Abstract
The physiological link between neuropathic pain and depression remains unknown despite a high comorbidity between these two disorders. A mouse model of spared nerve injury (SNI) was used to test the hypothesis that nerve injury precipitates depression through the induction of inflammation in the brain, and that prior exposure to stress exacerbates the behavioral and neuroinflammatory consequences of nerve injury. As compared with sham surgery, SNI induced mechanical allodynia, and significantly increased depressive-like behavior. Moreover, SNI animals displayed increased interleukin-1β (IL-1β) gene expression within the frontal cortex and concurrent increases in the expression of glial fibrillary acidic protein (GFAP) within the periaqueductal grey (PAG). Additionally, exposure to chronic restraint stress for 2 weeks before SNI exacerbated mechanical allodynia and depressive-like behavior, and resulted in an increase in IL-1β gene expression in the frontal cortex and brain-derived neurotrophic factor (BDNF) gene expression in PAG. Treatment with metyrapone (MET), a corticosteroid synthesis inhibitor, before stress eliminated deleterious effects of chronic stress on SNI. Finally, this study showed that interference with IL-1β signaling, through administration of IL-1 receptor antagonist (IL-1ra), ameliorated the effects of neuropathic pain on depressive-like behavior. Taken together, these data suggest that peripheral nerve injury leads to increased cytokine expression in the brain, which in turn, contributes to the development of depressive-like behavior. Furthermore, stress can facilitate the development of depressive-like behavior after nerve injury by promoting IL-1β expression.
Keywords: spared nerve injury, depressive-like behavior, interleukin-1β, stress, glucocorticoid, neuropathic pain
Introduction
Acute pain is often adaptive. It promotes survival by encouraging organisms to avoid threats and to protect existing injuries against further damage. In contrast, chronic or persistent pain can become maladaptive and debilitating, limiting physical activity and leading to psychopathology and reduced quality of life.1–3 Chronic pain disorders are a prevalent source of human suffering, afflicting over 50 million Americans and accounting for approximately 80% of all physician visits.4
Neuropathic pain, defined as pain derived from a lesion or dysfunction of nervous tissue,5 represents one of the more disabling chronic pain disorders. It can arise from a broad collection of pathological states, ranging from direct nerve trauma to diabetes and cancer.6,7 Despite the broad spectrum of causes, relatively little is known regarding the physiological mechanisms underlying neuropathic pain, and consequentially, relatively few effective treatments exist. Although much of the research on chronic pain has been focused at the level of the spinal cord, it has become increasingly apparent that supraspinal structures are intimately involved in both the development and maintenance of chronic pain states, including neuropathic pain.8–10 Indeed, neuropathic pain enhances synaptic transmission of nociceptive-specific input to the amygdala,11 induces long-term synaptic changes in the anterior cingulate,12 and upregulates proinflammatory cytokine gene expression in the prefrontal cortex and brainstem.13 The neuropathic pain-induced alteration in supraspinal structures, in turn, can facilitate nociceptive signal transmission through the activation of the efferent arm of the descending modulatory pain pathway, consisting of the midbrain periaqueductal grey (PAG) and rostroventral medulla (RVM). In the context of NP, the PAGRVM system is capable of facilitating nociceptive transmission through amplification of afferent spinal nociceptive signaling.9,14
The involvement of supraspinal structures in neuropathic pain provides a substrate through which emotional states are capable of altering pain perception and vice versa.15,16 Chronic pain conditions are associated with an increased risk of mood disorders, and >50% of the individuals suffering from chronic pain conditions have symptoms of depression.17 One possible link between neuropathic pain and depression is the induction of neuroinflammation. Apart from their role in regulating immune responses, proinflammatory cytokines are potent modulators of behavior and affect.18 Both exogenous and endogenous proinflammatory cytokines (that is interleukin-1β, IL-1β) induce depressive-like behavior in animals and therapeutic administration of the proinflammatory cytokine interferon-α leads to depression in up to 50% of clinical patients.18 In addition to the induction of depression, inflammatory cytokines also lead to hyperalgesia (hypersensitivity to noxious stimuli).19
This study will also examine a role for stress in exacerbating allodynia (a state in which typically innocuous stimuli elicit a pain response) and depressive-like behavior after nerve injury. Chronic stress is one of the more prominent risk factors for clinical depression.20 and is known to exacerbate various chronic pain conditions.21,22 Similarly, animals exposed to chronic stress develop hyperalgesia.23 Such findings are not surprising given the broad effects of chronic stress on brain structure and function. Stress, and subsequent exposure to high levels of glucocorticoids, dramatically alters the activity of corticolimbic structures known to modulate PAG-RVM-dependent pain facilitation.24,25 Thus, significant overlap exists between the brain structures involved in chronic pain, major depression, and chronic stress, which may explain the comorbidity of such conditions.
The goals of this study were (1) to determine whether peripheral nerve injury precipitates depressive-like behavior in a mouse model of neuropathic pain and (2) to elucidate the mechanism through which chronic stress impacts the behavioral and physiological responses to nerve injury. The primary hypothesis was that chronic stress before induction of nerve injury would decrease the nociceptive threshold, and increase the development of depressive-like behaviors. Additionally, we hypothesized that stress would increase the expression of genes associated with pain facilitation and depressive-like behavior.
Materials and methods
Adult male C57/BL6 mice (23–30 g; Charles River, Wilmington, MA, USA) were maintained on a 14:10 light/dark cycle and individually housed within a temperature and humidity-controlled vivarium. Water and food were available ad libitum throughout the study. The study was conducted in accordance with NIH guidelines for the care and use of animals and under protocols approved by the OSU Institutional Animal Care and Use Committee.
Experimental protocols
Study 1: Stress effects on mechanical allodynia, depressive-like behavior and supraspinal mRNA expression
To assess the effects of chronic stress on spared nerve injury (SNI), 40 animals were randomly assigned to one of four main groups: sham surgery with no exposure to stress (Sham-No Stress; n = 10), sham surgery with exposure to chronic stress (Sham-Stress; n = 10), SNI with no stress (SNI-No Stress; n = 10), or SNI with exposure to chronic stress (SNI-Stress; n = 10). At 1 day before surgery, baseline measures of mechanical allodynia were taken and re-assessed on days 1, 3, and 7 after surgery. At 6 days after surgery, locomotor activity was assessed in an automated open field chamber. At postoperative day 7, depressive-like behavior was assessed through the forced swim test and blood and tissue samples were collected (8–10 h after conclusion of the swim task).
Spared nerve injury
The SNI and sham-surgery (Sham) were performed on anesthetized mice (isoflurane) using sterile surgical technique and an earlier described procedure for inducing neuropathic pain in mice.26 Briefly, the right hind limb was immobilized in a lateral position and slightly elevated. The three peripheral branches (sural, common peroneal, and tibial nerves) of the sciatic nerve were exposed. The tibial and common peroneal nerves were ligated using a 6.0 silk suture and transected (1.5mm sections removed). The sural nerve was carefully preserved. The sham procedure consisted of the same surgery without ligation or transection of any nerves; instead, a 3 mm long thread of 6.0 silk was placed longitudinally at the level of the trifurcation.
Restraint stress
This stressor consisted of placing a mouse in a well-ventilated 50-ml transparent tube for 2 h per day for 14 consecutive days; the final restraint session occurred immediately before the surgery. The nonstressed control mice were left undisturbed in their home cages. Restraint occurred at random time points throughout the light phase.
Behavioral testing
Behavioral testing was conducted during the dark (active) phase of the daily light–dark cycle. The individual conducting and scoring behavioral tests was uninformed of experimental assignments, and all animals were tested using the same apparatus (cleaned between animals with 70% ethyl alcohol) under consistent conditions. The mice were habituated to the room for 15 min before testing.
