Abstract
Background and purpose
To investigate the efficacy of chiropractic spinal manipulative therapy (CSMT) for migraineurs.
Methods
This was a prospective three‐armed, single‐blinded, placebo, randomized controlled trial (RCT) of 17 months duration including 104 migraineurs with at least one migraine attack per month. The RCT was conducted at Akershus University Hospital, Oslo, Norway. Active treatment consisted of CSMT, whereas placebo was a sham push manoeuvre of the lateral edge of the scapula and/or the gluteal region. The control group continued their usual pharmacological management. The RCT consisted of a 1‐month run‐in, 3 months intervention and outcome measures at the end of the intervention and at 3, 6 and 12 months follow‐up. The primary end‐point was the number of migraine days per month, whereas secondary end‐points were migraine duration, migraine intensity and headache index, and medicine consumption.
Results
Migraine days were significantly reduced within all three groups from baseline to post‐treatment (P < 0.001). The effect continued in the CSMT and placebo group at all follow‐up time points, whereas the control group returned to baseline. The reduction in migraine days was not significantly different between the groups (P > 0.025 for interaction). Migraine duration and headache index were reduced significantly more in the CSMT than the control group towards the end of follow‐up (P = 0.02 and P = 0.04 for interaction, respectively). Adverse events were few, mild and transient. Blinding was strongly sustained throughout the RCT.
Conclusions
It is possible to conduct a manual‐therapy RCT with concealed placebo. The effect of CSMT observed in our study is probably due to a placebo response.
Keywords: chiropractic, headache, migraine, randomized controlled trial, spinal manipulative therapy
Introduction
The socio‐economic costs of migraine are enormous due to its high prevalence and disability during attacks 1, 2, 3. Acute pharmacological treatment is usually the first treatment option for migraine in adults. Migraineurs with frequent attacks, insufficient effect and/or contraindication to acute medication are potential candidates for prophylactic treatment. Migraine prophylactic treatment is often pharmacological, but manual therapy is not unusual, especially if pharmacological treatment fails or if the patient wishes to avoid medicine 4. Research has suggested that spinal manipulative therapy may stimulate neural inhibitory systems at different spinal cord levels because it might activate various central descending inhibitory pathways 5, 6, 7, 8, 9, 10.
Pharmacological randomized controlled trials (RCTs) are usually double‐blinded, but this is not possible in manual‐therapy RCTs, as the interventional therapist cannot be blinded. At present there is no consensus on a sham procedure in manual‐therapy RCTs that mimics placebo in pharmacological RCTs 11. Lack of a proper sham procedure is a major limitation in all previous manual‐therapy RCTs 12, 13. Recently, we developed a sham chiropractic spinal manipulative therapy (CSMT) procedure, where participants with migraine were unable to distinguish between real and sham CSMT evaluated after each of 12 individual interventions over a 3‐month period 14.
The first objective of this study was to conduct a manual‐therapy three‐armed, single‐blinded, placebo RCT for migraineurs with a methodological standard similar to that of pharmacological RCTs.
The second objective was to assess the efficacy of CSMT versus sham manipulation (placebo) and CSMT versus controls, i.e. participants who continued their usual pharmacological management.
Methods
Study design
The study was a three‐armed, single‐blinded, placebo RCT over 17 months. The RCT consisted of a 1‐month baseline, 12 treatment sessions over 3 months with follow‐up measures at the end of intervention, 3, 6 and 12 months later.
Participants were, before baseline, randomized equally into three groups: CSMT, placebo (sham manipulation) and control (continued their usual pharmacological management).
The design of the study conformed to the recommendations of the International Headache Society (IHS) and CONSORT (Appendix S1) 1, 15, 16. The Norwegian Regional Committee for Medical Research Ethics and the Norwegian Social Science Data Services approved the project. The RCT was registered at ClinicalTrials.gov (ID no: NCT01741714). The full trial protocol has been published previously 17.
Participants
Participants were recruited from January to September 2013 primarily through the Department of Neurology, Akershus University Hospital. Some participants were also recruited through General Practitioners from Akershus and Oslo Counties or media advertisement. All participants received posted information about the project followed by a telephone interview.
Eligible participants were migraineurs of 18–70 years old with at least one migraine attack per month and were allowed to have concomitant tension‐type headache but no other primary headaches. All participants were diagnosed by a chiropractor with experience in headache diagnostics during the interview and according to the International Classification of Headache Disorders‐II (ICHD‐II) 2. A neurologist had diagnosed all migraineurs from Akershus University Hospital.
