ABSTRACT
Natural killer (NK) cells are known to have effector and cytolytic properties to kill virus infected or tumor cells spontaneously. Due to these properties, NK cells have been used as an adoptive cellular therapy to control tumor growth in various clinical trials but have shown limited clinical benefits. This indicates that our knowledge about phenotypic and functional differences in NK cells within the tumor microenvironment and secondary lymphoid tissues is incomplete. In this work, we report that B16F10 cell-induced melanoma recruits the CD11b+CD27+ subset of NK cells at a very early stage during tumor progression. These intratumoral NK cells showed increased expression of CD69, reduced inhibitory receptor KLRG1, and decreased proliferative ability. As compared to splenic NK cells, intratumoral NK cells showed decreased expression of activating receptors NKG2D, Ly49D and Ly49H; increased inhibitory receptors, NKG2A and Ly49A; decreased cytokines IFNγ and GM-CSF; decreased cytokine receptors IL-21R, IL-6Rα, and CD122 expression. Depletion of NK cells led to decrease peripheral as well as intratumoral effector CD4+T-bet+ cells (Th1), and increased tumor growth. Furthermore, purified NK cells showed increased differentiation of Th1 cells in an IFNγ-dependent manner. Anti-NKG2D in the culture promoted differentiation of effector Th1 cells. Collectively, these observations suggest that intratumoral NK cells possess several inhibitory functions that can be partly reversed by signaling through the NKG2D receptor or by cytokine stimulation, which then leads to increased differentiation of effector Th1 cells.
KEYWORDS: Natural killer (NK) cells, NKG2D; Th1 cells, tumor microenvironment, tolerance
Introduction
NK cells have the ability to recognize and kill tumor or virus-infected cells. Based on the surface expression of CD27 and CD11b, murine and human NK cells can be classified into four distinct populations, such as CD27−CD11b−, CD27+CD11b−, CD27+CD11b+, and CD27−CD11b+, and each of these subsets show variable tissue distribution.1,2 Among the four subsets, the CD27+CD11b+ NK cells show higher cytotoxicity, cytokine production, and are known to control dendritic cell (DC) function in mice.1 CD27−CD11b− and CD27+CD11b− NK cells display immature phenotype, and have the potential to further differentiate into functionally mature NK subsets in both mice and humans.2,3 Mature CD27−CD11b+ NK cells were reported as one of the dominant NK cell subset in the lung, liver, blood and spleen, whereas the CD27+CD11b− NK cell subset was found to be predominant in the bone marrow and lymph nodes (LN).1 The ability of NK cells to recognize target cell is mediated by signaling through activating receptors such as NK group 2 member D (NKG2D), Ly49D, DNAM-1, and natural cytotoxicity receptors (NCRs), whereas tolerance to healthy cells is regulated through inhibitory receptors such as CD94, NKG2A, several killer immunoglobulin-like receptors (KIRs), and Ly49 family molecules. The fine balance and integration of signaling from activating and inhibitory receptors regulates the cytotoxic activity of NK cells. Besides a direct cytotoxic effect, NK cell-derived cytokines also play an important role in regulating the function of other immune cells like DCs and CD4+ T cells. NK:DC interactions predominantly lead to the functional activation of DCs, which increases their ability to enhance priming of the CD4+ T cell response and promotes Th1 polarization.4
Infiltration of NK cells has been observed in several types of tumors such as fibrosarcoma, renal cell carcinoma, gastrointestinal sarcoma, and breast tumors.5,6 The CD27−CD11b− NK cells were the dominant population reported in lung tumors7 whereas CD27+CD11b+ cells were more prevalent in fibrosarcoma.5 Despite the infiltration of cytotoxic NK cells in the tumor microenvironment, tumors continue to grow, suggesting that the tumor microenvironment, which includes stromal cells and tumor-derived factors, can modulate the phenotype and function of NK cells. Pietra et al. have demonstrated that melanoma cell lines inhibit the cytolytic activity of NK cells in vitro and that this impairment was mediated by melanoma cells-derived IDO (Indoleamine 2, 3-dioxygenase) and PGE2 (Prostaglandin E2).8 Melanoma-associated fibroblasts have also been reported to suppress the cytotoxic activity of NK cells in both contact-dependent and contact-independent manner.9 Several other suppressive cells in the tumor microenvironment, such as myeloid-derived dendritic cells (MDSCs), CD4+ regulatory T cells and M2 macrophages are also known to inhibit the cytolytic function of NK cells through secretion of inhibitory factors like IL-10 and TGF-β.10-12 In contrast to these suppressive cytokines, several cytokines such as IL-2, IL-12, IL-15, IL-18, and IL-21 are known to activate NK cells both in vitro and in vivo, and are proposed to increase the therapeutic potential of these cells for adoptive therapy.13
Due to their natural cytotoxic property in the peripheral blood,14 the use of NK cells has been identified as one of the promising adoptive cellular immunotherapies to control cancer.15 However, although NK cells use several strategies to eliminate tumor cells, NK cell immunotherapy has shown very limited clinical benefits.16 The kinetics and phenotypes of NK cell subsets present in the melanoma, and how they might modulate the adaptive immune response in a tolerogenic tumor microenvironment is not clearly understood. In the present study, we investigated the kinetics of the recruitment of various NK cell subsets in the melanoma tumor and their phenotype (expression of activating and inhibitory receptors). We further showed that depletion of NK cells led to a reduction in the Th1 cells in the peripheral tissues and tumor, and which then resulted in increased tumor growth. Our in vitro data further supported that splenic and intratumoral NK cell promoted the differentiation of Th1 cells in an IFNγ-dependent manner. Anti-NKG2D mAb further enhanced the differentiation of Th1 cells, suggesting that signaling through these receptors in NK cells can modulate the differentiation of effector Th1 cells.
Materials and methods
Mice
Six to 8 weeks-old C57BL/6 male mice were used. These mice were procured from The Jackson Laboratory (Maine, USA), and bred in our experimental animal facility. All experimental animal procedures were approved by the Institutional Ethics Committee of Animals usage (reference number EAF/2011/B-166 and EAF/2016/B-256).
Tumor transplantation
The B16F10 (mouse melanoma) cell line was maintained in complete culture medium [high glucose DMEM medium (Invitrogen, Carlsbad, CA) containing 10% FBS (Gibco), NaHCO3 (1.5 g/L), penicillin (50 units/mL), streptomycin (50 μg/mL) and sodium pyruvate (1 mM)] at 37°C in a humidified 5% CO2 incubator. B16F10 cells (1 × 106 cells/mouse in 200 μL PBS) were subcutaneously (s.c.) injected into the right flank of C57BL/6 mice. Tumor growth was monitored every alternate day, and tumor area was measured with the help of a caliper using the formula A = L × W, where L = length of tumor (mm), W = width of tumor (mm), A = Area (mm2).
Antibodies and other reagents
FITC-CD3ϵ (17A2), Alexa fluor 647-CD3ϵ (17A2), Brilliant violet 421-CD3ε (17A2), Alexa fluor 488-CD3ε (145-2C11), Alexa fluor 647 CD49b (DX5), Pacific blue-CD49b (DX5), PE-NK1.1 (PK136), Brilliant violet 421-NK1.1 (PK136), Alexa fluor 488 NK1.1 (PK136), PE/Cy7-CD27 (LG.3A10), Biotin-CD11b (M1/70), Brilliant violet 421-CD11b (M1/70, APC/Cy7-B220 (RA3-6B2), FITC-B220 (RA3-6B2), Biotin-CD4 (GK1.5), Alexa fluor 488-CD4 (GK1.5), APC-eFlour 780-CD4 (GK1.5), PE/Cy5-CD4 (GK1.5), PE-FoxP3 (FJK-16s), APC-TCRγδ (GL3), FITC-F4/80 (BM8), Pacific blue-CD11c (N418), Biotin Gr-1 (RB6-8C5), PE/Cy5-IL-21R (4A9), PE-IL-21R (4A9), Biotin-IFNγ-Rα (2E2), APC-IL-6Rα (D7715A7), Brilliant violet 421-CD25 (PC61), PE-CD25 (PC61), PE-NKG2D (CX5), Biotin-NKG2D (C7), Alexa fluor 647-Ly49D (4E5), Pacific blue-Ly49A (YE1/48.10.6), PE-CD107a (1D4B), Biotin-NKG2A (16A11), Alexa fluor 647-Ly49H (3D10), FITC-KLRG1 (2F1/KLRG1), Biotin-CD122 (5H4), purified anti-mouse NKG2D (C7), purified armenian hamster IgG isotype control (HTK888), purified anti-mouse CD159a (NKG2AB6) (16A11), purified mouse IgG2b, k isotype control (MG2b-57), purified rat IgG2a, k isotype control (RTK2758), purified anti-mouse Ly49D (4E5), purified anti-mouse Ly49H (3D10), PE/Cy7-IFNγ (XMG1.2), PE-GM-CSF (MP1-22E9), Pacific blue-TNF-α (MP6-XT22), PercCP/Cy5.5-CD69 Biotin-BrdU (Bu20a), FITC-Ki67 (16A8), Alexa fluor 647-streptavidin and APC-Cy7-Streptavidin and PE-Cy7-Streptavidin were purchased from Biolegend (San Diego, CA). Biotin-CD27 (LG.7F9), APC-eFlour 780-CD4 (GK1.5), APC-RORγt (AFKJS-9), PE-RORγt (AFKJS-9), APC-T-bet (4B10) and PE/Cy7-T-bet (4B10) were procured from eBioscience (San Diego, CA). PE/Cy7-CD11b (M1/70) was from BD Bioscience (San Jose, CA). Anti-mouse NK1.1 (PK136), mouse IgG2a isotype control (C1.18.4), and anti-mouse IFNγ (XMG1.2), were purchased from Bioxcell (West Lebanon, NH). Recombinant mouse IL-2, IFN-γ and IL-21 were purchased from Peprotech (Rehovot, Israel). Recombinant mouse IL-2 was purchased from Biolegend (San Diego, CA). Dylight549-strptavidin was from Jackson ImmunoResearch (West Grove, PA).