Measuring allodynia
Baseline von Frey monofilament testing (Stoelting Co, IL, USA) took place one day before SNI or SHAM surgery. Subsequent testing took place on days 1, 3, and 7 after surgery. Testing procedures were conducted as described earlier in Bourquin et al. (2006). Assessment began with the 8 mg monofilament and was followed by increasingly firm monofilaments until a positive response was determined. A positive response was defined as a flexion response (paw withdrawal) occurring twice in 10 applications of the respective filament being applied to the lateral side of both hind paws. After a positive response, the threshold (in milligrams) was noted and no further monofilaments were applied.27,28 A significant decrease in response threshold after surgery is interpreted as the development of allodynia, a state in which typically innocuous stimuli begin to elicit pain responses.
Open field
General activity and anxiety-like behavior were assessed during a 60-min session in an open field apparatus (40 × 40 × 37.5 cm) using Flex Field photobeam activity (San Diego Instruments, San Diego, CA, USA). The apparatus was enclosed in a sound-attenuating chamber equipped with a ventilating fan. Data were analyzed to determine general locomotor activity, and relative amount of activity occurring in the periphery vs the center of the apparatus (anxiety-like behavior).
Forced swim task
Mice were placed into an opaque cylinder tank (24 cm diameter, 53 cm height) filled to a depth of 30cm with water maintained at 29 °C. The water was changed after each animal and the tank was thoroughly cleaned. Swimming behavior was recorded for 5 min and scored for time spent actively swimming vs floating (no leg or tail movement contributing to forward movement). Quantification of float vs swim time was performed with Observer software (Version 5, Exeter Software, Setauket, NY, USA). An increase in floating is interpreted as an increase in depressive-like behavior.29,30
Gene expression
On postsurgical day 7, the mice were killed through rapid cervical dislocation and tissue collected. Samples from the periaqueductal gray and frontal cortex were dissected from whole brain and total RNA was extracted using a homogenizer (Ultra-Turrax T8, IKA Works, Wilmington, NC, USA) and an RNeasy Mini Kit (Qiagen, Valencia CA, USA) according to the manufacturer’s protocol. Extracted RNA was suspended in 30 µL of RNase-free water and RNA concentration was determined by a spectrophotometer (NanoDrop ND-1000, Wilmington, DE, USA). The following inventoried primers and probes (Applied Biosystems, Foster City, CA, USA) were used: glial fibrillary acidic protein (GFAP), MAC-1, IL-6 and IL-1β, tumor necrosis factor α (TNF-α), cholecystokinin, serotonin receptor-4, and brain-derived neurotrophic factor (BDNF). A TaqMan 18S rRNA primer and probe set (labeled with VIC dye: Applied Biosystems, Foster City, CA) was used as a control gene for relative quantification. Amplification was performed on an ABI 7000 Sequencing Detection System by using Taqman Universal PCR master mix. The universal two-step RT-PCR cycling conditions used were: 50 °C for 2 min, 95 °C for 10 min followed by 40 cycles of 95 °C for 15 s, and 60 °C for 1 min.
Corticosterone
Trunk blood samples were collected at the time of euthanasia and placed on ice. Clots were removed and the samples were centrifuged at 6000 rpm for 30 min at 4 °C; sera was collected and stored at −80 °C until assayed. Corticosterone (CORT) concentrations were determined by using an I125 corticosterone kit (MP Biomedical, Solon, OH); all samples were run in a single assay. The standard curve was run in triplicate and samples were run in duplicate. The lower limit of the assay is 7.7 ng ml−1 corticosterone, the cross reactivity with other steroids is low (< 1%), and the intra-assay coefficient of variation for this assay was 6.4%.
Study 2: Role of stress-induced corticosterone in modulating SNI outcome
To determine the functional significance of stress-induced increases in CORT concentrations on SNI outcome, 80 mice were randomly assigned to one of the four experimental groups that were treated with vehicle or MET (100 mg kg−1 MET; details provided below), a corticosteroid synthesis inhibitor: SNI with no exposure to stress, but treated daily with vehicle (SNI-No Stress-Vehicle), SNI with exposure to chronic stress, and treated daily with vehicle (SNI-Stress-Vehicle), SNI with no exposure to stress, but treated daily with MET (SNI-No Stress-MET), or SNI with exposure to chronic stress and treated daily with MET (SNI-Stress-MET). Two different regimens for MET administration were used to determine whether amelioration of stress effects on post-SNI behavior require preventing the rise in corticosterone that typically accompanies stress (Study 2a), or the suppression of corticosterone after nerve injury (Study 2b). In Study 2a (n = 10 per group), the MET or vehicle was administered 1 h before each restraint session (or at an equivalent time for animals in the No Stress groups), and in Study 2b (n = 10 per group), the MET or vehicle was administered daily beginning 24 h after SNI. All other aspects of the study were identical to Study 1, including the stress exposure, behavioral analyses, and physiological analyses. Briefly, 1 day before surgery, baseline measures of mechanical allodynia were taken and re-sampled on days 1, 3, and 7 after surgery. At 6 days after surgery, locomotor activity was measured in an automated open field chamber. At postoperative day 7, depressive-like behavior was assessed through the forced swim test and blood and tissue samples were collected (8–10 h after swim task).
Inhibition of corticosterone synthesis
To modulate glucocorticoid effects, groups of animals were injected IP with the glucocorticoid synthesis inhibitor MET [2-methyl-1, 2-di-3-pyridyl-1-propanone] (MET, Sigma; 100 mg kg−1) or vehicle (isotonic saline). The dose of MET used was chosen based on earlier studies showing that this dose is sufficient to block GC synthesis without causing long-term dysregulation of the HPA axis.31
Study 3: The functional significance of increased IL-1β levels in SNI animals
To determine the functional significance of increased IL-1β in SNI animals, mice were cannulated and IL-1 receptor antagonist (IL-1ra; 1.8 µg in 2µl) or artificial cerebral spinal fluid (2 µl) was administered intra-cerebroventricularly (ICV) to sham (n = 6 per group) and SNI (n = 7 per group) animals on days 6 and 7 after surgery. A guide cannula, targeting the left lateral ventricle, was implanted 1 week before surgery. The mice were anesthetized with 1–1.5% isofluorane in oxygen-enriched air and placed in a stereotaxic apparatus (David Kopf Instruments, Tujunga, CA, USA). The cannula (2.00 mm below the pedestal, Plastics One, Roanoke, VA, USA) was positioned at +0.02 mm posterior and +0.95 mm lateral to bregma, and secured with glue. The FST was conducted approximately 30 min after ICV injection on day 7. The dose was selected based on earlier reports showing its efficacy.32 At conclusion of the study, accuracy of cannula placement was confirmed.
Statistical analysis
The data are expressed as means ± standard error of the mean. Testing of statistical significance was performed using ANOVA. When a significant overall treatment effect was reported (P < 0.05), post hoc analyses were conducted using the Tukey test. Levels of significance were taken at P < 0.05. Von Frey responses for all experiments were analyzed using two-way repeated measures ANOVA assessing effects of time and group. CORT concentrations, mRNA expression, FST, and open field data were analyzed using one-way ANOVAs. In the case of von Frey responses, conditions of normality were not met, so the data were subsequently log transformed, which succeeded in normalizing the data.