Exclusion criteria were contraindication to spinal manipulative therapy, spinal radiculopathy, pregnancy, depression and CSMT within the previous 12 months. Participants who received manual therapy 18, changed their prophylactic migraine medicine or became pregnant during the RCT were informed that they would be withdrawn from the study at that time and regarded as drop‐outs. Participants were allowed to continue and change acute migraine medication throughout the study period.
Eligible participants were invited to an interview and physical assessment including meticulous spinal column investigation by a chiropractor (A.C.). Participants randomized to the CSMT or the placebo group had a full spine radiographic examination.
Randomization and masking
After written consent was obtained, participants were equally randomized into one of the three study arms by drawing one single lot. Numbered sealed lots with the three study arms were each subdivided into four subgroups by age and gender, i.e. 18–39 or 40–70 years, and men or women.
After each treatment session, the participants in the CSMT and the placebo group completed a questionnaire on whether they believed CSMT treatment was received, and how certain they were that active treatment was received on a 0–10 numeric rating scale, where 10 represented absolute certainty 14.
Both the block randomization and the blinding questionnaire were exclusively administered by a single external party.
Interventions
The CSMT group received spinal manipulative therapy using the Gonstead method, a specific contact, high‐velocity, low‐amplitude, short‐lever spinal with no post‐adjustment recoil that was directed to spinal biomechanical dysfunction (full spine approach) as diagnosed by standard chiropractic tests at each individual treatment session 19.
The placebo group received sham manipulation, a broad non‐specific contact, low‐velocity, low‐amplitude sham push manoeuvre in a non‐intentional and non‐therapeutic directional line of the lateral edge of the scapula and/or the gluteal region 14. All of the non‐therapeutic contacts were performed outside the spinal column with adequate joint slack and without soft tissue pre‐tension so that no joint cavitations occurred. The sham manipulation alternatives were pre‐set and equally interchanged among the placebo participants according to protocol during the 12‐week treatment period to strengthen the study validity. The placebo procedure is described in detail in the available trial protocol 17.
Each intervention session lasted for 15 min and both groups underwent the same structural and motion assessments prior to and after each intervention. No other intervention or advice was given to participants during the trial period. Both groups received interventions at Akershus University Hospital by a single experienced chiropractor (A.C.).
The control group continued their usual pharmacological management without receiving manual intervention by the clinical investigator.
Outcomes
The participants filled in a validated diagnostic headache diary throughout the study and returned them on a monthly basis 20. In the case of unreturned diaries or missing data, the participants were contacted by phone to secure compliance.
The primary end‐point was number of migraine days per month (30 days/month). At least 25% reduction of migraine days from baseline to end of intervention, with the same level maintained at 3, 6 and 12 months follow‐up was expected in the CSMT group.
Secondary end‐points were migraine duration, migraine intensity and headache index (HI), and medicine consumption. At least 25% reduction in duration, intensity and HI, and at least 50% reduction in medicine consumption were expected from baseline to end of intervention, with the same level maintained at 3, 6 and 12 months follow‐up in the CSMT group.
No change was expected for primary and secondary end‐point in the placebo and the control group.
A migraine day was defined as a day on which migraine with aura, migraine without aura or probable migraine occurred. Migraine attacks lasting for >24 h were calculated as one attack unless pain‐free intervals of ≥48 h had occurred 21. If a patient fell asleep during a migraine attack and woke up without a migraine, in accordance with the ICHD‐III β, the duration of the attack was recorded as persisting until the time of awakening 22. The minimum duration of a migraine attack was 4 h unless a triptan or drug containing ergotamine was used, in which case we specified no minimum duration. HI was calculated as mean migraine days per month (30 days) × mean migraine duration (h/day) × mean intensity (0–10 numeric rating scale).
The primary and secondary end‐points were chosen based on the Task Force of the IHS Clinical Trial Subcommittee's clinical trial guidelines 1, 15. Based on previous reviews on migraine, a 25% reduction was considered to be a conservative estimate 12, 13.
The outcome analyses were calculated during the 30 days after the last intervention session and 30 days after the follow‐up time points, i.e. 3, 6 and 12 months, respectively.
All adverse events (AEs) were recorded after each intervention in accordance with the recommendations of CONSORT and the IHS Task Force on AEs in migraine trials 16, 23.
Statistical analysis
We based the power calculation on a recent study of topiramate in migraineurs 24. We hypothesized the average difference in reduction of number of migraine days per month between the active and the placebo, and between the active and the control groups of 2.5 days, with SD of 2.5 for reduction in each group. As primary analysis includes two group comparisons, the significance level was set at 0.025. For the power of 80%, a sample size of 20 patients was required in each group to detect a significant difference in reduction of 2.5 days.