Intracellular cytokine staining
For cytokine analysis, the cells were stimulated with 81nM PMA, 1.34 μM ionomycin, 10.6 μM brefeldin and 2 μM monensin in RPMI medium containing 10% FBS for 6 h at 37°C in 5% CO2 incubator. Cells were surface-stained using saturating concentrations of specific antibodies at 4°C for 30 min; washed and incubated with appropriate secondary antibodies (1:500 dilution) for 30 min on ice. For intracellular staining of cytokines and transcription factors, cells were fixed and permeabilized with the Foxp3 fixation permeabilization kit (Biolegend, San Diego, CA) according to the manufacturer's instructions.
ELISA
IFNγ ELISA from culture supernatants were performed using mouse ELISA Max Deluxe kit as per manufacturer's guidelines (Biolegend).
Immunohistochemical staining and analysis
For immunohistochemical staining, tissues were embedded in OCT tissue freezing medium (Sakura Finetek, Torrance, CA), snap frozen in liquid nitrogen and stored in −80°C. Tissue sections (8 μm thick) were fixed in chilled acetone for 5 min, air dried, washed with cold PBS, and blocked with 10% horse serum (Jackson ImmunoResearch, West Grove, PA) for 30 min at room temperature (RT). Sections were washed with PBS and incubated with indicated fluorochrome-conjugated primary Abs (1:200 dilution) for 45 min; washed three times with cold PBS, incubated with secondary antibodies (1:1000 dilution) for 30 min; washed three times with PBS, fixed with 1% paraformaldehydre, and mounted in aqueous mounting medium containing DAPI (Electron Microscopy Sciences, Hatfield, PA). Immunofluorescence images were captured using the Leica DMI 6000 fluorescent microscope (Leica Microsystems, Germany).
Quantitative real-time RT-PCR (qRT-PCR) analysis
Total RNA was isolated from purified cells using Trizol reagent (Invitrogen). cDNA was prepared using Superscript II RT kit (Invitrogen) and oligo (dT)14–16 primers. Real-time PCR were performed using SYBR green dye (Invitrogen) on Bio-Rad CFX 96 Real-Time System (Bio-Rad, Germany). The PCR cycle consisted of denaturation for 10-15 min at 95°C, followed by 40 cycles of 15 sec at 95°C, 20 sec at 56°C and 30 sec at 72°C. The relative mRNA expression of a specific gene was calculated as: 2(Ct of control gene−Ct of specific gene). The relative expression was normalized to the housekeeping gene, cyclophilin A or GAPDH. Primer sequences are listed in supplementary Table 1.
In vivo depletion of NK cells
NK cells were depleted by intravenous (i.v.) injection of anti-NK1.1 monoclonal antibody (clone PK136; 100 μg/injection/mouse; Bioxcell, West Lebanon, NH) in C57BL6 mice on days −3, 0, +1, +4, +9, +14, and +19 with respect to tumor cell injection. Mice were sacrificed either on day 13 or 23, and NK cell depletion was checked by flow cytometry.
Preparation of single cell suspension and staining
Tumors were excised, manually disrupted into small pieces using fine forceps, re-suspended in 1X Hanks balanced salt solution (HBSS) containing collagenase type I (0.1 mg/mL, Gibco) and collagenase type IV (0.1 mg/mL, Sigma), hyaluronidase (0.06 mg/mL, Sigma), DNase I (0.02 mg/mL, Sigma) and soybean trypsin inhibitor (0.1 mg/mL, Sigma), and incubated for 30–90 min at 37°C in a shaking water bath. Single cell suspensions were obtained by passing through a 70 µm pore-size cell strainer (BD Biosciences, San Jose, CA). Following removal of RBCs by ACK lysis buffer and washing with RPMI medium, the cell suspensions were subjected to flow cytometry sorting (FACS Aria III, BD Bioscience) or analysis (FACS Canto II, BD Bioscience). Single cell suspension of spleen was prepared by mechanical disruption, and passed the cell suspensions through a 70 µm pore-size strainer, removed RBCs by ACK lysis buffer, washed with RPMI medium, stained and re-suspended in RPMI medium for flow cytometry sorting or flow cytometric analysis. Purity of sorted cells were >95%.
NK cell degranulation assay
For NK cell degranulation assay, NK cells were purified from naive mice spleen (Nsp), tumor-bearing mice spleen (Tsp), and tumor tissues (Tu) using flow cytometry sorting (FACS Aria II, BD Bioscience, San Jose, CA). NK cells (1 × 105 cells) were cultured with B16F10 as target cells at E:T ratio of 20:1 in 200 μL complete RPMI medium in the presence of anti-CD107a mAb (1:100 dilution) in 96 well U-bottom plates, and incubated at 37°C in a 5% CO2 incubator for 5 h. Cells were harvested, stained with indicated mAb and analyzed by flow cytometry (FACS Canto II, or FACSAria III). Data were analyzed using FlowJo software (Tree Star Inc., Ashland, OR).
In vitro culture of CD4+ T cells
Naive CD4+ T cells (CD4+CD25−CD44−) and T cell-depleted splenocytes were purified from C57BL/6 mice spleen by flow cytometry sorting. CD3−NK1.1+ NK cells were purified from spleen and tumor by flow cytometry sorting. Naive CD4+ T cells (5 × 104 cells/well) and NK cells (2.5 × 104 cells/well) were cultured along with irradiated T cell-depleted splenocytes (5 × 104 cells/well, irradiated 800 rad) in 200 μL of complete RPMI media in the presence of IL-2 (20 ng/mL), anti-CD3ε (5 μg/mL) in 96 well U-bottom plates. In some cases, purified anti-mouse IFNγ (10 μg/mL) antibodies was added in the cultures. Purified anti-mouse NKG2D (10 μg/mL), purified anti-mouse Ly49D (10 μg/mL), purified anti-mouse Ly49H (10 μg/mL), purified anti-mouse NKG2A (10 μg/mL) or purified isotype control IgG (10 μg/mL) were added in indicated culture conditions. Cells were cultured at 37°C in a 5% CO2 incubator for 4 d. Cells were harvested and stained with anti-CD4, anti-CD25 and anti-T-bet mAb, and analyzed using flow cytometry.
In vitro culture of NK cells
CD27+CD11b−, CD27+CD11b+, and CD27−CD11b+ NK cell subsets were purified from spleen and tumor using flow cytometry sorting. NK cells (1 × 104 cells/well) were cultured in 200 μL of complete RPMI media in the presence of IL-2 (20 ng/mL) or IL-2/IL-21 (20 ng/mL) into 96 well U-bottom plate at 37°C in a 5% CO2 incubator for 5 d. Cells were harvested and stained with anti-NK1.1, anti-NKG2D, and anti-Ly49D, and analyzed using flow cytometry.
Statistical analysis
Unpaired two-tailed Student's t-test was used to compare two independent groups. One-way ANOVA followed by Tukey's post hoc procedure was used to compare three independent groups. A p value of less than 0.05 was considered statistically significant. All statistical analyses were performed using GraphPad Prism 6 software (GraphPad Software, San Diego, CA).
Results
Selective recruitment of CD27+CD11b+ NK cells into the tumor microenvironment
Subcutaneous injection of B16F10 melanoma cells in syngeneic C57BL/6 mice showed progression of tumor growth with increased infiltration of mononuclear cells, including NK cells (Fig. 1A). CD3−NK1.1+ NK cells in the tumor constituted about 25% of the total lymphocytes as early as day 5 (Fig. 1B), and this infiltration of NK cells was maintained even at later stages (day 13) (Fig. 1B). Injection of tumor cells resulted in a significant increase in the percentage of NK cells in the spleen at day 5, as compared to the naive mouse spleen, and returned to similar levels as in the naïve mouse spleen at day 13 (Fig. 1B). No significant changes were observed in the size of spleen or absolute number of NK cells in the spleen of naive mice, day 5 and day13 tumor-bearing mice (data not shown). The absolute numbers of NK cells increased significantly from day 5 to day 13 in the tumor (Fig. 1B). These results indicated that NK cells migrated very early into the growing tumor and maintained their numbers in the tumor microenvironment. Based on the surface expression of CD11b and CD27, NK cells can be divided into four phenotypic subsets.1 From these, we observed a high frequency of CD27+CD11b+ NK cell subsets in the tumor at day 5 and day 13 (Fig. 1C). The absolute number of all NK cell subsets increased from day 5 to day 13 in the tumors (Fig. 1D). These results suggest that specific subsets of NK cells (represented by CD27+CD11b+ subset) are preferentially recruited into the developing tumor.
Figure 1.