Results
Study 1: Stress effects on mechanical allodynia, depressive-like behavior, and supraspinal mRNA expression
Allodynia
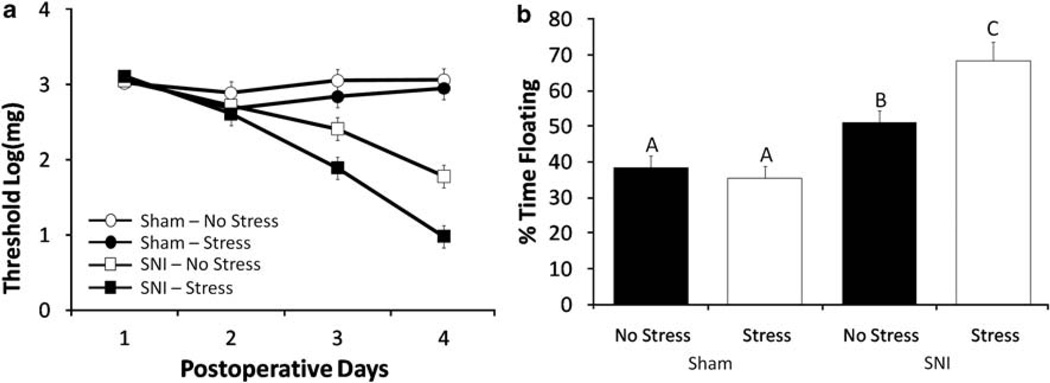
As expected, both SNI groups exhibited an increase in mechanical allodynia as measured by von Frey response threshold on postsurgical day 7 (Figure 1a, F3,57 = 61.34, P < 0.05), whereas neither of the Sham-operated groups exhibited mechanical allodynia at any of the postsurgical time points (P > 0.05). Furthermore, exposure to 14 consecutive days of restraint stress before SNI surgery (SNI-Stress) significantly reduced von Frey response threshold at postsurgical day 7 relative to the SNI-No Stress group and the two Sham groups (Figure 1, F3,36 = 35.05, P < 0.05, post hoc = P < 0.05), suggesting that stress exposure exacerbates SNI-induced allodynia. In contrast, von Frey response thresholds were similar for the Sham-No Stress and Sham-Stress groups at all time points, thereby showing that exposure to chronic stress did not produce allodynia in the absence of nerve injury in this study.
Figure 1.
Assessment of allodynia and depressive-like behaviors. (a) SNI significantly decreases paw withdrawal threshold as compared with sham-operated animals on postsurgical days 3 and 7. Exposure to 14 consecutive days of chronic stress exacerbates SNI effects on threshold on post-SNI days 3 and 7. Chronic stress does not alter threshold in Sham animals. (b) SNI significantly increases the percentage time spent floating during a 5 min forced swim test conducted on day 7 after surgery. Exposure to 14 consecutive days of chronic stress exacerbates SNI effects on the forced swim test. Similar letters above error bars indicate no significant difference between groups (P > 0.05), whereas different letters represent a significant difference between groups (P < 0.05). Data are presented as mean ± s.e.m.
Depressive-like and anxiety-like behavior
Overall, the SNI groups spent significantly more time floating during the forced swim test than the two Sham groups, suggesting an increase in depressive-like behavior among SNI animals (Figure 1b, F1,38 = 25.04, P < 0.05). Presurgical exposure to stress further increased floating behavior in the SNI-Stress group relative to the other three experimental groups (Figure 1b, P < 0.05), but did not influence floating behavior among the Sham groups. All four experimental groups exhibited similar levels of general locomotor activity (P > 0.05, data not shown) thereby suggesting that SNI does not produce a general motor deficit. Furthermore, central tendency in the open field, a measure of anxiety-like behavior, was similar among groups (P > 0.05; data not shown).
Gene expression
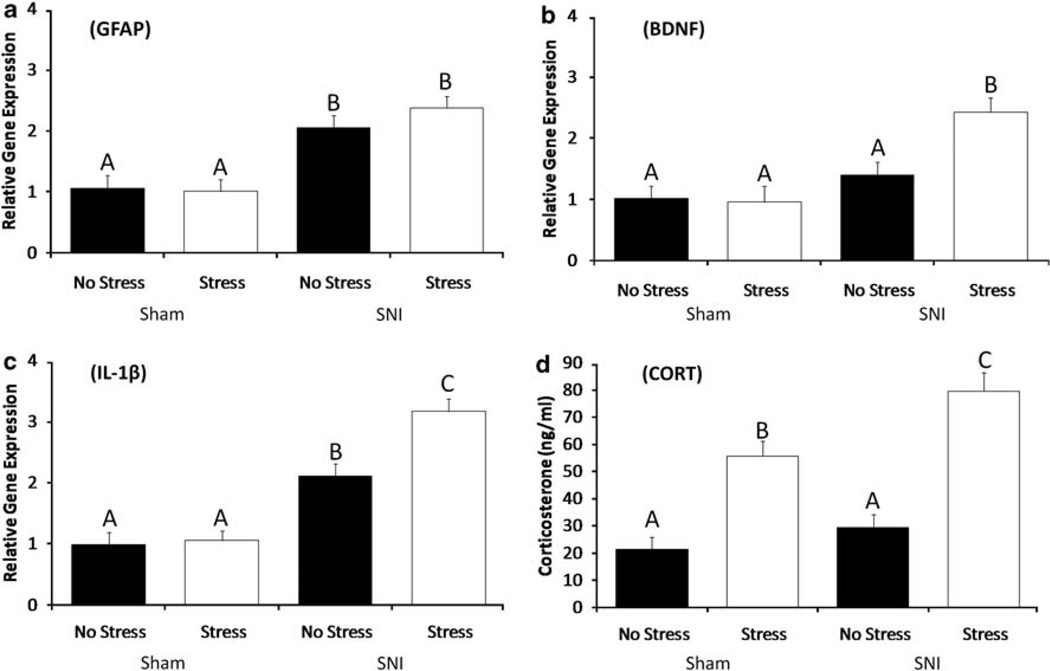
Gene expression analysis of the PAG on postsurgical day 7 revealed increased levels of GFAP, an intermediate filament protein that is up-regulated in astrocytes after SNI (F1,38 = 9.74, P < 0.05), although stress did not significantly alter GFAP expression in either the Sham or SNI groups (Figure 2a). BDNF mRNA expression in PAG (Figure 2b) was significantly greater in the Stress-SNI group than the other three groups (F1,36 =12.05, P < 0.05), which did not differ from one another. SNI animals had significantly higher levels of IL-1β mRNA within the prefrontal cortex (Figure 2c, F1,38 = 25.63, P < 0.05) than the SHAM animals (P < 0.05). Stress did not alter IL-1β mRNA within the Sham group, but IL-1β gene expression was significantly greater in the Stress-SNI group than the No Stress-SNI group (F1,36 = 11.14, P < 0.05). No significant differences among experimental groups were found in mRNA expression of GFAP or BDNF in the PFC, IL-1β in the PAG, or IL-6, TNF-α, cholecystokinin, MAC-1 or serotonin receptor-4 in the PAG or frontal cortex (P > 0.05).
Figure 2.
SNI influences on supraspinal gene expression and CORT levels on day 7. SNI significantly increases mRNA expression of GFAP (a) and BDNF (b) within the PAG and IL-1β (c) within the PFC. Chronic stress does not influence the expression of GFAP within the PAG (a). Chronic stress increased the expression of PAG BDNF (b) and IL-1β (c) among SNI animals. No chronic stress effects are detected in Sham-operated animals. (d) SNI alone does not alter CORT concentration at 7 days after surgery. Exposure to 14 days of chronic stress before surgery lead to significant increases in CORT levels within Sham and SNI animals, with SNI-chronic stress animals having the highest levels of CORT. Similar letters above error bars indicate no significant difference between groups (P > 0.05), whereas different letters represent a significant difference between groups (P < 0.05). Data are presented as mean ± s.e.m.