Patient characteristics at baseline were presented as means and SD or frequencies and percentages in each group and compared by independent samples t‐test and χ 2 test.
Time profiles of all end‐points were compared between the groups. Due to repeated measurements for each patient, linear mixed models accounting for the intra‐individual variations were estimated for all end‐points. Fixed effects for (non‐linear) time, group allocation and interaction between the two were included. Random effects for patients and slopes were entered into the model. As the residuals were skewed, the bootstrap inference based on 1000 cluster samples was used. Pairwise comparisons were performed by deriving individual time point contrasts within each group at each time point with the corresponding P‐values and 95% confidence intervals. Medicine consumption within groups was reported by mean doses with SD, and groups were compared by an independent samples median test. A dose was defined as a single administration of a triptan or ergotamine; paracetamol 1000 mg ± codeine; non‐steroidal anti‐inflammatory drugs (tolfenamic acid, 200 mg; diclofenac, 50 mg; aspirin, 1000 mg; ibuprofen, 600 mg; naproxen, 500 mg); and morphinomimetics (tramadol, 50 mg). None of the patients changed study arm and none of the drop‐outs filled in headache diaries after withdrawal from the study. Hence, only per protocol analysis was relevant.
The analyses were blinded to treatment allocation and conducted in SPSS v22 (IBM Corporation, Armonk, NY, USA) and STATA v14 (JSB) (StataCorp LP, College Station, TX, USA). A significance level of 0.025 was applied for the primary end‐point, whereas elsewhere a level of 0.05 was used.
Ethics
Good clinical practice guidelines were followed 25. Oral and written information about the project was provided in advance of inclusion and group allocation. Written consent was obtained from all participants. Participants in the placebo and control group were promised CSMT treatment after the RCT, if the active intervention was found to be effective. Insurance was provided through the Norwegian System of Compensation to Patients (Patient Injury Compensation), an independent national body that compensates patients injured by treatments provided by the Norwegian health service. A stopping rule was defined for withdrawing participants from this study in accordance with the recommendations in the CONSORT extension for Better Reporting of Harms 26. All AEs were monitored during the intervention period and acted on as they occurred according to the recommendations of CONSORT and the IHS Task Force on AEs in migraine trials 16, 23. In case of severe AE, the participant would be withdrawn from the study and referred to the General Practitioner or hospital emergency department depending on the event. The investigator (A.C.) was available by mobile phone at any time throughout the study treatment period.
Results
Figure 1 shows a flow chart of the 104 migraineurs included in the study. Baseline and demographic characteristics were similar across the three groups (Table 1).
Figure 1.
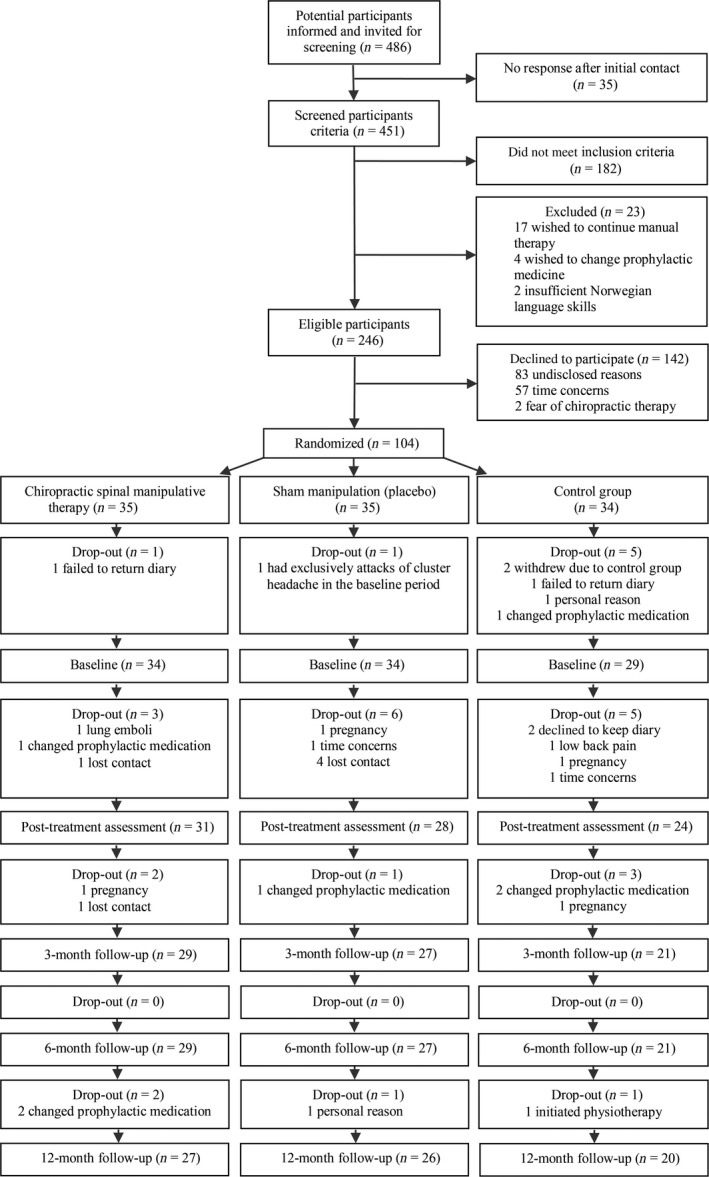
Study flow chart.