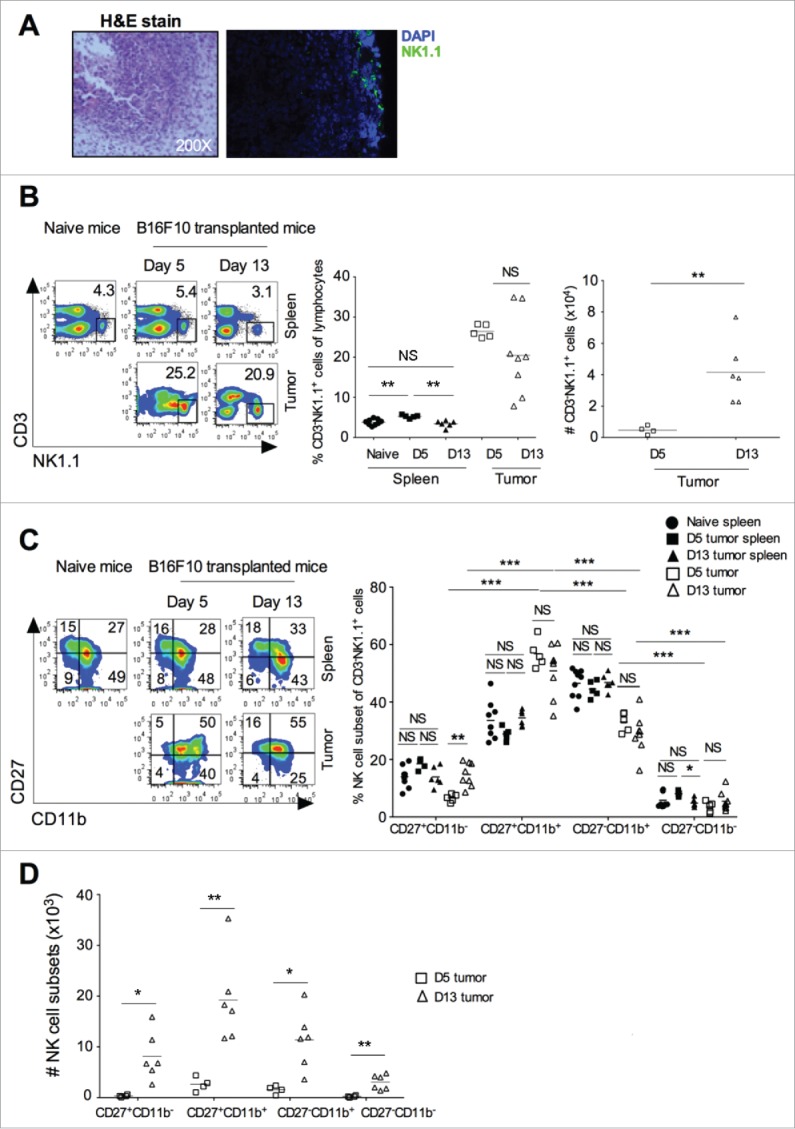
B16F10 induced tumor showed early recruitment of NK cells. B16F10 cells (1×106 cells/mouse) were s.c. injected in the naive C57BL/6 mice. (A) At day 13, tumor was harvested, representative H&E staining is shown (left), and a representative image of NK1.1+ NK cells staining in the tumor shown (right). (B) Tumor and spleen was harvested at days 5 and 13 after B16F10 injection, and CD3−NK1.1+ cells were analyzed after gating on lymphocytes and singlet population using flow cytometry (left). Numbers indicate the percentage of cells in the gated region. Mean percentage of NK cells in the spleen and tumor were plotted (middle). The absolute number of CD3−NK1.1+ cells was calculated and plotted (right). Data are representative of 2-5 independent experiments. (C) Based on CD11b and CD27 staining, NK cell subsets were analyzed by flow cytometry after gating on CD3−NK1.1+ cells (left). Mean percentage of indicated NK cell subsets were plotted (right). (D) The absolute numbers of NK cell subset in the tumor at day 5 and day 13 were counted and plotted. From (B)–(D), the bars represent mean and each dot represents an individual mouse (n = 5–9 mice/group for naive and day 5, and n = 6–8 mice/group for day 13; one-way ANOVA followed by Tukey's post hoc analysis). Data are representative 2-5 independent experiments. In all panels, *p < 0.05; **p < 0.01; NS, not significant.
Intratumoral NK cells showed reduced KLRG1 expression, increased CD69 expression, and lower proliferation
Having observed accumulation of a specific subset of NK cells in the tumor, we further investigated whether these NK cells also proliferate within the tumor microenvironment. KLRG1, an inhibitory C-type lectin receptor, has been shown to regulate homeostasis and maturation of NK cells.17 Terminally mature NK cell subsets (CD27−CD11b+) have been shown to express KLRG1 and early activation antigen-CD69.1 Our results showed that intratumoral NK cells had lower expression of KLRG1 and higher expression of CD69 (Fig. 2A). Furthermore, both intermediate CD27+CD11b+ and terminally mature CD27−CD11b+ subset of NK cells in the tumor microenvironment showed significantly reduced expression of KLRG1, as compared to splenic subsets (Fig. 2B). Since KLRG1+ NK cells have been reported to have reduced proliferative capacity and turnover rate than KLRG1− NK cells,17 the lower KLRG1 expression on tumor-infiltrating NK cells suggests that these cells might have the higher proliferation capacity than their splenic counterparts. However, the analysis of bromo-deoxyuridine (BrdU) incorporation in DNA and Ki67 expression showed significantly less BrdU incorporation and reduced Ki67 expression in tumor NK cells, indicating that they had a lower proliferating rate than the splenic counterparts (Fig. 2C). Furthermore, our data also showed reduced Ki67 and BrdU staining in tumor infiltrating CD27+CD11b− CD27+CD11b+ and CD27−CD11b+ NK subsets, as compared to splenic populations (Fig. 2D). Together, these results clearly suggest that tumor NK cells express CD69 and show reduced proliferation, as compared to splenic subsets.
Figure 2.
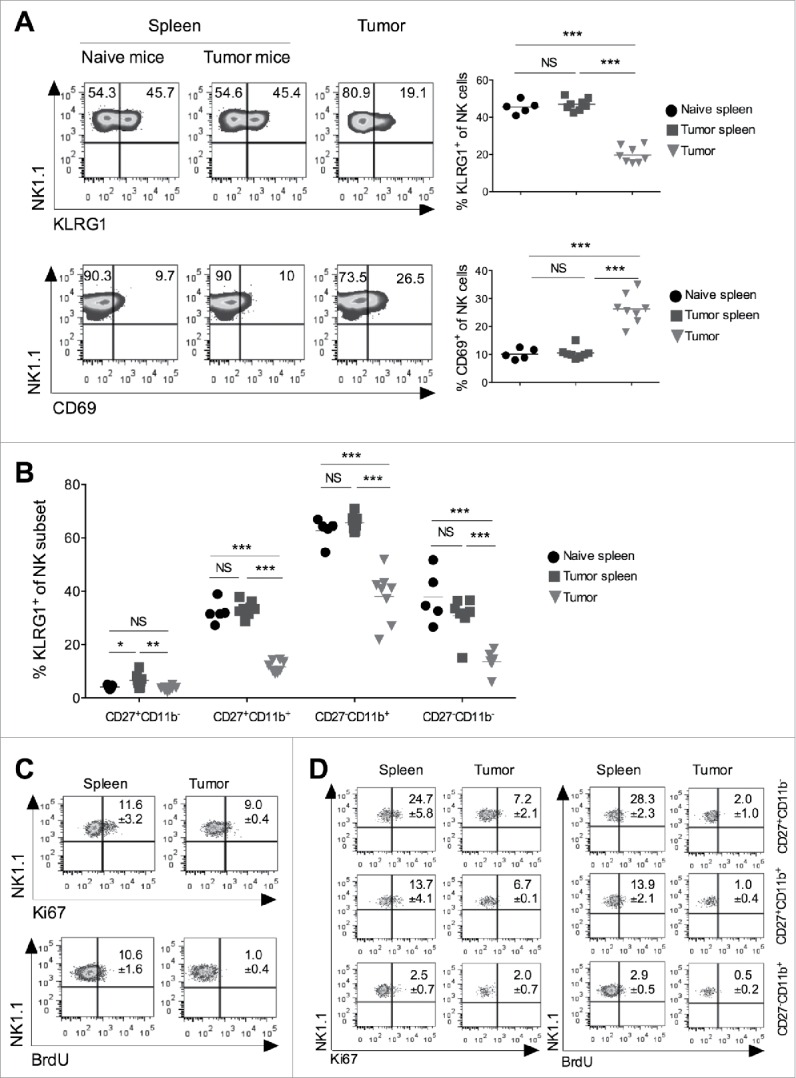
Tumor infiltrating NK cells show reduced KLRG1 expression and lower proliferation. B16F10 cells (1 × 106 cells/mouse) were s.c. injected into the naive C57BL/6 mice. (A) At day 13, spleen and tumors were harvested, and expression of KLRG1 and CD69 on total CD3−NK1.1+ NK cells were monitored using flow cytometry. Numbers in the quadrant represent percentage of cells in the indicated region. Plots represent mean percentage of KLRG1+ and CD69+ NK cells. The bar represents the mean and each dot represents an individual mouse (n = 5–8 mice/group; one-way ANOVA followed by Tukey's post hoc analysis). (B) At day 13, expression of KLRG1 on indicated subsets of NK cells was analyzed by flow cytometry. Plots represent KLRG1+ NK cell subsets after gating on CD3−NK1.1+ cells. The bar represents the mean and each dot represents an individual mouse (n = 5–8 mice/group; one-way ANOVA followed by Tukey's post hoc analysis). (C) B16F10 cells (1 × 106 cells/mouse) were s.c. injected in the naïve C57BL/6 mice. 5-Bromo-2′-deoxyuridine (BrdU; 150 μg/mouse) was injected i.p. twice a day on days 12, 13, and 14 with respect to B16F10 cell injection. Animals were sacrificed on day 15, spleen and tumor tissues from individual mice were harvested (n = 3 mice). Cells were stained with anti-BrdU and anti-Ki67 mAb, and analyzed by flow cytometry after gating on NK1.1+CD3ε− cells. (D) Expression of Ki67 and BrdU on NK cell subsets were analyzed after gating based on CD27 and CD11b staining using flow cytometry. Numbers in the plot indicate mean percentage of cells ± SEM, n = 3 mice/group. In all panels, *p < 0.05; **p < 0.01; ***p < 0.001; NS, not significant.