Corticosterone
On day 7 after SNI or Sham surgery, corticosterone concentrations were similar between the SNI-No Stress and Sham-No Stress groups, and both of these groups had significantly lower corticosterone concentrations than the SNI-Stress and Sham-Stress groups (F1,38 = 22.32, P < 0.05). Furthermore, corticosterone concentrations were greater in the Stress-SNI group than the Stress-Sham group (P < 0.05). Thus, prior exposure to chronic stress, regardless of nerve injury, was sufficient to produce a prolonged elevation in corticosterone concentrations, but that the greatest increase occurred among the mice that were exposed to both stress and SNI.
Study 2: Role of stress-induced corticosterone in modulating SNI outcome
Study 2a: Administration of MET before stress Allodynia
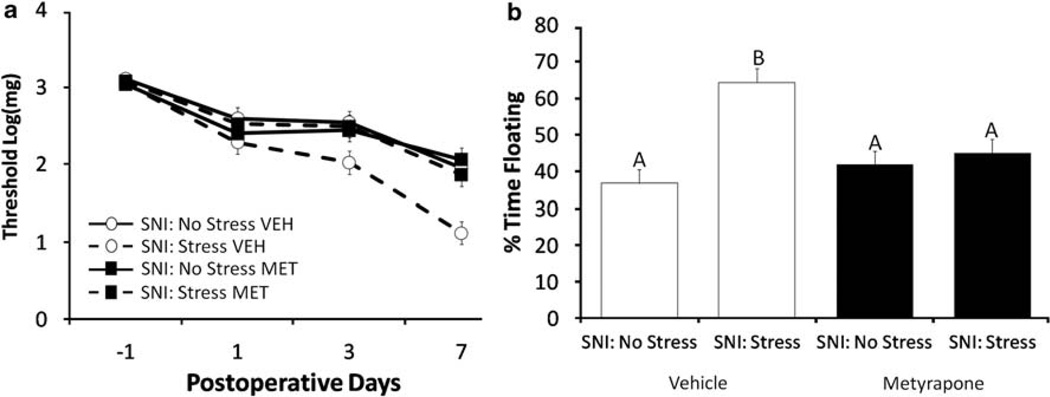
After SNI surgery, all experimental groups exhibited allodynia (F3,117 = 78.43, P < 0.05). Paw withdrawal thresholds at all four time points were similar for the SNI-No Stress-Vehicle, SNI-No Stress-MET, and SNI-Stress-MET groups (P > 0.05). Paw withdrawal thresholds were not statistically different for the SNI-Stress-Vehicle group and the other three groups at baseline, or on post-SNI days 1 or 3 (Figure 3a, P > 0.05). However, by post-SNI day 7, paw withdrawal threshold was significantly lower (F3,36 = 7.56, P < 0.05, post hoc = P < 0.05) for the SNI-Stress-Vehicle group than the other three experimental groups. Thus, in the absence of stress, blockade of corticosterone synthesis for 2 weeks before SNI had no impact on the development of allodynia. In contrast, preventing the stress-induced increase in CORT for 2 weeks before SNI eliminated the stress-induced increase in allodynia.
Figure 3.
(a) Modification of stress effects on SNI-induced allodynia and depressive-like behavior by metyrapone. SNI significantly decreases threshold in all groups. Vehicle (VEH)-treated animals exposed to chronic stress before SNI surgery have significantly lower paw withdrawal thresholds. Inhibition of stress-induced CORT elevation through treatment with metyrapone (MET; 100 mg kg−1) before restraint eliminates the effect of stress on SNI outcome. Data are presented as mean ± s.e.m. (b) Vehicle-treated chronic stress mice spend more time floating in the FST at day 7. Inhibition of stress-induced CORT elevation through treatment with metyrapone (MET, 100 mg kg−1) eliminates the deleterious effect of chronic stress on SNI-induced depressive-like behavior. Similar letters above error bars indicate no significant difference between groups (P > 0.05), whereas different letters represent a significant difference between groups (P < 0.05). Data are presented as mean ± s.e.m.
Depressive-like behavior
Time spent floating in the Porsolt forced swim test was significantly greater among mice in the SNI-Stress-Vehicle group than the other three experimental groups (Figure 3b, F1,36 = 7.21, P < 0.05, post hoc = P < 0.05). Inhibition of CORT synthesis through administration of MET before stress, eliminated the effects of stress on depressive-like behavior in the swim test; time spent floating was similar for the SNI-Stress-MET group and the SNI-No Stress-MET and SNI-No Stress-Vehicle groups (Figure 3b).
Gene expression
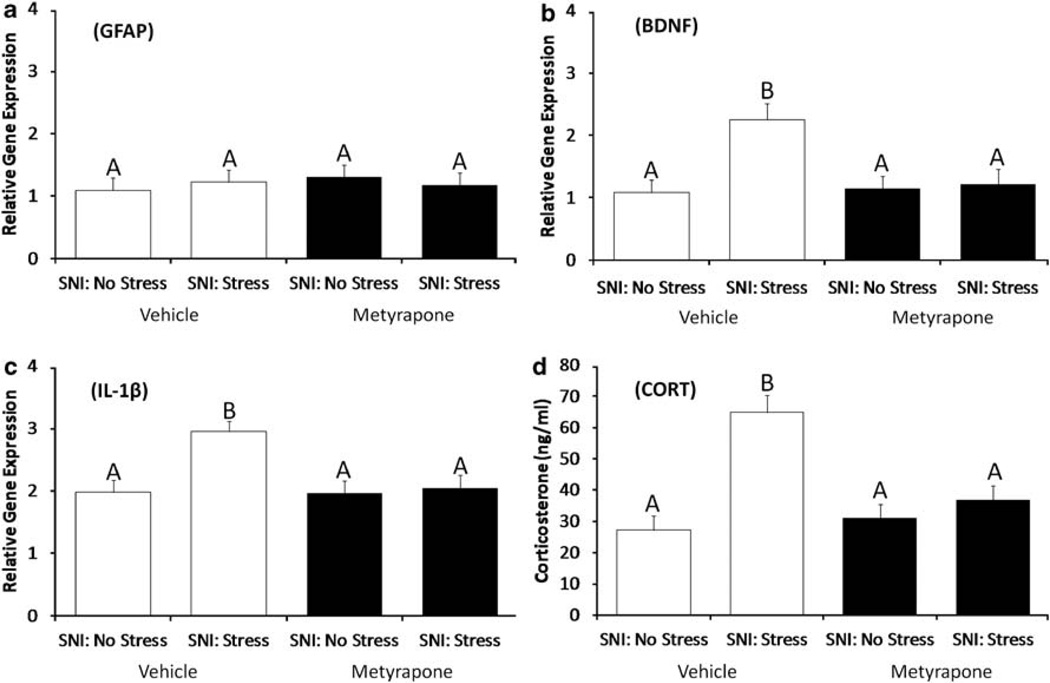
There were no group differences detected for GFAP (Figure 4a, P > 0.05) within the PAG of SNI animals. Among vehicle-treated mice, exposure to stress and SNI significantly increased BDNF (P < 0.05) mRNA expression in the PAG (Figure 4b) and IL-1β in the PFC (Figure 4c) relative to the SNI-No Stress group. Inhibition of CORT synthesis with MET before stress eliminated the effects of stress on SNI-induced BDNF and IL-1β mRNA expression such that expression of these two genes was similar for the SNI-Stress-MET group and the SNI-No Stress-MET and SNI-No Stress-Vehicle groups (Figure 4b and c).
Figure 4.
Modification of stress effects on SNI-induced gene expression and depressive-like behavior by metyrapone. Neither chronic stress nor MET treatment influences (a) PAG GFAP mRNA expression. Inhibition of stress-induced CORT elevation through treatment with metyrapone (MET, 100 mg kg−1), eliminates the effects of chronic stress on (b) PAG BNDF and (c) PFC IL-1β and mRNA expression. Inhibition of CORT synthesis during chronic stress eliminates the effects of pre-SNI stress on CORT levels measured a week after the final restrain session (d). Similar letters above error bars indicate no significant difference between groups (P > 0.05), whereas different letters represent a significant difference between groups (P < 0.05). Data are presented as mean ± s.e.m.