Table 1.
Baseline demographic and clinical characteristics
| CSMT | Sham manipulation (placebo) | Control group | |
|---|---|---|---|
| Number of participants | 34 | 34 | 29 |
| Malesb | 6 (18%) | 5 (15%) | 3 (10%) |
| Femalesb | 28 (82%) | 29 (85%) | 26 (90%) |
| Age ± SD (years) (range)a | 41.0 ± 11.3 (19–63) | 39.6 ± 9.8 (18–65) | 38.7 ± 11.1 (20–58) |
| Migraine without aurab | 32 (94%) | 30 (88%) | 26 (90%) |
| Migraine with aurab | 9 (26%) | 12 (35%) | 6 (21%) |
| Duration (years with migraine ± SD)a | 21.9 ± 13.2 | 21.4 ± 11.2 | 20.8 ± 10.5 |
| Migraine days (30 days/month) in the run‐in period ± SDa | 6.5 ± 3.3 | 8.3 ± 5.6 | 7.8 ± 6.0 |
| Co‐morbid tension‐type headache (%)b | 24 (71%) | 26 (76%) | 22 (76%) |
| Tension‐type headache days (30 days/month) in the run‐in period ± SDa | 1.0 ± 2.0 | 2.1 ± 3.5 | 0.9 ± 1.8 |
| Diagnosed at hospital by a neurologistb | 26 (76%) | 26 (76%) | 21 (72%) |
| Diagnosed by neurologistb | 5 (15%) | 7 (21%) | 4 (14%) |
| Diagnosed by general practitioner aloneb | 3 (9%) | 1 (3%) | 4 (14%) |
| Previously received CSMT (%)b | 11 (32%) | 13 (38%) | 16 (55%) |
| Previously experienced cervical painb | 29 (85%) | 28 (82%) | 20 (69%) |
| Previously experienced thoracic painb | 24 (71%) | 26 (76%) | 16 (55%) |
| Previously experienced lumbar painb | 24 (71%) | 26 (76%) | 18 (62%) |
Data are presented as means and SDs or frequencies and percentages in each group and compared by aindependent samples t‐test and b χ 2 test. No significant group differences were seen between chiropractic spinal manipulative therapy (CSMT) versus placebo and CSMT versus control (all P > 0.05).
Outcome measures
The results on all end‐points are presented in Fig. 2a–d and Tables 2, 3, 4.
Figure 2.
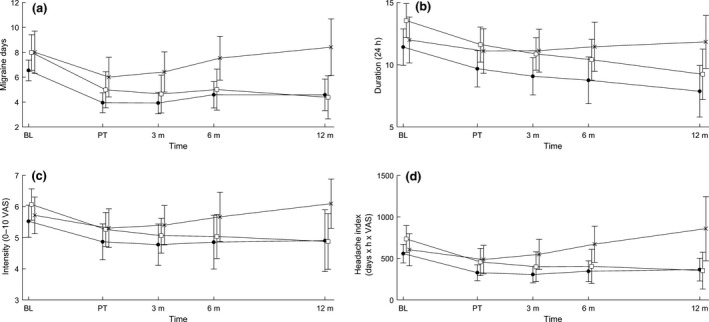
(a) Headache days; (b) headache duration; (c) headache intensity; (d) headache index. Time profiles in primary and secondary end‐points, means and error bars represent 95% confidence intervals. BL, baseline; control, control group (×); CSMT, chiropractic spinal manipulative therapy (●); placebo, sham manipulation (□); PT, post‐treatment; 3 m, 3‐month follow‐up; 6 m, 6‐month follow‐up; 12 m, 12‐month follow‐up; VAS, visual analogue scale.
