOI] [PubMed] [Google Scholar]
- 19.Michelson H, Bolund C, Nilsson B, Brandberg Y. Health-related quality of life measured by the EORTC QLQ-C30: reference values from a large sample of Swedish population. Acta Oncol. 2000;39:477–484. doi: 10.1080/028418600750013384. [DOI] [PubMed] [Google Scholar]
- 20.Mor V, Allen S, Malin M. The psychosocial impact of cancer on older versus younger patients and their families. Cancer. 1994;74(7 suppl):2118–2127. doi: 10.1002/1097-0142(19941001)74:7+<2118::AID-CNCR2820741720>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 21.Parker PA, Baile WF, de Moor Cd, Cohen L. Psychosocial and demographic predictors of quality of life in a large sample of cancer patients. Psychooncology. 2003;12:183–193. [DOI] [PubMed]
- 22.Parkerson HA, Zvolensky MJ, Asmundson GJ. Understanding the relationship between smoking and pain. Expert Rev Neurother. 2013;13:1407–1414. doi: 10.1586/14737175.2013.859524. [DOI] [PubMed] [Google Scholar]
- 23.Quan H, Li B, Couris CM, Fushimi K, Graham P, Hider P, Januel JM, Sundararajan V. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173:676–682. doi: 10.1093/aje/kwq433. [DOI] [PubMed] [Google Scholar]
- 24.Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med. 2001;33:337–343. doi: 10.3109/07853890109002087. [DOI] [PubMed] [Google Scholar]
- 25.Rustøen T, Moum T, Padilla G, Paul S, Miaskowski C. Predictors of quality of life in oncology outpatients with pain from bone metastasis. J Pain Symptom Manage. 2005;30:234–242. doi: 10.1016/j.jpainsymman.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 26.Tong E, Deckert A, Gani N, Nissim R, Rydall A, Hales S, Rodin G, Lo C. The meaning of self-reported death anxiety in advanced cancer. Palliat Med. 2016;30:772–779. doi: 10.1177/0269216316628780. [DOI] [PubMed] [Google Scholar]
- 27.Wan GJ, Counte MA, Cella DF. The influence of personal expectations on cancer patients’ reports of health-related quality of life. Psychooncology. 1997;6:1–11. doi: 10.1002/(SICI)1099-1611(199703)6:1<1::AID-PON230>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 28.Westhoff PG, de Graeff A, Monninkhof EM, Pomp J, van Vulpen M, Leer JW, Marijnen CA, van der Linden YM, Dutch Bone Metastasis Study Group. Quality of life in relation to pain response to radiation therapy for painful bone metastases. Int J Radiat Oncol Biol Phys. 2015;93:694–701. [DOI] [PubMed]
- 29.Wong E, Chow E, Zhang L, Bedard G, Lam K, Fairchild A, Vassiliou V, Alm El-Din MA, Jesus-Garcia R, Kumar A, Forges F, Tseng LM, Hou MF, Chie WC, Bottomley A. Factors influencing health related quality of life in cancer patients with bone metastases. J Palliat Med. 2013;16:915–921. doi: 10.1089/jpm.2012.0623. [DOI] [PubMed] [Google Scholar]
- 30.Wu J, Zheng W, Xiao JR, Sun X, Liu WZ, Guo Q. Health-related quality of life in patients with spinal metastases treated with or without spinal surgery: a prospective, longitudinal study. Cancer. 2010;116:3875–3882. doi: 10.1002/cncr.25126. [DOI] [PubMed] [Google Scholar]
- 31.Wyatt G, Sikorskii A, Tamkus D, You M. Quality of life among advanced breast cancer patients with and without distant metastasis. Eur J Cancer Care (Engl). 2013;22:272–280. doi: 10.1111/ecc.12028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zimmermann C, Burman D, Swami N, Krzyzanowska MK, Leighl N, Moore M, Rodin G, Tannock I. Determinants of quality of life in patients with advanced cancer. Support Care Cancer. 2011;19:621–629. doi: 10.1007/s00520-010-0866-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.