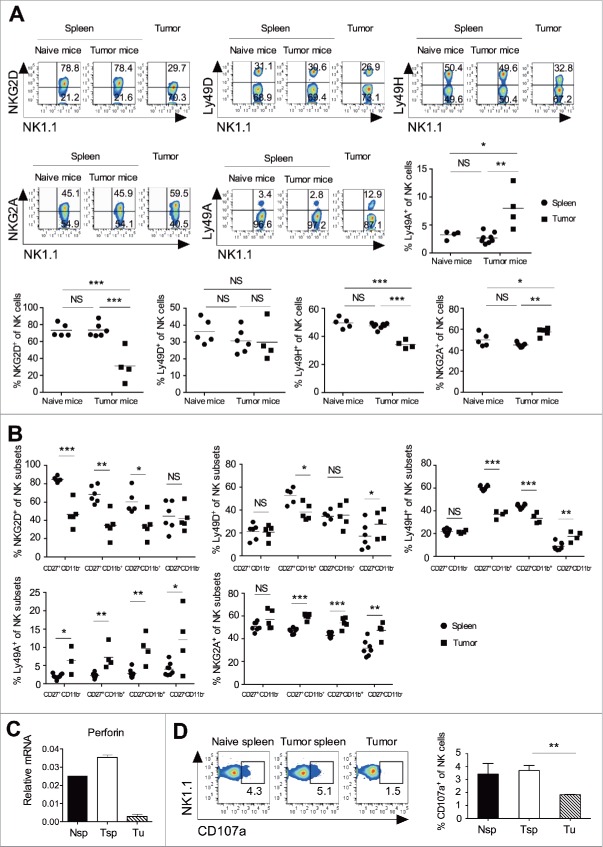
Tumor NK cells showed altered activating and inhibitory receptors, and reduced cytolytic functions
Activating receptors promote the cytolytic function of NK cells, whereas inhibitory receptors help in maintaining a tolerogenic microenvironment. The cumulative signals from these receptors dictate the cytolytic function of NK cells.18 Our findings showed that intratumoral NK cells had increased inhibitory receptor Ly49A and NKG2A, and decreased activating receptor NKG2D and Ly49H expression, whereas Ly49D expression was unaltered as compared to the splenic population (Fig. 3A). To test the role of B16F10 induced changes in the expression of activating receptors, we cultured the splenocytes in 50% B16F10 conditioned media for 72 h. Results showed significant reduction in the expression of NKG2D and Ly49H on conditioned media exposed NK cells as compared to without conditioned media (data not shown). Except the CD27−CD11b− subset, all other NK cell subsets in the tumor showed significantly decreased NKG2D expression as compared to those in the spleen (Fig. 3B). The CD27+CD11b+ NK subset showed significantly decreased Ly49D expression in the tumor, as compared to the spleen (Fig. 3B). The CD27+CD11b+ and CD27−CD11b+ tumor NK cells showed significantly reduced Ly49H expression as compared to the splenic subsets (Fig. 3B). The majority of NK subsets showed increased NKG2A and Ly49A expression in the tumor, as compared to the splenic subsets (Fig. 3B). These results clearly suggest that intratumoral NK cells have increased inhibitory receptors and reduced activating receptors, which might have a cumulative effect on their reduced effector and cytolytic function. In fact, we found reduced perforin expression in the intratumoral NK cells as compared to splenic NK cells (Fig. 3C), and the intratumoral NK cells showed reduced cytolytic activity as measured by flow cytometry based degranulation assay19 (Fig. 3D). Together, these results suggest that as compared to the splenic population of NK cells, intratumoral NK cells have increased inhibitory receptors, reduced activating receptors, and show poor cytolytic activity against tumor cells.
Figure 3.
Tumor infiltrating NK cell display an altered expression of activating and inhibitory receptor and impaired cytolytic function. B16F10 cells (1×106 cells/mouse) were s.c. injected in the naive C57BL/6 mice. (A) At day 13, spleen and tumors were harvested, and frequencies of NKG2D, NKG2A, Ly49D, Ly49H, and Ly49A expression on CD3ε−NK1.1+ cells were analyzed using flow cytometry. Numbers in each region represent percentage of cells. The bar represents mean and each dot represents an individual mouse (n = 4–8 mice per group; one-way ANOVA followed by Tukey's post hoc analysis. (B) At day 13, the frequency of NKG2D, NKG2A, Ly49D, Ly49H, and Ly49A on the indicated of NK subsets were analyzed after gating on CD3−NK1.1+ cells using flow cytometry. The bar represents mean and each dot represents an individual mouse (n = 4–8 mice; Student's t-test). (C) At day 13, CD3−CD49b+ NK cells were purified from pooled naïve mouse spleen (Nsp), tumor bearing mouse spleen (Tsp) and tumor (Tu) using flow cytometry sorting. Perforin mRNA expression was analyzed using qRT-PCR. Data shows the Mean ± SD of duplicate wells, and data are representative of two independent experiments. (D) At day 13, NK cells were purified from pooled spleen and tumor using flow cytometry sorting, and degranulation of NK cells against B16F10 tumor cells were performed as described in materials and methods. The numbers represent the percentage of cells in the region. Mean ± SEM shown, n = 3 independent experiments. In all the panels, *p < 0.05, **p < 0.01, ***p < 0.001, NS, not significant.
Intratumoral NK cells display altered cytokine receptors and cytokines expression
Activated NK cells are known to produce an array of cytokines, chemokines and growth factors that can modulate the function of NK cells as well as other immune cells in the inflamed tissue or secondary lymphoid tissue. We analyzed pro-inflammatory and anti-inflammatory cytokines produced by the intratumoral and splenic NK cells. The results showed that IFNγ and GM-CSF expression by the intratumoral NK cells was significantly less as compared to the splenic population (Figs. 4A and S1A). Concurrently, we also observed significant lower expression of transcription factor T-bet by the intratumoral NK cells, as compared to the splenic NK cells (Fig. S1B). No significant differences were observed between intracellular TNF-α expression in splenic and intratumoral NK cells (Fig. 4A). The expression of IL-10 and IL-6 mRNA was upregulated in intratumoral NK cells, as compared to the splenic population (Fig. S1C). Our results suggest that intratumoral NK cell downregulate several pro-inflammatory cytokines, while upregulate anti-inflammatory cytokines, which might hamper antitumor immune response in the tumor microenvironment.
Figure 4.
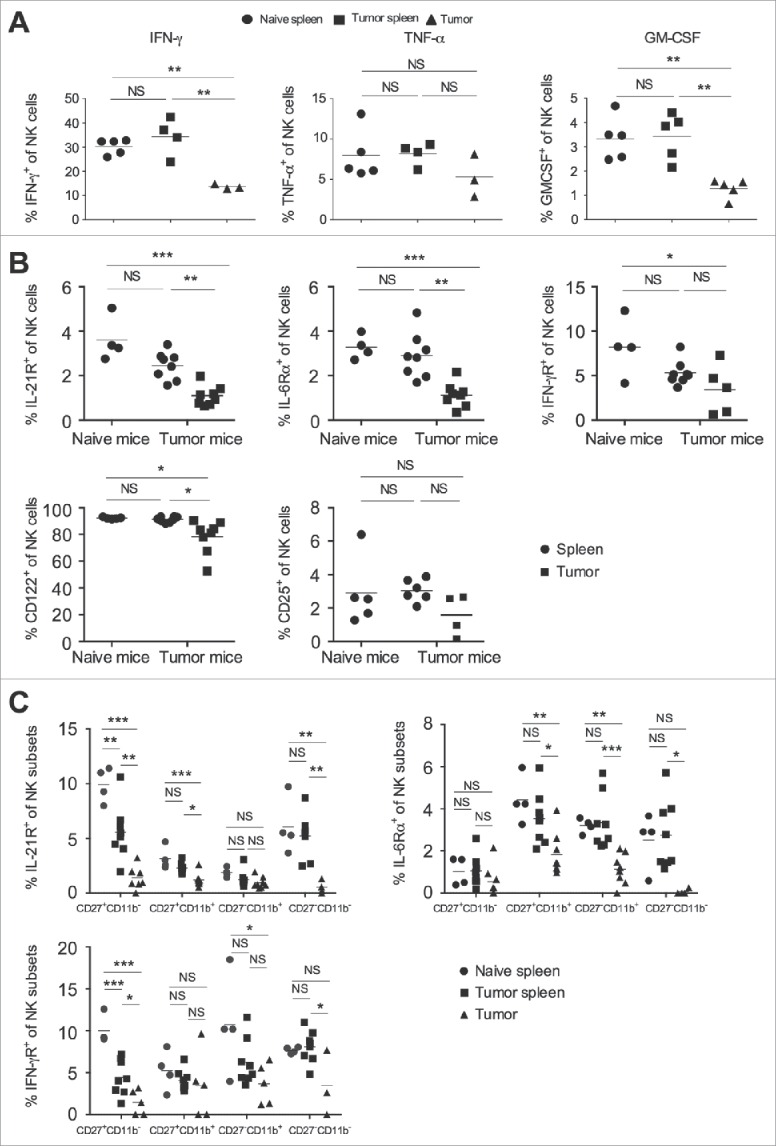
Tumor infiltrating NK cells shows altered expression of cytokine and cytokine receptor. B16F10 cells (1×106 cells/mouse) were s.c. injected in the naïve C57BL/6 mice. (A) At day 13, spleen and tumors were harvested, and single cell suspension made. Cells were stimulated with cell stimulation cocktail as mentioned in materials and methods, and intracellular cytokine expression was analyzed after gating on CD3−NK1.1+ cells using flow cytometry and plotted. The bar represents mean and each dot represents an individual mouse (one-way ANOVA followed by Tukey's post hoc analysis). (B) On day 13, single cell suspension from spleen and tumors were made, surface stained for indicating molecules, and their expression on NK cells was analyzed after gating on CD3−NK1.1+ cells using flow cytometry. The bar represents mean and each dot represents an individual mouse (n = 4–8 mice per group; one-way ANOVA followed by Tukey's post hoc analysis). Data are representative of two independent experiments (C) The frequencies of cytokine receptor on cell surface were analyzed on the indicated NK cell subsets based on CD11b and CD27 staining using flow cytometry. The bar represents mean and each dot represents an individual mouse (n = 4–8 mice per group; one-way ANOVA followed by Tukey's post hoc analysis). In all panels, *p < 0.05; **p < 0.01; ***p < 0.001; NS, not significant.
To investigate the NK cell response to various cytokines that might be present in the tumor microenvironment, we monitored the expression of various cytokine receptors on NK cells. Intratumoral NK cells showed reduced frequency of IL-21R, IL-6Rα, IFN-γRα, and CD122 (IL-2Rβ) expression as compared to the splenic population, while CD25 (IL-2Rα) expression was similar (Fig. 4B). Since our results showed that the majority of NK cells in the tumor are CD27+CD11b+ and CD27−CD11b+, we tested the profile of expression of various cytokine receptors on different subsets of NK cell in the spleen and tumor. The frequency of IL-21R was significantly lower on CD27+CD11b−, CD27+CD11b+, and CD27−CD11b− subsets, whereas the expression of IL-6Rα was decreased on the CD27+CD11b+ and CD27−CD11b+ subsets of NK cells in the tumor, as compared to the spleen (Fig. 4C). The frequency of IFN-γRα was significantly lower on the immature CD27−CD11b− and CD27+CD11b− subsets of tumor infiltrating NK cells, as compared to splenic subsets (Fig. 4C). No differences were observed in IFN-γRα expression on mature CD27+CD11b+ and CD27−CD11b+ subsets of NK cells from the tumor and the spleen (Fig. 4C). Collectively, these results suggest that reduced expression of several cytokine receptors in intratumoral NK cells might lead to altered effector function in the tumor.