Corticosterone concentration
Among vehicle-treated mice, exposure to stress before SNI was associated with a significant increase in circulating CORT on postsurgical day 7; the SNI-Stress-Vehicle group had significantly higher circulating CORT concentrations than the SNI-No Stress-Vehicle, SNI-No Stress-MET and SNI-Stress-MET groups (F1,36 = 11.21, P < 0.05, Tukey = P < 0.05; Figure 4d).
Study 2b: Administration of MET beginning 24 h after SNI
In contrast to Study 2a, inhibition of CORT synthesis through daily administration of MET beginning 24 h after SNI had no effect on allodynia (VEH: 1.83 + 0.22 vs MET: 1.93 + 0.16 log(mg) threshold), performance in the FST (SNI-Stress-VEH: 63% vs SNI-Stress-MET: 59% time floating), CORT (SNI-Stress-VEH: 68 + 13 pg ml−1 vs SNI-Stress-MET: 77 + 11 pg ml−1), or gene expression (PFC IL-1β: SNI-Stress-VEH: 2.73 + 0.37 vs SNI-Stress-MET: 2.59 + 0.31).
Study 3: The functional significance of increased IL-1β levels in the prefrontal cortex of SNI animals
Depressive-like behavior
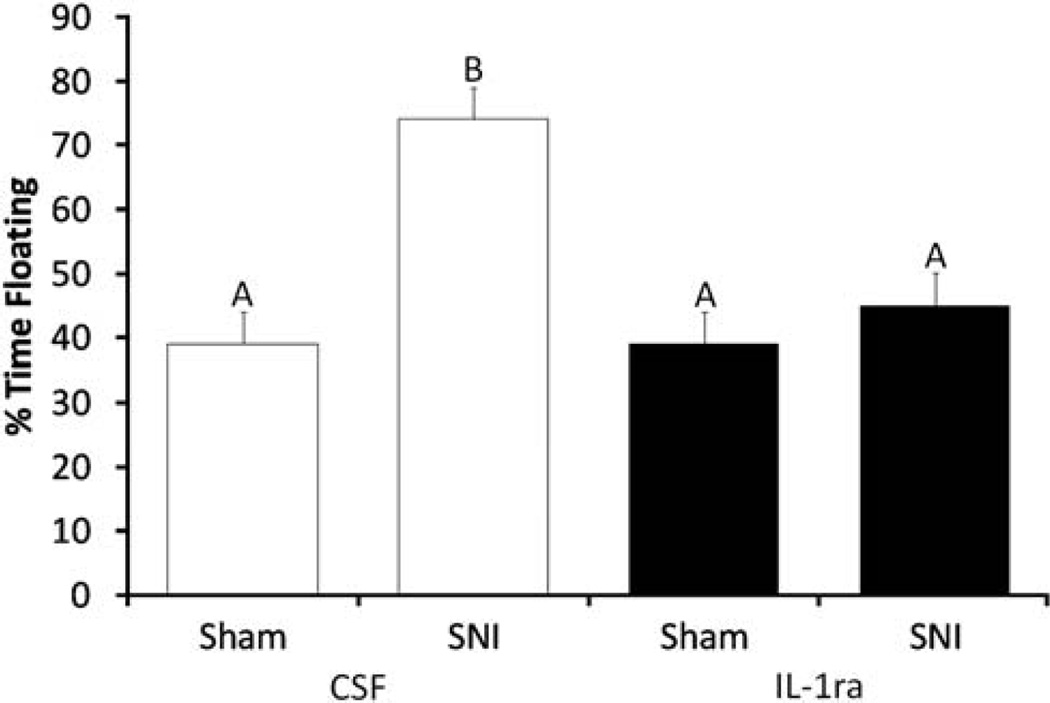
Similar to the outcome of study 1, SNI-vehicle-treated animals displayed increased depressive-like behavior as measured by increased floating in the forced swim test relative to Shams (Figure 5, P < 0.05). However, IL-1ra treatment on postsurgical days 6 and 7 significantly reduced floating behavior in SNI animals (Figure 5, F1,22 = 14.89, P < 0.05, Tukey = P < 0.05). IL-1ra administration did not influence floating behavior in Sham animals.
Figure 5.
Influence of IL-1ra on SNI-induced depressive-like behavior. Percentage time spent floating during a 5-min forced swim test conducted on day 7 after surgery was used as an index of depressive-like behavior. The mice were fitted with a cannula targeting the left lateral ventricle 1 week before SNI or Sham surgery. IL-1ra (1.8 µg) or the vehicle (VEH; 2 µl aCSF) was administered on postoperative days 6 and 7. SNI animals treated with CSF spent more time floating in FST than the other three experimental groups. Administration of IL-1ra eliminated the effect of SNI on time spent floating, such that behavior in this group was not significantly different than either Sham group. Similar letters above error bars indicate no significant difference between groups (P > 0.05), whereas different letters represent a significant difference between groups (P < 0.05). Data are presented as mean ± s.e.m.
Discussion
This study establishes that peripheral nerve injury induces the expression of IL-1β in the brain, which in turn contributes to the development of depressive-like behavior in mice. A causal relationship between IL-1β response to nerve injury and the change in affect is confirmed through ICV administration of IL-1ra, which returns depressive-like behavior after SNI to Sham levels. Furthermore, stress has a robust influence on the expression of IL-1β and behavioral responses to nerve injury. Exposure to chronic stress increases IL-1β expression in the frontal cortex, and potentiates allodynia, and depressive-like behavior. These effects are reversed when the stress-induced increase in corticosterone is prevented with concurrent MET treatment. It is well established that IL-1β expression at the level of the spinal cord contributes to the development of pain behaviors; however, this study provides the first evidence that a concomitant increase in supraspinal IL-1β expression modulates the induction of depressive-like behavior after nerve injury.
SNI, stress, and allodynia
The SNI procedure produces a significant increase in allodynia, which is exacerbated by prior exposure to stress (Figure 1a). However, in the absence of nerve injury (Sham), stress has no effect on pain threshold (Figure 1a), thereby suggesting that some component of the pathophysiological response to SNI (likely IL-1β, discussed below) is necessary for stress to induce allodynia. Furthermore, it is likely that stress-induced increases in circulating corticosterone underlie the effects of stress on allodynia after SNI. Treating mice with MET, a corticosteroid synthesis inhibitor, 1 h before stress eliminates the effect of stress on pain threshold (Figure 3a) and normalizes corticosterone concentrations on postsurgical day 7 (Figure 4d). In the absence of stress exposure, treatment with MET has no effect on SNI-induced allodynia (Figure 3a) or postsurgical CORT concentrations (Figure 4d). These data support and extend an earlier study, which showed that stress exacerbates SNI-induced allodynia, and that treatment with a glucocorticoid receptor antagonist at the time of stress exposure eliminates the effects of stress on allodynia.33 As circulating corticosterone concentrations are elevated on postsurgical day 7 among the mice that had been exposed to stress for 2 weeks before SNI or Sham surgery (Figure 2d), we tested the hypothesis that post-SNI elevations in corticosterone mediate the effects of stress on the physiological and behavioral responses to SNI. However, delaying treatment with MET until after SNI is ineffective in decreasing pain responses among mice that were exposed to stress before SNI.