Table 2.
Regression coefficients and SE from linear mixed models
| Variable | Migraine days | Duration | Intensity | Headache index | ||||
|---|---|---|---|---|---|---|---|---|
| Regression coefficient (SE) | P‐value | Regression coefficient (SE) | P‐value | Regression coefficient (SE) | P‐value | Regression coefficient (SE) | P‐value | |
| Intercept | 6.54 (0.42) | <0.001 | 11.42 (0.75) | <0.001 | 5.53 (0.26) | <0.001 | 557.24 (56.93) | <0.001 |
| Time | −0.04 (0.006) | <0.001 | −0.02 (0.01) | 0.05 | −0.009 (0.004) | 0.02 | −3.22 (0.72) | <0.001 |
| Time × Time | 2 × 10−3 (3 × 10−4) | <0.001 | 6 × 10−4 (7 × 10−4) | 0.38 | 3 × 10−4 (3 × 10−4) | 0.19 | 0.01 (0.004) | 0.005 |
| Time × Time × Time | −2 × 10−6 (5 × 10−7) | <0.001 | −6 × 10−7 (1 × 10−6) | 0.51 | −4 × 10−7 (4 × 10−7) | 0.36 | −1 × 10−4 (7 × 10−5) | 0.04 |
| CSMT – ref. | 0 | – | 0 | – | 0 | – | 0 | – |
| Placebo | 1.44 (0.80) | 0.07 | 2.13 (1.02) | 0.04 | 0.54 (0.38) | 0.16 | 176.75 (93.30) | 0.06 |
| Control | 1.46 (0.93) | 0.12 | 0.57 (1.15) | 0.62 | 0.19 (0.40) | 0.64 | 46.83 (112.79) | 0.68 |
| CSMT – ref. | 0 | – | 0 | – | 0 | – | 0 | – |
| Placebo × Time | −0.003 (0.002) | 0.04 | −0.002 (0.003) | 0.61 | −0.001 (0.001) | 0.41 | −0.39 (0.20) | 0.05 |
| Control × Time | 0.005 (0.003) | 0.06 | 0.007 (0.003) | 0.02 | 0.002 (0.001) | 0.11 | 0.93 (0.46) | 0.04 |
Control, control group; CSMT, chiropractic spinal manipulative therapy; placebo, sham manipulation; ref., reference group.
Table 3.
Means and SD, not adjusted for intra‐patient correlations, at baseline (BL) and follow‐up for primary end‐point (migraine days) and secondary end‐points (duration, intensity and headache index) by group
| CSMT | Placebo | Control | CSMT versus placebo | CSMT versus control | |
|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | P‐values | P‐values | |
| Migraine days | |||||
| BL | 6.5 (3.3) | 8.3 (5.6) | 7.8 (6.0) | 0.07 | 0.12 |
| Post‐treatment | 3.9 (3.1) | 4.1 (5.7) | 6.1 (5.9) | 0.20 | 0.02 |
| 3‐month follow‐up | 4.5 (3.6) | 4.6 (5.7) | 6.2 (5.6) | 0.39 | 0.005 |
| 6‐month follow‐up | 4.1 (3.9) | 5.1 (6.4) | 6.8 (6.3) | 0.65 | 0.003 |
| 12‐month follow‐up | 4.4 (4.2) | 4.1 (6.0) | 8.0 (8.2) | 0.85 | 0.002 |
| BL to post‐treatment | 2.6 (0.4) | 3.0 (0.4) | 2.0 (0.4) | 0.04 | 0.06 |
| Duration | |||||
| BL | 11.7 (5.9) | 14.0 (4.7) | 11.1 (6.1) | 0.04 | 0.62 |
| Post‐treatment | 9.2 (5.8) | 10.4 (7.0) | 13.1 (6.5) | 0.04 | 0.20 |
| 3‐month follow‐up | 9.5 (6.9) | 10.6 (7.2) | 10.8 (6.9) | 0.06 | 0.07 |
| 6‐month follow‐up | 7.3 (7.1) | 11.6 (7.4) | 11.3 (6.7) | 0.12 | 0.03 |
| 12‐month follow‐up | 8.1 (7.3) | 8.9 (7.7) | 11.8 (5.9) | 0.34 | 0.01 |
| BL to post‐treatment | 1.7 (0.6) | 1.9 (0.6) | 0.9 (0.6) | 0.61 | 0.009 |
| Intensity | |||||
| BL | 5.7 (1.7) | 6.1 (1.7) | 5.6 (2.0) | 0.16 | 0.64 |
| Post‐treatment | 4.7 (2.8) | 5.0 (3.0) | 5.7 (2.5) | 0.27 | 0.26 |
| 3‐month follow‐up | 5.0 (3.0) | 4.9 (2.8) | 5.4 (2.8) | 0.46 | 0.13 |
| 6‐month follow‐up | 4.4 (3.6) | 5.2 (2.9) | 5.7 (2.5) | 0.69 | 0.09 |
| 12‐month follow‐up | 5.1 (3.5) | 4.4 (3.2) | 6.1 (2.4) | 0.97 | 0.06 |