IL-2 and IL-21 are known to activate NK cell function and promote tumor rejection.20-22 Our data suggest that tumor infiltrating NK cells have reduced IL-21R (Fig. 4B and C) and activation receptors NKG2D, Ly49D, and Ly49H (Fig. 3A and B). Therefore, we investigated the effect of IL-2 and IL-21 on the expression of Ly49D and NKG2D on various subsets of NK cells present in the spleen and tumor. Our results showed that in vitro stimulation of NK cells with purified IL-2 increased the expression of activating receptor NKG2D on all subsets of intratumoral NK cells, and this induced expression was either similar or slightly higher than the splenic subsets (Fig. S2). The addition of IL-21 along with IL-2 only marginally increased the expression of NKG2D on the CD27+CD11b− and CD27−CD11b+ subsets of tumor NK cells. IL-2 and/or IL-21 treatment did not affect the expression of Ly49D on the different subsets of NK cells (Fig. S2). Therefore, the tumor microenvironment may have contributed to the observed expression of cytokine receptors in the subsets of intratumoral NK cells, which may have an influence on the function of these cells. Our findings suggest that stimulation with cytokines like IL-2 could potentially boost the function of intratumoral NK cells.
NK cells control differentiation of Th1 cells
In order to understand the importance of NK cells in regulating tumor growth, we depleted NK cells by intravenous injection of anti-mouse NK1.1 antibody. Treatment with anti-NK1.1 antibody depleted about 90% of NK cells in the spleen (Fig. S3A) but did not alter the frequency of Foxp3+CD4+ Treg, γδ T cells, F4/80+ macrophages, MDSCs, and DCs in the secondary lymphoid organs (Fig. S3B). Our results further showed that NK cell depletion led to a significant enhancement in tumor growth, as compared to tumor in the control IgG treated animals, suggesting that NK cells are involved in controlling tumor growth (Fig. 5A). Since NK cell depletion increased tumor growth (Fig. 5A), we investigated the effect of NK cell depletion on the differentiation of effector CD4+ T cells. Our results showed that depletion of NK cells significantly decreased the percentage of T-bet+CD4+ T cells (Th1) in the spleen of tumor-bearing mice at day 13 (Fig. 5B) as well as at a later time-point (day 24) (Fig. 5C). Furthermore, depletion of NK cells also decreased the expression of T-bet and IFNγ mRNA in the intratumoral CD4+ T cells (Fig. 5D). Immunohistochemical analysis of spleen, LN, and tumor showed a decreased number of Th1 cells in NK cell-depleted mice, as compared to control IgG-treated mice (Fig. 5E). These results clearly indicate a positive association between NK cells and differentiation of Th1 cells in the spleen and tumor.
Figure 5.
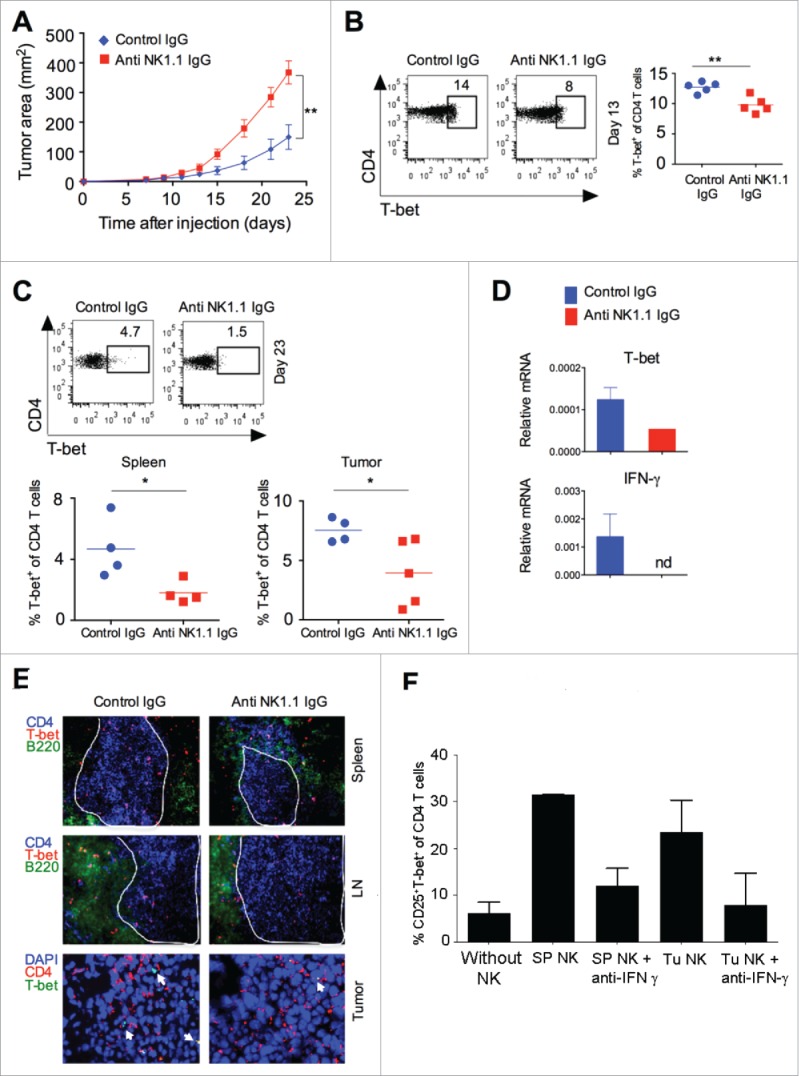
NK cell depletion affects T cell differentiation and result in increased tumor growth. B16F10 cells (1 × 106 cells/mouse) were s.c. injected in the naive C57BL/6 mice, and NK cells were depleted by i.v injection of NK1.1 mAb (PK136; 100 μg/mouse/injection) or isotype control IgG2a on days −3, +1, +4, +9, +14 and +19 with respect to tumor cell injection. (A) Tumor growth was monitored and plotted (n = 4–5 mice/group; Student's t-test, *p < 0.05; **p < 0.01). (B) At day 13, CD4+T-bet+ T cells (Th1) in the spleen were analyzed after gating on CD4+ cells using flow cytometry (left panel). The numbers represent percentage of cells in the region. The mean percentages of CD4+T-bet+ T cells (Th1) are plotted (right panel). The bar represents mean and each dot represents an individual mouse. (C) At day 23, CD4+T-bet+ T cells (Th1) in the spleen and tumor were analyzed after gating on CD4+ cells using flow cytometry (upper panel). The numbers represents percentage of cells in the region. The mean percentages of CD4+T-bet+ T cells (Th1) in the spleen and tumors are plotted (bottom panel). The bar represents mean and each dot represents an individual mouse. (D) At day 13, CD4+ T cells were purified from tumor using flow cytometry. T-bet and IFNγ mRNA expression was analyzed using qRT-PCR. Bar graph represents the expression of the indicated gene relative to GAPDH. Data shown are the Mean ± SD; nd, not detected. (E) At day 23, CD4+T-bet+ T cells (Th1) in the spleen, lymph nodes (LN) and tumors were analyzed by immunohistochemistry. Original magnification 400× for spleen and LN; and 630× for tumor. White line indicates CD4+ T cell zone in spleen and lymph node tissues. White arrow in the tumor tissue indicates T-bet+ cells. (F) Naive CD4+ T cells (5 × 104 cells/well) were cultured with purified splenic or tumor NK cells (2.5 × 104 cells/well) and irradiated T cell depleted syngeneic splenocytes (5 ×104 cells/well) along with recombinant IL-2 (20 ng/mL) and anti-mouse CD3ε (5 μg/mL) in the presence or absence of anti-IFNγ mAb (10 μg/mL) for 4 d at 37°C in U-bottomed 96 well plates. Expression of T-bet was analyzed after gating on CD4+ cells by flow cytometry. The mean percentages of cells were plotted and error bas represent SEM; n = 2 experiments (Student's t-test, *p < 0.05; **p < 0.01).
To further investigate whether NK cells directly regulate the differentiation of effector CD4+ T cells, we cultured naive CD4+ T cells (CD4+CD25−CD44− cells) together with purified splenic or intratumoral NK cells. NK cells promoted the in vitro differentiation of naive CD4+ T cells into CD25+T-bet+CD4+ (Th1) cells in an IFNγ-dependent manner (Fig. 5F). To further test that intratumoral IFNγ can modulate the Th1 response and affect the phenotype of NK cells, intratumoral injection of recombinant IFNγ was given into the tumor, and after 5 d Th1 response and phenotype of NK cells were monitored. Results showed that IFNγ promoted intratumoral Th1 response (Fig. S4A), and also increased the activating receptor NKG2D and Ly49D on NK cells, whereas decreased the expression of inhibitory receptor NKG2A on NK cells (Fig. S4B). These results suggest that NK cells promote the differentiation of effector Th1 cells and depletion of NK cells leads to reduced frequency of effector Th1 cells in the secondary lymphoid tissues, leading to an increase in tumor growth.