SNI, stress, and depression
Chronic pain conditions within clinical populations are associated with a high incidence of affective disorders, including depression.1,2 Mirroring this pattern, this study shows that SNI increases depressive-like behavior, as indicated by increased floating in the forced swim test (Figure 1b). Exposure to chronic restraint for 2 weeks before SNI, further increases depressive-like behavior (Figure 1b). Inhibition of CORT synthesis through pretreatment with MET eliminates the effect of stress exposure on floating behavior in the forced swim test, but does not influence floating behavior among mice that were not exposed to stress before SNI (Figure 3b). In addition, in the absence of the nerve injury, the repeated stress does not influence behavior in the forced swim test. Importantly, there were no significant concomitant differences among the experimental groups in general locomotor activity or anxiety-like behavior.
Hypercortisolism is a common feature of depression,34 and pharmacological blockade of glucocorticoid receptors ameliorates depressive symptoms in many such patients.35 In this study, corticosterone concentrations on postoperative day 7 are significantly elevated after stress in both the SNI and Sham groups. However, the finding that behavior in the FST is similar between Sham groups despite differential exposure to stress and elevated corticosterone concentrations indicates that these two factors alone are not sufficient to induce depressive-like behavior under the experimental parameters used in this study. In contrast, the MET experiment shows that exposure to stress-induced increases in corticosterone before SNI, increases the expression of depressive-like behavior (Figure 3b). The stress-induced increase in depressive-like behavior after SNI, but not Sham surgery, is likely because stress exposure exacerbates the pathophysiological (neuroinflammatory) response to nerve injury, which in turn, influences the expression of depressive-like behavior. Corticosteroids are typically characterized as having anti-inflammatory properties, but several recent studies suggest stress and treatment with exogenous glucocorticoids can be proinflammatory within the CNS.36–38
SNI, stress, and Supraspinal gene expression
On postsurgical day 7, tissue was collected from the PAG and prefrontal cortex. The PAG, through its projections to the RVM, is capable of both inhibitory and facilitatory regulation of nociceptive information through direct modulation of spinal cord signal transmission that, together with primary afferent input, ultimately determines the excitability of spinal neurons.8,9 The frontal cortex was chosen because of its prominent role in mediating depressive-like behaviors.19 SNI produces a significant increase in GFAP mRNA expression in the PAG relative to Sham, but there is no effect of prior stress exposure on GFAP expression among the SNI or Sham groups (Figure 2a). Increased glial activity within the RVM-PAG system contributes to descending pain facilitation.39 Thus, increased GFAP mRNA expression within the PAG-RVM system of SNI animals may contribute to allodynia after nerve injury through modulation of supraspinal descending pain pathways; additional studies will need to be conducted to determine how GFAP expression may be influencing the development and maintenance of pain-related behaviors.
The combination of stress exposure and SNI increases BDNF expression in PAG, but there are no independent effects of stress or SNI on expression of this gene (Figure 2b). This observation provides a potential physiological mechanism for the enhanced nociceptive states in stressed neuropathic animals. Large quantities of BDNF mRNA are present within the PAG40,41 and interference of BDNF signaling within the PAG inhibits hyperalgesia, whereas microinjection of exogenous BDNF produces hyperalgesia.39 Furthermore, administration of MET before stress completely abolishes the effect of chronic stress on mechanical allodynia and BDNF mRNA expression within the PAG. Thus, corticosterone-mediated increases in BDNF mRNA levels within the PAG of chronically stressed neuropathic animals may represent one physiological mechanism underlying the stress-induced increases in mechanical allodynia and other behaviors.
In this study, there are no group differences in IL-1β mRNA levels in the PAG, but expression of this gene in the frontal cortex is significantly increased after SNI and further increased by the combination of stress and SNI (Figure 2c). Treatment with MET before stress eliminates the effects of stress on SNI-induced IL-1β gene expression (Figure 4c), which suggests that the increase in corticosterone during stress augments expression of this gene after nerve injury. Elevated gene expression of IL-1β within the PFC is particularly interesting given the ability of proinflammatory cytokines to induce hyperalgesia, as well as depressive-like behavior, in mice without nerve injury.19,42 Within the spinal cord, proinflammatory cytokines, including IL-1β, are central to the development and maintenance of neuropathic pain43,44 and disruption of cytokine signaling attenuates neuropathic pain.43,45 Centrally administered IL-1β induces hyperalgesia,46,47 whereas IL-1ra reduces pain behavior in enhanced pain states.48 Additionally, IL-1β expression within supraspinal regions, including prefrontal cortex, covary with measures of allodynia,13,49,50 and administration of an IL-1β neutralizing antibody into the red nucleus of the rostral midbrain, prevents the development of allodynia after nerve injury.50 Although this study does not directly test the functional consequences of IL-1β on mechanical allodynia, the existing literature suggests that the stress-induced increase in IL-1β expression in this study may contribute to the stress-induced increase in allodynia.
The concomitant increase in IL-1β gene expression in the prefrontal cortex (Figure 2a) and depressive-like behavior (Figure 1b), paired with the elimination of SNI-induced depressive-like behavior after treatment with IL-1ra (Figure 5), supports the hypothesis that the increase in the expression of proinflammatory cytokine IL-1β after SNI is responsible for the display of depressive-like behavior. Within the past decade, numerous studies have implicated inflammatory mediators, including IL-1β, in the pathophysiology of depression.42,51,52 Individuals suffering from medical conditions characterized by inflammation and increased IL-1β production within the brain have significantly higher rates of major depression.53,54 Additionally, the risk of depression is elevated in individuals with heightened levels of IL-1β55,56 and those with specific polymorphisms in genes within the IL-1 family.57 The finding that IL-1ra administration significantly reversed the effects of SNI on depressive-like behavior suggests a causative role for the elevated PFC IL-1β mRNA levels. Furthermore, chronic stress is capable of increasing central IL-1β expression,58 a process thought to mediate chronic stress-induced depression.59 Although chronic stress in the absence of nerve injury did not influence behavioral or immunological outcomes in this study, when combined with SNI it led to a synergistic increase in depressive-like behavior and IL-1β mRNA within the PFC as compared with no-stress neuropathic animals. The chronic stress-induced increases in depressive-like behavior and IL-1β mRNA among SNI animals was mediated by corticosterone, as administration of MET before each stress session entirely blocked the effects on behavior and gene expression. Thus, in conjunction with earlier reports on the functional significance of proinflammatory states in both depression and chronic pain, these data suggest treatments focused on reducing CNS inflammation may prove beneficial in NP and major depression in clinical settings.
In sum, this study shows that in conjunction with its effects on mechanical allodynia, SNI leads to significant increases in mRNA expression of GFAP within the PAG and IL-1β within the prefrontal cortex, as well as depressive-like behavior. The increased IL-1β mRNA expression has a causative function in the expression of post-SNI depressive-like behavior as administration of IL-1ra blocks the effects of SNI on floating behavior in the FST. In line with earlier studies, the SNI procedure did not significantly alter circulating corticosterone levels, anxiety-like behavior, or overall locomotor activity. However, exposure to 2 weeks of daily 2 h restraint stress potentiated the behavioral and proinflammatory cytokine effects of SNI in a glucocorticoid-dependent manner. Together, the data suggest that the neuroinflammatory response to peripheral nerve injury can precipitate a depressive-like state, and that psychosocial factors that modulate inflammatory processes can, in turn, influence injury-induced depression.