| BL to post‐treatment | 0.7 (0.2) | 0.8 (0.2) | 0.4 (0.2) | 0.41 | 0.11 |
| Headache index | |||||
| BL | 557.5 (458.2) | 762.5 (639.0) | 581.6 (635.0) | 0.06 | 0.68 |
| Post‐treatment | 295.5 (348.1) | 330.1 (602.3) | 547.6 (649.7) | 0.16 | 0.10 |
| 3‐month follow‐up | 338.0 (350.8) | 399.6 (582.0) | 526.8 (641.1) | 0.32 | 0.02 |
| 6‐month follow‐up | 313.0 (395.6) | 402.8 (595.1) | 562.6 (740.1) | 0.56 | 0.009 |
| 12‐month follow‐up | 350.8 (451.6) | 322.9 (668.8) | 872.0 (1475.6) | 0.92 | 0.009 |
| BL to post‐treatment | 229.7 (42.7) | 276.9 (50.9) | 118.2 (55.1) | 0.05 | 0.04 |
P‐values are based on linear mixed‐model analysis. P<0.025 for change from BL to post‐treatment in primary end‐point (migraine days); P<0.05 for secondary end‐points denotes significant finding. Control, control group; CSMT, chiropractic spinal manipulative therapy; placebo, sham manipulation.
Table 4.
Mean (SD) doses of medications at baseline (BL) and follow‐up by group
| CSMT | Placebo | Control | CSMT versus placebo | CSMT versus control | |
|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | P‐values | P‐values | |
| Triptan | |||||
| BL | 4.2 (4.8) | 4.4 (6.7) | 3.9 (4.6) | 0.63 | 0.81 |
| Post‐treatment | 2.5 (3.6) | 1.1 (2.6) | 2.5 (3.5) | 0.33 | 0.81 |
| 3‐month follow‐up | 2.6 (3.8) | 1.6 (2.7) | 2.2 (3.1) | 1.00 | 0.82 |
| 6‐month follow‐up | 2.8 (3.9) | 1.8 (2.9) | 1.8 (2.9) | 0.63 | 0.95 |
| 12‐month follow‐up | 2.6 (4.0) | 1.6 (2.7) | 2.0 (3.1) | 0.46 | 0.96 |
| BL to post‐treatment | 1.8 (4.3) | 2.9 (4.9) | 1.4 (3.5) | 0.62 | 0.35 |
| Ergotamine | |||||
| BL | 0.1 (0.7) | 0 | 0.1 (0.6) | 1.00 | 0.89 |
| Post‐treatment | 0 | 0 | 0.1 (0.4) | – | 0.94 |
| 3‐month follow‐up | 0 | 0 | 0 | – | – |
| 6‐month follow‐up | 0 | 0 | 0 | – | – |
| 12‐month follow‐up | 0 | 0 | 0 | – | – |
| BL to post‐treatment | 0.1 (0.7) | 0 | 0.1 (0.6) | 1.00 | 0.54 |
| Paracetamol | |||||
| BL | 1.1 (2.8) | 2.1 (2.7) | 1.7 (2.6) | 0.08 | 0.07 |
| Post‐treatment | 0.4 (1.2) | 0.8 (2.3) | 1.1 (2.4) | 0.17 | 0.34 |
| 3‐month follow‐up | 0.6 (2.0) | 0.7 (1.7) | 1.4 (3.4) | 1.00 | 0.64 |
| 6‐month follow‐up | 0.4 (1.4) | 1.0 (3.0) | 1.9 (3.6) | 0.76 | 0.16 |
| 12‐month follow‐up | 0.4 (2.1) | 0.8 (2.4) | 2.2 (4.5) | 0.04 | 0.03 |
| BL to post‐treatment | 0.7 (2.8) | 1.4 (2.2) | 0.6 (1.6) | 0.20 | 0.33 |
| Paracetamol + codeine | |||||
| BL | 0.4 (1.1) | 0.3 (0.7) | 0.6 (3.0) | 1.00 | 0.82 |
| Post‐treatment | 0.1 (0.3) | 0.2 (0.8) | 0.8 (4.3) | 1.00 | 0.85 |
| 3‐month follow‐up | 0.03 (0.2) | 0.1 (0.5) | 0.8 (3.9) | 0.61 | 0.84 |
| 6‐month follow‐up | 0.03 (0.1) | 0.1 (0.4) | 0.6 (3.1) | 1.00 | 0.78 |
| 12‐month follow‐up | 0.01 (0.1) | 0.04 (0.3) | 1.0 (5.3) | 0.47 | 0.84 |
| BL to post‐treatment | 0.3 (1.1) | 0.1 (1.1) | −0.2 (1.3) | 1.00 | 0.16 |
| Non‐steroidal anti‐inflammatory drugs | |||||
| BL | 1.9 (4.3) | 1.2 (2.1) | 2.3 (3.3) | 1.00 | 0.11 |
| Post‐treatment | 1.1 (2.7) | 1.0 (3.5) | 1.6 (2.9) | 0.78 | 0.48 |
| 3‐month follow‐up | 0.9 (2.3) | 0.8 (1.9) | 1.8 (3.7) | 0.58 | 0.26 |
| 6‐month follow‐up | 1.3 (4.2) | 0.4 (1.3) | 2.0 (3.7) | 1.00 | 0.15 |