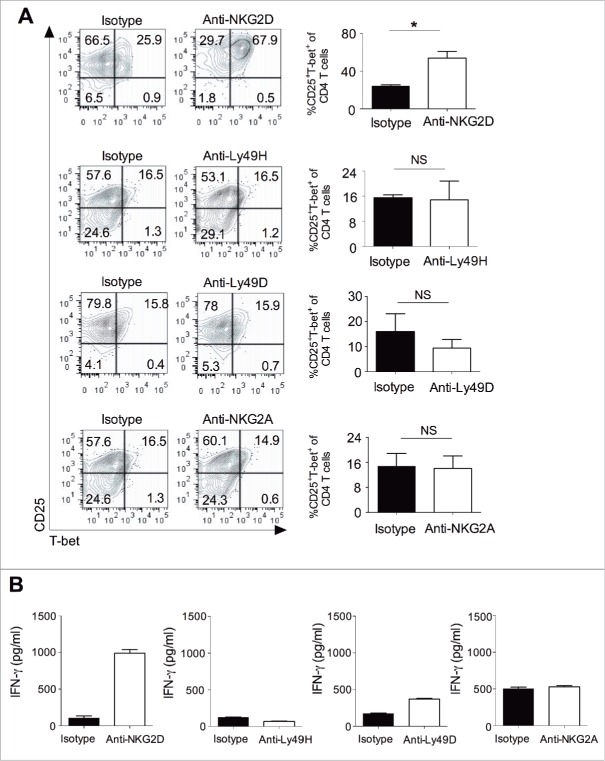
NK cell activating receptors control the differentiation of Th1 cells
To further explore whether signaling from activating receptors in NK cells affects CD4+ T cell differentiation, we cultured naive CD4+ T cells with splenic NK cells in the presence of soluble antibodies against NKG2D, Ly49H, Ly49D, and NKG2A. Signaling from the activating receptors NKG2D resulted in increased ability of NK cells to promote the differentiation of Th1 cells (CD4+CD25+T-bet+ cells) (Fig. 6A), and increased IFNγ secretion (Fig. 6B), as compared to the isotype control. There were no changes in T-bet expression or IFNγ secretion observed with anti-Ly49H, anti-Ly49D, or anti-NKG2A treatment (Fig. 6A and B). These results collectively suggest that signaling from activating receptor NKG2D in NK cells can increase their potential to drive the differentiation of Th1 cells, which could have an effect on tumor growth.
Figure 6.
Activating receptor signaling on NK cell control the differentiation of Th1 cells. Naive CD4+ T cells (5 × 104 cells/well) were cultured with purified splenic NK cells (2.5 × 104 cells/well) and irradiated T cell depleted syngeneic splenocytes (5 × 104 cells/well) along with purified recombinant IL-2 (20 ng/mL) and anti-mouse CD3ε mAb (5 μg/mL) in the presence or absence of anti-NKG2D, anti-Ly49H, anti-Ly49D, anti-NKG2A, or isotype control antibody (10 μg/mL) at 37°C in U-bottomed 96 well plates for 4 d. (A) Expression of T-bet in the CD4+ T cells was analyzed after gating on CD4+ cells using flow cytometry (left). The bar graph represent the percentage of CD25+T-bet+ cells gated on total CD4+ T cells (right). Mean ± SEM shown, n = 3–4 independent experiments (Student's t-test, *p < 0.05; NS, not significant). (B) Culture supernatants were harvested from the above experiment and secretion of IFNγ was analyzed by ELISA. Mean ± SD of duplicate wells are shown and data are representative of one of the two independent experiments.
Discussion
One of the important prerequisites for NK cell-based cancer immunotherapy is achieving efficient early trafficking of a significant number of activated NK cells into the tumor for mounting an effective immune response. Several studies have provided a correlation between high NK cell infiltration, better prognosis, and improved survival.23,24 Although CD27+CD11b+ NK cells are less mature than the CD27−CD11b+ subset, CD27+CD11b+ NK cells are known to display higher cytotoxicity, require a lower signaling threshold for activation as compared to CD27−CD11b+ NK cells,1 and are also known to prevent metastasis.6 Our data have shown for the first time that a significantly high number of CD27+CD11b+ NK cells are preferentially recruited into the B16F10-induced melanoma tumor microenvironment at an early stage. Our data are in contrast with previous report by Lakshmikanth et al. which observed very few NK cells among leukocytes in mouse primary melanomas.25 The observed discrepancy might be due to the stages at which the tumors were analyzed, since a loss of NK cell infiltration can occur at a later stage when the tumor cells escape NK cell-mediated immune surveillance. Previous studies have shown that Tregs and MDSC could suppress NK cell proliferation and their cytotoxic activity in contact-dependent and contact-independent manners.26,27 This explains our observation regarding reduced proliferation of intratumoral NK cells. Furthermore, Chen et al. have shown that CD62L expression on NK cells is required for their migration into inflamed LN to control tumor metastasis, and adoptively transferred wild-type NK cells have shown reduced tumor burden in CD62L−/− mice.28 Chemokine receptors such as CXCR3 and CX3CR1 have been previously shown to promote infiltration of NK cells in the tumor microenvironment.29,30 Our results suggest that NK cells use CCR2, CXCR4, and CXCR3 to migrate into the tumor microenvironment, since ligands for these chemokines were abundantly present in the tumor microenvironment (data not shown). However, it is also possible that immature CD27+CD11b− NK cells migrate into the tumor, and can potentially differentiate into the CD27+CD11b+ subset. Further studies attempting to dissect the regulation of chemokine and chemokine receptor expression on NK cells, and to develop strategies to modulate their expression and regulate the differentiation of NK cells in the tumor microenvironment would be interesting.
Although CD69 was earlier considered to be an early activation marker for lymphocytes, subsequent studies have shown CD69 to be a negative regulator of the immune response.31,32 The use of anti-CD69 antibody that down-regulates surface CD69 without depleting the CD69+ immune cells, and CD69−/− mice is reported to have improved NK cell cytotoxic activity through inhibition of TGF-β production.31,32 In the light of these reports, our data showing that tumor infiltrating NK cells have increased CD69 expression suggest that these cells may contribute to establishing a suppressive tumor microenvironment by inducing TGF-β production. Efficient activation of NK cells depends on signals received from activation receptors like NKG2D. Previous studies have shown no differences in NKG2D expression between peripheral blood mononuclear cells (PBMCs) and LN NK cells in melanoma patients and healthy individuals.33-36 However, these studies did not examine the NKG2D expression in tumor infiltrating NK cells. We observed significantly lower NKG2D expression in the intratumoral NK cells, but no difference in the splenic population, between naive and tumor-bearing mice. Murine NK cells have also been reported to eliminate melanoma cells in an NKG2D-independent fashion.25 NK cells express an array of other activating receptors such as Ly49D, Ly49H, Ly49P, NKp46, DNAM-1, and NKG2C.37 The Ly49 family of receptors is known to help in NK cell education, licensing to control cancer growth and immunosurveillance.38 Furthermore, Ly49H+ NK cells have been implicated to differentiate into long-lived memory NK cells during MCMV infection and provide better protection to the host.39 Our data showing significantly reduced Ly49D and Ly49H expression in tumor infiltrating CD27+CD11b+ NK cells suggest reduced antitumor properties in these populations. Whether tumor Ly49H+ NK cells also possess the memory phenotype is currently not known and needs to be further investigated.
Current NK cell immunotherapy aims at improving the effector function of NK cells in the tumor microenvironment. IFNγ has been shown to enhance tumor immunogenicity through the upregulation of MHC class I molecules, thereby, making tumor cells sensitive to cytotoxic T lymphocyte (CTL)-mediated elimination.40 Furthermore, IFNγ also has anti-proliferative, anti-angiogenic, and pro-apoptotic effect against tumor cells.41-43 Recent reports suggest that NK cells can modulate CD4+ T cell response,44 and NK cell-derived IFNγ has also been shown to promote Th1 differentiation in secondary lymphoid organs in both mice and humans, providing protection against infection.45-47 We also observed that NK cell depletion caused a significant reduction of Th1 cells in the spleen of tumor-bearing mice. Th1 and Th17 cells have been reported to display antitumor activity and regulate tumor growth.48-51 The effect on NK cell depletion on CD4+ T cell response could be due to the loss of NK-T interaction/NK cell secreted cytokines or may be due to rapid tumor growth in the absence of NK cells. Our in vitro NK-T cell co-culture data suggest that both spleen- and tumor-infiltrating NK cells can partly promote Th1 differentiation in an IFNγ dependent manner and that direct contact between NK and CD4+ T cells may not be required to induce Th1 differentiation. However, we cannot rule out the possibility that within the tumor microenvironment, tumor cells or other stromal cells might directly interact with the infiltrating CD4+ T cells and modulate their phenotype. Together, these results suggest that the NK cell-mediated differentiation of Th1 plays an important role in mounting effective antitumor immunity. The anti-NK1.1 antibody (PK136 clone) used in this study is also known to deplete NKT cells, and it has been reported that collaborative NK and NKT cell function is required for host protective response.52 Further experiments focusing on the role of specific depletion of NK or NKT cells and its effect on modulating the adaptive immune response, and on crosstalk with other immune cells, which is a mechanism that controls tumor growth, would provide more insights in tumor immunology. In addition to IFNγ, GM-CSF has also been shown to have an antitumor effect against melanoma cells and can enhance the efficacy of anti-melanoma vaccines when used as adjuvant therapy, intratumoral monotherapy or in combination with chemotherapy.53 Therefore, upregulation of IL-10 and lower expression of IFNγ and GM-CSF by tumor infiltrating NK cells may permit tumor growth.
In NK cells, NKG2D acts as a primary activation signal and can override inhibitory signals received by other NK cell receptors.54,55 In the tumor microenvironment, Treg and MDSC were reported to downregulate NKG2D expression and inhibit IFNγ secretion in NK cells through membrane-bound TGF-β12. Here, we have shown that stimulation of splenic NK cells with anti-NKG2D mAb induces Th1 cell differentiation, whereas anti-NKG2A, anti-Ly49H or anti-Ly49D mAb has no effect. Therefore, lower expression of activating receptors and higher expression of inhibitory receptors on tumor infiltrating NK cells would possibly also compromise their ability to induce Th1 cell differentiation in the tumor microenvironment. Though, NK cell-based adoptive cellular therapy has not yielded very encouraging results15 so far, combining this cellular therapy with activating NK receptor-based immunotherapy could not only increase the cytolytic function of NK cells but could also improve the development of effector Th1 cells, thus, potentially providing a better strategy to control tumors.