Acknowledgments
We thank ZM Weil for technical support and assistance with data analysis. This work was supported by grants from the American Heart Association (predoctoral fellowship to KK), National Institute of Neurological Disorders and Stroke Behavioral Core Grant P30 NS045758 (to ACD), 5R01NR010806 SNI Grant (to ACD)
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Wise TN, Fishbain DA, Holder-Perkins V. Painful physical symptoms in depression: a clinical challenge. Pain Med. 2007;8(Suppl 2):S75–S82. doi: 10.1111/j.1526-4637.2007.00352.x. [DOI] [PubMed] [Google Scholar]
- 2.Strouse TB. The relationship between cytokines and pain/depression: a review and current status. Curr Pain Headache Rep. 2007;11:98–103. doi: 10.1007/s11916-007-0005-y. [DOI] [PubMed] [Google Scholar]
- 3.Jensen MP, Chodroff MJ, Dworkin RH. The impact of neuropathic pain on health-related quality of life: review and implications. Neurology. 2007;68:1178–1182. doi: 10.1212/01.wnl.0000259085.61898.9e. [DOI] [PubMed] [Google Scholar]
- 4.Gatchel RJT, et al. D.C. Psychological Approaches to Pain Management: A Practitioner’s Handbook. New York: Guilford Press; 1996. [Google Scholar]
- 5.Classification of Chronic Pain. Proceedings of the IASP Task Force on Taxonomy. Seattle: IASP Press; 1994. [Google Scholar]
- 6.Lesser H, Sharma U, LaMoreaux L, Poole RM. Pregabalin relieves symptoms of painful diabetic neuropathy: a randomized controlled trial. Neurology. 2004;63:2104–2110. doi: 10.1212/01.wnl.0000145767.36287.a1. [DOI] [PubMed] [Google Scholar]
- 7.Wilkie DJ, Huang HY, Reilly N, Cain KC. Nociceptive and neuropathic pain in patients with lung cancer: a comparison of pain quality descriptors. J Pain Symptom Manage. 2001;22:899–910. doi: 10.1016/s0885-3924(01)00351-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Urban MO, Gebhart GF. Supraspinal contributions to hyperalgesia. Proc Natl Acad Sci USA. 1999;96:7687–7692. doi: 10.1073/pnas.96.14.7687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vanegas H, Schaible HG. Descending control of persistent pain: inhibitory or facilitatory? Brain Res Brain Res Rev. 2004;46:295–309. doi: 10.1016/j.brainresrev.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 10.Gebhart GF. Descending modulation of pain. Neurosci Biobehav Rev. 2004;27:729–737. doi: 10.1016/j.neubiorev.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 11.Neugebauer V, Li W, Bird GC, Han JS. The amygdala and persistent pain. Neuroscientist. 2004;10:221–234. doi: 10.1177/1073858403261077. [DOI] [PubMed] [Google Scholar]
- 12.Xu H, Wu LJ, Wang H, Zhang X, Vadakkan KI, Kim SS, et al. Presynaptic and postsynaptic amplifications of neuropathic pain in the anterior cingulate cortex. J Neurosci. 2008;28:7445–7453. doi: 10.1523/JNEUROSCI.1812-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Apkarian AV, Lavarello S, Randolf A, Berra HH, Chialvo DR, Besedovsky HO, et al. Expression of IL-1beta in supraspinal brain regions in rats with neuropathic pain. Neurosci Lett. 2006;407:176–181. doi: 10.1016/j.neulet.2006.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fields HL, Basbaum AI. Central nervous system mechanisms of pain modulation. In: Wall PD, Melzack R, editors. Textbook of Pain. 4th. Edinburgh: Churchill Livingston; 1999. pp. 309–329. [Google Scholar]
- 15.Rhudy JL, Williams AE, McCabe KM, Nguyen MA, Rambo P. Affective modulation of nociception at spinal and supraspinal levels. Psychophysiology. 2005;42:579–587. doi: 10.1111/j.1469-8986.2005.00313.x. [DOI] [PubMed] [Google Scholar]
- 16.Villemure C, Bushnell MC. Cognitive modulation of pain: how do attention and emotion influence pain processing? Pain. 2002;95:195–199. doi: 10.1016/S0304-3959(02)00007-6. [DOI] [PubMed] [Google Scholar]
- 17.Dworkin RH, Gitlin MJ. Clinical aspects of depression in chronic pain patients. Clin J Pain. 1991;7:79–94. doi: 10.1097/00002508-199106000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Watkins LR, Maier SF. Immune regulation of central nervous system functions: from sickness responses to pathological pain. J Intern Med. 2005;257:139–155. doi: 10.1111/j.1365-2796.2004.01443.x. [DOI] [PubMed] [Google Scholar]
- 20.Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 21.McBeth J, Silman AJ, Gupta A, Chiu YH, Ray D, Morriss R, et al. Moderation of psychosocial risk factors through dysfunction of the hypothalamic-pituitary-adrenal stress axis in the onset of chronic widespread musculoskeletal pain: findings of a population-based prospective cohort study. Arthritis Rheum. 2007;56:360–371. doi: 10.1002/art.22336. [DOI] [PubMed] [Google Scholar]
- 22.Wood PB. Stress and dopamine: implications for the pathophysiology of chronic widespread pain. Med Hypotheses. 2004;62:420–424. doi: 10.1016/j.mehy.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 23.da Silva Torres IL, Cucco SN, Bassani M, Duarte MS, Silveira PP, Vasconcellos AP, et al. Long-lasting delayed hyperalgesia after chronic restraint stress in rats-effect of morphine administration. Neurosci Res. 2003;45:277–283. doi: 10.1016/s0168-0102(02)00232-8. [DOI] [PubMed] [Google Scholar]
- 24.Cerqueira JJ, Pego JM, Taipa R, Bessa JM, Almeida OF, Sousa N. Morphological correlates of corticosteroid-induced changes in prefrontal cortex-dependent behaviors. J Neurosci. 2005;25:7792–7800. doi: 10.1523/JNEUROSCI.1598-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mitra R, Sapolsky RM. Acute corticosterone treatment is sufficient to induce anxiety and amygdaloid dendritic hypertrophy. Proc Natl Acad Sci USA. 2008;105:5573–5578. doi: 10.1073/pnas.0705615105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bourquin AF, Suveges M, Pertin M, Gilliard N, Sardy S, Davison AC, et al. Assessment and analysis of mechanical allodynia-like behavior induced by spared nerve injury (SNI) in the mouse. Pain. 2006;122:14.e1–14.e14. doi: 10.1016/j.pain.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 27.Tal M, Bennett GJ. Extra-territorial pain in rats with a peripheral mononeuropathy: mechano-hyperalgesia and mechano-allodynia in the territory of an uninjured nerve. Pain. 1994;57:375–382. doi: 10.1016/0304-3959(94)90013-2. [DOI] [PubMed] [Google Scholar]
- 28.Decosterd I, Allchorne A, Woolf CJ. Differential analgesic sensitivity of two distinct neuropathic pain models. Anesth Analg. 2004;99:457–463. doi: 10.1213/01.ANE.0000131967.69309.4F. table of contents. [DOI] [PubMed] [Google Scholar]
- 29.Porsolt RD, Bertin A, Jalfre M. Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther. 1977;229:327–336. [PubMed] [Google Scholar]
- 30.Porsolt RD, Brossard G, Hautbois C, Roux S. Rodent models of depression: forced swimming and tail suspension behavioral despair tests in rats and mice. Curr Protoc Neurosci. 2001;Chapter 8(Unit 8 10A) doi: 10.1002/0471142301.ns0810as14. [DOI] [PubMed] [Google Scholar]