| 12‐month follow‐up | 0.9 (2.3) | 0.5 (1.2) | 2.9 (6.2) | 0.76 | 0.15 |
| BL to post‐treatment | 0.8 (3.9) | 0.3 (3.5) | 0.7 (1.6) | 0.78 | 0.21 |
| Morphinomimetics | |||||
| BL | 0 | 0 | 0.1 (0.4) | – | 0.94 |
| Post‐treatment | 0 | 0 | 0.1 (0.3) | – | 0.40 |
| 3‐month follow‐up | 0 | 0 | 0.04 (0.2) | – | 0.91 |
| 6‐month follow‐up | 0 | 0 | 0.04 (0.2) | – | 1.00 |
| 12‐month follow‐up | 0 | 0 | 0 | – | – |
| BL to post‐treatment | 0 | 0 | 0 | – | 0.94 |
P‐values are based on independent samples median test and <0.05 denotes statistically significant finding at 5% level. Control, control group; CSMT, chiropractic spinal manipulative therapy; placebo, sham manipulation.
Primary end‐point
Migraine days were significantly reduced within all groups from baseline to post‐treatment (P < 0.001). The effect continued in the CSMT and the placebo groups at 3, 6 and 12 months follow‐up, whereas migraine days reverted to baseline level in the control group (Fig. 2a). The linear mixed model showed no overall significant differences in change in migraine days between the CSMT and the placebo groups (P = 0.04) or between the CSMT and the control group (P = 0.06; Table 2). However, the pairwise comparisons at individual time points showed significant differences between the CSMT and the control group at all time points starting at post‐treatment (Table 3).
Secondary end‐points
There was a significant reduction from baseline to post‐treatment in migraine duration, intensity and HI in the CSMT (P = 0.003, P = 0.002 and P < 0.001, respectively) and the placebo (P < 0.001, P = 0.001 and P < 0.001, respectively) groups, and the effect continued at 3, 6 and 12 months follow‐up.
The only significant differences between the CSMT and control groups were change in migraine duration (P = 0.02) and in HI (P = 0.04; Table 2).
At 12 months follow‐up, change in consumption of paracetamol was significantly lower in the CSMT group as compared with the placebo (P = 0.04) and control (P = 0.03) groups (Table 4).
Blinding
After each of the 12 intervention sessions, >80% of the participants believed they had received CSMT regardless of group allocation. The odds ratio for believing that CSMT treatment was received was >10 at all treatment sessions in both groups (all P < 0.001).
Adverse effects
A total of 703 of the potential 770 intervention sessions were assessed for AEs (355 in the CSMT group and 348 in the placebo group). Reasons for missed AE assessment were drop‐out or missed intervention sessions. AEs were significantly more frequent in the CSMT than the placebo intervention sessions (83/355 vs. 32/348; P < 0.001). Local tenderness was the most common AE reported by 11.3% (95% CI, 8.4–15.0) in the CSMT group and 6.9% (95% CI, 4.7–10.1) in the placebo group, whereas tiredness on the intervention day and neck pain were reported by 8.5% and 2.0% (95% CI, 6.0–11.8 and 1.0–4.0), and 1.4% and 0.3% (95% CI, 0.6–3.3 and 0.1–1.9), respectively. All other AEs (lower back pain, face numbness, nausea, provoked migraine attack and fatigue in arms) were rare (<1%). No severe or serious AEs were reported.