Supplementary Material
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
SP and NK are Senior Research Fellow (SRF) of Council of Scientific and Industrial Research (CSIR). Shilpi is SRF of Indian Council of Medical Research (ICMR), Government of India. Authors also thank Dr. Jyoti Rao for critical reading and comments.
Funding
This work was supported by the NCCS intramural fund; Department of Biotechnology (DBT), Government of India (grant BT/RLF/Re-entry/41/2010 to GL).
Author contributions
SP performed the experiments and analyzed the data. NK and Shilpi performed the experiments. SP and GL designed experiments, interpreted data, and wrote the manuscript.
References
- 1.Hayakawa Y, Smyth MJ. CD27 dissects mature NK cells into two subsets with distinct responsiveness and migratory capacity. J Immunol 2006; 176:1517-24; PMID:16424180; http://dx.doi.org/ 10.4049/jimmunol.176.3.1517 [DOI] [PubMed] [Google Scholar]
- 2.Fu B, Wang F, Sun R, Ling B, Tian Z, Wei H. CD11b and CD27 reflect distinct population and functional specialization in human natural killer cells. Immunol 2011; 133:350-9; PMID:21506999; http://dx.doi.org/ 10.1111/j.1365-2567.2011.03446.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chiossone L, Chaix J, Fuseri N, Roth C, Vivier E, Walzer T. Maturation of mouse NK cells is a 4-stage developmental program. Blood 2009; 113:5488-96; PMID:19234143; http://dx.doi.org/ 10.1182/blood-2008-10-187179 [DOI] [PubMed] [Google Scholar]
- 4.Crouse J, Xu HC, Lang PA, Oxenius A. NK cells regulating T cell responses: mechanisms and outcome. Trends Immunol 2015; 36:49-58; PMID:25432489; http://dx.doi.org/ 10.1016/j.it.2014.11.001 [DOI] [PubMed] [Google Scholar]
- 5.Hayakawa Y, Sato-Matsushita M, Takeda K, Iwakura Y, Tahara H, Irimura T. Early activation and interferon-gamma production of tumor-infiltrating mature CD27 high natural killer cells. Cancer Sci 2011; 102:1967-71; PMID:21781225; http://dx.doi.org/ 10.1111/j.1349-7006.2011.02042.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ballas ZK, Buchta CM, Rosean TR, Heusel JW, Shey MR. Role of NK cell subsets in organ-specific murine melanoma metastasis. PLoS One 2013; 8:e65599; PMID:23776508; http://dx.doi.org/ 10.1371/journal.pone.0065599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jin J, Fu B, Mei X, Yue T, Sun R, Tian Z, Wei H. CD11b(-)CD27(-) NK cells are associated with the progression of lung carcinoma. PLoS One 2013; 8:e61024; PMID:23565296; http://dx.doi.org/ 10.1371/journal.pone.0061024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pietra G, Manzini C, Rivara S, Vitale M, Cantoni C, Petretto A, Balsamo M, Conte R, Benelli R, Minghelli S et al.. Melanoma cells inhibit natural killer cell function by modulating the expression of activating receptors and cytolytic activity. Cancer Res 2012; 72:1407-15; PMID:22258454; http://dx.doi.org/ 10.1158/0008-5472.CAN-11-2544 [DOI] [PubMed] [Google Scholar]
- 9.Balsamo M, Scordamaglia F, Pietra G, Manzini C, Cantoni C, Boitano M, Queirolo P, Vermi W, Facchetti F, Moretta A et al.. Melanoma-associated fibroblasts modulate NK cell phenotype and antitumor cytotoxicity. Proc Natl Acad Sci USA 2009; 106:20847-52; PMID:19934056; http://dx.doi.org/ 10.1073/pnas.0906481106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cekic C, Day YJ, Sag D, Linden J. Myeloid expression of adenosine A2A receptor suppresses T and NK cell responses in the solid tumor microenvironment. Cancer Res 2014; 74:7250-9; PMID:25377469; http://dx.doi.org/ 10.1158/0008-5472.CAN-13-3583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trzonkowski P, Szmit E, Mysliwska J, Dobyszuk A, Mysliwski A. CD4+CD25+ T regulatory cells inhibit cytotoxic activity of T CD8+ and NK lymphocytes in the direct cell-to-cell interaction. Clin Immunol 2004; 112:258-67; PMID:15308119; http://dx.doi.org/ 10.1016/j.clim.2004.04.003 [DOI] [PubMed] [Google Scholar]
- 12.Li H, Han Y, Guo Q, Zhang M, Cao X. Cancer-expanded myeloid-derived suppressor cells induce anergy of NK cells through membrane-bound TGF-β 1. J Immunol 2009; 182:240-9; PMID:19109155; http://dx.doi.org/ 10.4049/jimmunol.182.1.240 [DOI] [PubMed] [Google Scholar]
- 13.Ardolino M, Azimi CS, Iannello A, Trevino TN, Horan L, Zhang L, Deng W, Ring AM, Fischer S, Garcia KC et al.. Cytokine therapy reverses NK cell anergy in MHC-deficient tumors. J Clin Invest 2014; 124:4781-94; PMID:25329698; http://dx.doi.org/ 10.1172/JCI74337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Imai K, Matsuyama S, Miyake S, Suga K, Nakachi K. Natural cytotoxic activity of peripheral-blood lymphocytes and cancer incidence: an 11-year follow-up study of a general population. Lancet 2000; 356:1795-9; PMID:11117911; http://dx.doi.org/ 10.1016/S0140-6736(00)03231-1 [DOI] [PubMed] [Google Scholar]
- 15.Ljunggren HG, Malmberg KJ. Prospects for the use of NK cells in immunotherapy of human cancer. Nat Rev Immunol 2007; 7:329-39; PMID:17438573; http://dx.doi.org/ 10.1038/nri2073 [DOI] [PubMed] [Google Scholar]
- 16.Sutlu T, Alici E. Natural killer cell-based immunotherapy in cancer: current insights and future prospects. J Intern Med 2009; 266:154-81; PMID:19614820; http://dx.doi.org/ 10.1111/j.1365-2796.2009.02121.x [DOI] [PubMed] [Google Scholar]
- 17.Huntington ND, Tabarias H, Fairfax K, Brady J, Hayakawa Y, Degli-Esposti MA, Smyth MJ, Tarlinton DM, Nutt SL. NK cell maturation and peripheral homeostasis is associated with KLRG1 upregulation. J Immunol 2007; 178:4764-70; PMID:17404256; http://dx.doi.org/ 10.4049/jimmunol.178.8.4764 [DOI] [PubMed] [Google Scholar]
- 18.Long EO, Kim HS, Liu D, Peterson ME, Rajagopalan S. Controlling natural killer cell responses: integration of signals for activation and inhibition. Ann Rev Immunol 2013; 31:227-58; PMID:23516982; http://dx.doi.org/ 10.1146/annurev-immunol-020711-075005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mizutani T, Neugebauer N, Putz EM, Moritz N, Simma O, Zebedin-Brandl E, Gotthardt D, Warsch W, Eckelhart E, Kantner HP et al.. Conditional IFNAR1 ablation reveals distinct requirements of Type I IFN signaling for NK cell maturation and tumor surveillance. Oncoimmunol 2012; 1:1027-37; PMID:23170251; http://dx.doi.org/ 10.4161/onci.21284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Skak K, Frederiksen KS, Lundsgaard D. Interleukin-21 activates human natural killer cells and modulates their surface receptor expression. Immunology 2008; 123:575-83; PMID:18005035; http://dx.doi.org/ 10.1111/j.1365-2567.2007.02730.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brady J, Hayakawa Y, Smyth MJ, Nutt SL. IL-21 induces the functional maturation of murine NK cells. J Immunol 2004; 172:2048-58; PMID:14764669; http://dx.doi.org/ 10.4049/jimmunol.172.4.2048 [DOI] [PubMed] [Google Scholar]
- 22.Takaki R, Hayakawa Y, Nelson A, Sivakumar PV, Hughes S, Smyth MJ, Lanier LL. IL-21 enhances tumor rejection through a NKG2D-dependent mechanism. J Immunol 2005; 175:2167-73; PMID:16081783; http://dx.doi.org/ 10.4049/jimmunol.175.4.2167 [DOI] [PubMed] [Google Scholar]
- 23.Ishigami S, Natsugoe S, Tokuda K, Nakajo A, Xiangming C, Iwashige H, Aridome K, Hokita S, Aikou T. Clinical impact of intratumoral natural killer cell and dendritic cell infiltration in gastric cancer. Cancer Letters 2000; 159:103-8; PMID:10974412; http://dx.doi.org/ 10.1016/S0304-3835(00)00542-5 [DOI] [PubMed] [Google Scholar]
- 24.Villegas FR, Coca S, Villarrubia VG, Jimenez R, Chillon MJ, Jareno J, Zuil M, Callol L. Prognostic significance of tumor infiltrating natural killer cells subset CD57 in patients with squamous cell lung cancer. Lung Cancer 2002; 35:23-8; PMID:11750709; http://dx.doi.org/ 10.1016/S0169-5002(01)00292-6 [DOI] [PubMed] [Google Scholar]