- 31.Rotllant D, Armario A. A single dose of metyrapone caused long-term dysregulation of the hypothalamic-pituitary-adrenal axis in the rat. Neuroscience. 2005;130:427–434. doi: 10.1016/j.neuroscience.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 32.Craft TK, DeVries AC. Role of IL-1 in poststroke depressive-like behavior in mice. Biol Psychiatry. 2006;60:812–818. doi: 10.1016/j.biopsych.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 33.Alexander JK, DeVries AC, Popovich PG. Stress effects on neuropathic pain. Annual Meeting of the Society for Neuroscience; 2006; Atlanta, GA. [Google Scholar]
- 34.Gillespie CF, Nemeroff CB. Hypercortisolemia and depression. Psychosom Med. 2005;67(Suppl 1):S26–S28. doi: 10.1097/01.psy.0000163456.22154.d2. [DOI] [PubMed] [Google Scholar]
- 35.DeBattista C, Belanoff J. The use of mifepristone in the treatment of neuropsychiatric disorders. Trends Endocrinol Metab. 2006;17:117–121. doi: 10.1016/j.tem.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 36.Dinkel K, MacPherson A, Sapolsky RM. Novel glucocorticoid effects on acute inflammation in the CNS. J Neurochem. 2003;84:705–716. doi: 10.1046/j.1471-4159.2003.01604.x. [DOI] [PubMed] [Google Scholar]
- 37.de Pablos RM, Villaran RF, Arguelles S, Herrera AJ, Venero JL, Ayala A, et al. Stress increases vulnerability to inflammation in the rat prefrontal cortex. J Neurosci. 2006;26:5709–5719. doi: 10.1523/JNEUROSCI.0802-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.MacPherson A, Dinkel K, Sapolsky R. Glucocorticoids worsen excitotoxin-induced expression of pro-inflammatory cytokines in hippocampal cultures. Exp Neurol. 2005;194:376–383. doi: 10.1016/j.expneurol.2005.02.021. [DOI] [PubMed] [Google Scholar]
- 39.Guo W, Robbins MT, Wei F, Zou S, Dubner R, Ren K. Supraspinal brain-derived neurotrophic factor signaling: a novel mechanism for descending pain facilitation. J Neurosci. 2006;26:126–137. doi: 10.1523/JNEUROSCI.3686-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ceccatelli S, Ernfors P, Villar MJ, Persson H, Hokfelt T. Expanded distribution of mRNA for nerve growth factor, brain-derived neurotrophic factor, and neurotrophin 3 in the rat brain after colchicine treatment. Proc Natl Acad Sci USA. 1991;88:10352–10356. doi: 10.1073/pnas.88.22.10352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Conner JM, Lauterborn JC, Yan Q, Gall CM, Varon S. Distribution of brain-derived neurotrophic factor (BDNF) protein and mRNA in the normal adult rat CNS: evidence for anterograde axonal transport. J Neurosci. 1997;17:2295–2313. doi: 10.1523/JNEUROSCI.17-07-02295.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wolf G, Gabay E, Tal M, Yirmiya R, Shavit Y. Genetic impairment of interleukin-1 signaling attenuates neuropathic pain, autotomy, and spontaneous ectopic neuronal activity, following nerve injury in mice. Pain. 2006;120:315–324. doi: 10.1016/j.pain.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 44.Scholz J, Woolf CJ. The neuropathic pain triad: neurons, immune cells and glia. Nat Neurosci. 2007;10:1361–1368. doi: 10.1038/nn1992. [DOI] [PubMed] [Google Scholar]
- 45.Sweitzer S, Martin D, DeLeo JA. Intrathecal interleukin-1 receptor antagonist in combination with soluble tumor necrosis factor receptor exhibits an anti-allodynic action in a rat model of neuropathic pain. Neuroscience. 2001;103:529–539. doi: 10.1016/s0306-4522(00)00574-1. [DOI] [PubMed] [Google Scholar]
- 46.Oka T, Aou S, Hori T. Intracerebroventricular injection of interleukin-1 beta induces hyperalgesia in rats. Brain Res. 1993;624:61–68. doi: 10.1016/0006-8993(93)90060-z. [DOI] [PubMed] [Google Scholar]
- 47.Tonosaki Y, Nishiyama K, Roubos EW, Sugiura Y. alpha-Melanophorestimulating hormone (alpha-MSH) antagonizes interleukin-1beta-induced hyperalgesia and Fos expression in the paraventricular and arcuate nucleus of the rat. Neuroendocrinology. 2005;81:167–173. doi: 10.1159/000086888. [DOI] [PubMed] [Google Scholar]
- 48.Zhang RX, Li A, Liu B, Wang L, Ren K, Zhang H, et al. IL-1ra alleviates inflammatory hyperalgesia through preventing phosphorylation of NMDA receptor NR-1 subunit in rats. Pain. 2008;135:232–239. doi: 10.1016/j.pain.2007.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xie W, Luo S, Xuan H, Chou C, Song G, Lv R, et al. Betamethasone affects cerebral expressions of NF-kappaB and cytokines that correlate with pain behavior in a rat model of neuropathy. Ann Clin Lab Sci. 2006;36:39–46. [PubMed] [Google Scholar]
- 50.Wang Z, Wang J, Li X, Yuan Y, Fan G. Interleukin-1 beta of Red nucleus involved in the development of allodynia in spared nerve injury rats. Exp Brain Res. 2008;188:379–384. doi: 10.1007/s00221-008-1365-1. [DOI] [PubMed] [Google Scholar]
- 51.Schiepers OJ, Wichers MC, Maes M. Cytokines and major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:201–217. doi: 10.1016/j.pnpbp.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 52.Koo JW, Duman RS. IL-1beta is an essential mediator of the antineurogenic and anhedonic effects of stress. Proc Natl Acad Sci USA. 2008;105:751–756. doi: 10.1073/pnas.0708092105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Levine J, Barak Y, Chengappa KN, Rapoport A, Rebey M, Barak V. Cerebrospinal cytokine levels in patients with acute depression. Neuropsychobiology. 1999;40:171–176. doi: 10.1159/000026615. [DOI] [PubMed] [Google Scholar]
- 54.Hayley S, Poulter MO, Merali Z, Anisman H. The pathogenesis of clinical depression: stressor- and cytokine-induced alterations of neuroplasticity. Neuroscience. 2005;135:659–678. doi: 10.1016/j.neuroscience.2005.03.051. [DOI] [PubMed] [Google Scholar]
- 55.Anisman H, Ravindran AV, Griffiths J, Merali Z. Endocrine and cytokine correlates of major depression and dysthymia with typical or atypical features. Mol Psychiatry. 1999;4:182–188. doi: 10.1038/sj.mp.4000436. [DOI] [PubMed] [Google Scholar]
- 56.Thomas AJ, Davis S, Morris C, Jackson E, Harrison R, O’Brien JT. Increase in interleukin-1beta in late-life depression. Am J Psychiatry. 2005;162:175–177. doi: 10.1176/appi.ajp.162.1.175. [DOI] [PubMed] [Google Scholar]
- 57.Yu YW, Chen TJ, Hong CJ, Chen HM, Tsai SJ. Association study of the interleukin-1 beta (C-511T) genetic polymorphism with major depressive disorder, associated symptomatology, and antidepressant response. Neuropsychopharmacology. 2003;28:1182–1185. doi: 10.1038/sj.npp.1300172. [DOI] [PubMed] [Google Scholar]
- 58.Tanebe K, Nishijo H, Muraguchi A, Ono T. Effects of chronic stress on hypothalamic lnterleukin-1beta, interleukin-2, and gonadotrophin-releasing hormone gene expression in ovariectomized rats. J Neuroendocrinol. 2000;12:13–21. doi: 10.1046/j.1365-2826.2000.00414.x. [DOI] [PubMed] [Google Scholar]
- 59.Goshen I, Kreisel T, Ben-Menachem-Zidon O, Licht T, Weidenfeld J, Ben-Hur T, et al. Brain interleukin-1 mediates chronic stressinduced depression in mice via adrenocortical activation and hippocampal neurogenesis suppression. Mol Psychiatry. 2008;13:717–728. doi: 10.1038/sj.mp.4002055. [DOI] [PubMed] [Google Scholar]