- 25.Lakshmikanth T, Burke S, Ali TH, Kimpfler S, Ursini F, Ruggeri L, Capanni M, Umansky V, Paschen A, Sucker A, et al.. NCRs and DNAM-1 mediate NK cell recognition and lysis of human and mouse melanoma cell lines in vitro and in vivo. J Clin Invest 2009; 119:1251-63; PMID:19349689; http://dx.doi.org/ 10.1172/JCI36022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pedroza-Pacheco I, Madrigal A, Saudemont A. Interaction between natural killer cells and regulatory T cells: perspectives for immunotherapy. Cell Mol Immunol 2013; 10:222-9; PMID:23524654; http://dx.doi.org/ 10.1038/cmi.2013.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoechst B, Voigtlaender T, Ormandy L, Gamrekelashvili J, Zhao F, Wedemeyer H, Lehner F, Manns MP, Greten TF, Korangy F. Myeloid derived suppressor cells inhibit natural killer cells in patients with hepatocellular carcinoma via the NKp30 receptor. Hepatology 2009; 50:799-807; PMID:19551844; http://dx.doi.org/ 10.1002/hep.23054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen S, Kawashima H, Lowe JB, Lanier LL, Fukuda M. Suppression of tumor formation in lymph nodes by L-selectin-mediated natural killer cell recruitment. J Exp Med 2005; 202:1679-89; PMID:16352740; http://dx.doi.org/ 10.1084/jem.20051473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wendel M, Galani IE, Suri-Payer E, Cerwenka A. Natural killer cell accumulation in tumors is dependent on IFN-gamma and CXCR3 ligands. Cancer Res 2008; 68:8437-45; PMID:18922917; http://dx.doi.org/ 10.1158/0008-5472.CAN-08-1440 [DOI] [PubMed] [Google Scholar]
- 30.Lavergne E, Combadiere B, Bonduelle O, Iga M, Gao JL, Maho M, Boissonnas A, Murphy PM, Debré P, Combadière C. Fractalkine mediates natural killer-dependent antitumor responses in vivo. Cancer Res 2003; 63:7468-74; PMID:14612547 [PubMed] [Google Scholar]
- 31.Esplugues E, Sancho D, Vega-Ramos J, Martinez C, Syrbe U, Hamann A, Engel P, Sánchez-Madrid F, Lauzurica P. Enhanced antitumor immunity in mice deficient in CD69. J Exp Med 2003; 197:1093-106; PMID:12732655; http://dx.doi.org/ 10.1084/jem.20021337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Esplugues E, Vega-Ramos J, Cartoixa D, Vazquez BN, Salaet I, Engel P, Lauzurica P. Induction of tumor NK-cell immunity by anti-CD69 antibody therapy. Blood 2005; 105:4399-406; PMID:15692061; http://dx.doi.org/ 10.1182/blood-2004-10-3854 [DOI] [PubMed] [Google Scholar]
- 33.Konjevic G, Mirjacic Martinovic K, Jurisic V, Babovic N, Spuzic I. Biomarkers of suppressed natural killer (NK) cell function in metastatic melanoma: decreased NKG2D and increased CD158a receptors on CD3-CD16+ NK cells. Biomarkers 2009; 14:258-70; PMID:19489688; http://dx.doi.org/ 10.1080/13547500902814658 [DOI] [PubMed] [Google Scholar]
- 34.Markel G, Seidman R, Besser MJ, Zabari N, Ortenberg R, Shapira R, Treves AJ, Loewenthal R, Orenstein A, Nagler A et al.. Natural killer lysis receptor (NKLR)/NKLR-ligand matching as a novel approach for enhancing anti-tumor activity of allogeneic NK cells. PloS One 2009; 4:e5597; PMID:19440333; http://dx.doi.org/ 10.1371/journal.pone.0005597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fregni G, Messaoudene M, Fourmentraux-Neves E, Mazouz-Dorval S, Chanal J, Maubec E, Marinho E, Scheer-Senyarich I, Cremer I, Avril MF et al.. Phenotypic and functional characteristics of blood natural killer cells from melanoma patients at different clinical stages. Plos One 2013; 8:e76928; PMID:24204708; http://dx.doi.org/ 10.1371/journal.pone.0076928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Messaoudene M, Fregni G, Fourmentraux-Neves E, Chanal J, Maubec E, Mazouz-Dorval S, Couturaud B, Girod A, Sastre-Garau X, Albert S et al.. Mature cytotoxic CD56(bright)/CD16(+) natural killer cells can infiltrate lymph nodes adjacent to metastatic melanoma. Cancer Res 2014; 74:81-92; PMID:24225017; http://dx.doi.org/ 10.1158/0008-5472.CAN-13-1303 [DOI] [PubMed] [Google Scholar]
- 37.Kadri N, Luu Thanh T, Hoglund P. Selection, tuning, and adaptation in mouse NK cell education. Immunol Rev 2015; 267:167-77; PMID:26284477; http://dx.doi.org/ 10.1111/imr.12330 [DOI] [PubMed] [Google Scholar]
- 38.Tu MM, Mahmoud AB, Wight A, Mottashed A, Belanger S, Rahim MM, Abou-Samra E, Makrigiannis AP. Ly49 family receptors are required for cancer immunosurveillance mediated by natural killer cells. Cancer Res 2014; 74:3684-94; PMID:24802191; http://dx.doi.org/ 10.1158/0008-5472.CAN-13-3021 [DOI] [PubMed] [Google Scholar]
- 39.Sun JC, Beilke JN, Lanier LL. Adaptive immune features of natural killer cells. Nature 2009; 457:557-61; PMID:19136945; http://dx.doi.org/ 10.1038/nature07665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaplan DH, Shankaran V, Dighe AS, Stockert E, Aguet M, Old LJ, Schreiber RD. Demonstration of an interferon gamma-dependent tumor surveillance system in immunocompetent mice. Proc Natl Acad Sci USA 1998; 95:7556-61; PMID:9636188; http://dx.doi.org/ 10.1073/pnas.95.13.7556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chin YE, Kitagawa M, Su WC, You ZH, Iwamoto Y, Fu XY. Cell growth arrest and induction of cyclin-dependent kinase inhibitor p21 WAF1/CIP1 mediated by STAT1. Science 1996; 272:719-22; PMID:8614832; http://dx.doi.org/ 10.1126/science.272.5262.719 [DOI] [PubMed] [Google Scholar]
- 42.Beatty G, Paterson Y. IFN-gamma-dependent inhibition of tumor angiogenesis by tumor-infiltrating CD4+ T cells requires tumor responsiveness to IFN-gamma. J Immunol 2001; 166:2276-82; PMID:11160282; http://dx.doi.org/ 10.4049/jimmunol.166.4.2276 [DOI] [PubMed] [Google Scholar]
- 43.Chawla-Sarkar M, Lindner DJ, Liu YF, Williams BR, Sen GC, Silverman RH, Borden EC. Apoptosis and interferons: role of interferon-stimulated genes as mediators of apoptosis. Apoptosis 2003; 8:237-49; PMID:12766484; http://dx.doi.org/ 10.1023/A:1023668705040 [DOI] [PubMed] [Google Scholar]
- 44.Crome SQ, Lang PA, Lang KS, Ohashi PS. Natural killer cells regulate diverse T cell responses. Trends Immunol 2013; 34:342-9; PMID:23601842; http://dx.doi.org/ 10.1016/j.it.2013.03.002 [DOI] [PubMed] [Google Scholar]
- 45.Martin-Fontecha A, Thomsen LL, Brett S, Gerard C, Lipp M, Lanzavecchia A, Sallusto F. Induced recruitment of NK cells to lymph nodes provides IFN-gamma for T(H)1 priming. Nat Immunol 2004; 5:1260-5; PMID:15531883; http://dx.doi.org/ 10.1038/ni1138 [DOI] [PubMed] [Google Scholar]
- 46.Morandi B, Bougras G, Muller WA, Ferlazzo G, Munz C. NK cells of human secondary lymphoid tissues enhance T cell polarization via IFN-gamma secretion. Eur J Immunol 2006; 36:2394-400; PMID:16917961; http://dx.doi.org/ 10.1002/eji.200636290 [DOI] [PubMed] [Google Scholar]
- 47.Laouar Y, Sutterwala FS, Gorelik L, Flavell RA. Transforming growth factor-beta controls T helper type 1 cell development through regulation of natural killer cell interferon-gamma. Nat Immunol 2005; 6:600-7; PMID:15852008; http://dx.doi.org/ 10.1038/ni1197 [DOI] [PubMed] [Google Scholar]
- 48.Ye ZJ, Zhou Q, Gu YY, Qin SM, Ma WL, Xin JB, Tao XN, Shi HZ. Generation and differentiation of IL-17-producing CD4+ T cells in malignant pleural effusion. J Immunol 2010; 185:6348-54; PMID:20952674; http://dx.doi.org/ 10.4049/jimmunol.1001728 [DOI] [PubMed] [Google Scholar]
- 49.Gnerlich JL, Mitchem JB, Weir JS, Sankpal NV, Kashiwagi H, Belt BA, Porembka MR, Herndon JM, Eberlein TJ, Goedegebuure P et al.. Induction of Th17 cells in the tumor microenvironment improves survival in a murine model of pancreatic cancer. J Immunol 2010; 185:4063-71; PMID:20805420; http://dx.doi.org/ 10.4049/jimmunol.0902609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Martin-Orozco N, Muranski P, Chung Y, Yang XO, Yamazaki T, Lu S, Hwu P, Restifo NP, Overwijk WW, Dong C. T helper 17 cells promote cytotoxic T cell activation in tumor immunity. Immunity 2009; 31:787-98; PMID:19879162; http://dx.doi.org/ 10.1016/j.immuni.2009.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Muranski P, Boni A, Antony PA, Cassard L, Irvine KR, Kaiser A, Paulos CM, Palmer DC, Touloukian CE, Ptak K et al.. Tumor-specific Th17-polarized cells eradicate large established melanoma. Blood 2008; 112:362-73; PMID:18354038; http://dx.doi.org/ 10.1182/blood-2007-11-120998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smyth MJ, Crowe NY, Godfrey DI. NK cells and NKT cells collaborate in host protection from methylcholanthrene-induced fibrosarcoma. Int Immunol 2001; 13:459-63; PMID:11282985; http://dx.doi.org/ 10.1093/intimm/13.4.459 [DOI] [PubMed] [Google Scholar]
- 53.Kaufman HL, Ruby CE, Hughes T, Slingluff CL Jr. Current status of granulocyte-macrophage colony-stimulating factor in the immunotherapy of melanoma. J Immunother Cancer 2014; 2:11; PMID:24971166; http://dx.doi.org/ 10.1186/2051-1426-2-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gilfillan S, Ho EL, Cella M, Yokoyama WM, Colonna M. NKG2D recruits two distinct adapters to trigger NK cell activation and costimulation. Nat Immunol 2002; 3:1150-5; PMID:12426564; http://dx.doi.org/ 10.1038/ni857 [DOI] [PubMed] [Google Scholar]
- 55.Spear P, Wu MR, Sentman ML, Sentman CL. NKG2D ligands as therapeutic targets. Cancer Immunity 2013; 13:8; PMID:23833565 [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.