Discussion
To our knowledge, this is the first manual‐therapy RCT with a documented successful blinding. Our three‐armed, single‐blinded, placebo RCT evaluated the efficacy of CSMT in the treatment of migraine versus placebo (sham chiropractic) and control (usual pharmacological treatment). The results showed that migraine days were significantly reduced within all three groups from baseline to post‐treatment. The effect continued in the CSMT and placebo groups at all follow‐up time points, whereas the control group returned to baseline. AEs were mild and transient, which is in accordance with previous studies.
The study design adhered to the recommendations for pharmacological RCTs as given by the IHS and CONSORT 1, 15, 16. Manual‐therapy RCTs have three major obstacles as compared with pharmacological RCTs. Firstly, it is impossible to blind the investigator in relation to the applied treatment. Secondly, consensus on an inert placebo treatment is lacking 11. Thirdly, previous attempts to include a placebo group have omitted validating the blinding, thus, it remains unknown whether active and placebo treatment were concealed 27. Due to these challenges we decided to conduct a three‐armed, single‐blinded RCT, which also included a control group that continued usual pharmacological treatment in order to obtain an indication of the magnitude of the placebo response.
It has been suggested that, in pharmacological double‐blind placebo RCTs, only 50% will believe that they receive active treatment in each group, if the blinding is perfect. However, this may not be true in manual‐therapy RCTs, because the active and placebo physical stimulus might be more convincing than a tablet 28. A single investigator reduces inter‐investigator variability by providing similar information to all participants and it is generally recommended that the placebo intervention should resemble the active treatment in terms of procedure, treatment frequency and time spent with the investigator to allow for similar expectations in both groups 28. The importance of our successful blinding is emphasized by the fact that all previous manual‐therapy RCTs on headache lack placebo. Thus, we believe that our results discussed below are valid at the same level as a pharmacological RCT 14.
Prospective data are more reliable than retrospective data in terms of recall bias; however, non‐compliance can be a challenge, especially at the end of the study. We believe the frequent contact between participants and the investigator, including monthly contact in the follow‐up period, probably maintained high compliance throughout our study.
Although our study sample ended with 104 participants in the three groups, the power calculation assumption and the high completion rate support the data achieved being valid for the investigated population. The Gonstead method is used by 59% of chiropractors 19 and, thus, the results are generalizable for the profession. Diagnostic certainty is one of our major strengths as nearly all of the participants had been diagnosed by a neurologist according to the ICHD‐II 2. In contrast to previous chiropractic migraine RCTs that recruited participants through media such as newspapers and radio advertisement 12, the majority of our participants were recruited from the Department of Neurology, Akershus University Hospital, indicating that the migraineurs may have more frequent/severe attacks that are difficult to treat than the general population, as they were referred by their General Practitioner and/or practicing neurologist. Thus, our study is representative of primarily the tertiary clinic population, and the outcome might have been different if participants had been recruited from the general population. The percentage of neck pain has been found to be high in patients with migraine 29 and, thus, the high percentage of non‐radicular spinal pain in our study might be a confounder for which effect was seen on migraine days.
Three pragmatic chiropractic manual‐therapy RCTs using the diversified technique have previously been conducted for migraineurs 12, 30, 31, 32. An Australian RCT showed within‐group reduction in migraine frequency, duration and intensity of 40%, 43% and 36%, respectively, at 2 months follow‐up 30. An American study found migraine frequency and intensity to reduce within‐group by 33% and 42%, respectively, at 1 month follow‐up 31. Another Australian study, which was the only RCT to include a control group, i.e. detuned ultrasound, found a within‐group reduction of migraine frequency and duration of 35% and 40%, respectively, at 2 months follow‐up in the CSMT group, as compared with a within‐group reduction of 17% and 20% in the control group, respectively 32. The reduction in migraine days was similar to ours (40%) in the CSMT group from baseline to 3 months follow‐up, whereas migraine duration and intensity were less reduced at 3 months follow‐up, i.e. 21% and 14%, respectively. Long‐term follow‐up comparisons are impossible as neither of the previous studies included a sufficient follow‐up period. Our study design including strong internal validity allows us to interpret the effect seen as a placebo response.
Our RCT had fewer AEs as compared with previous manual‐therapy studies, but of similar transient and mild character 33, 34, 35, 36, 37, 38, 39. However, it was not sufficiently powered to detect uncommon serious AEs. In comparison, AEs in pharmacological migraine prophylactic placebo RCTs are common including non‐mild and non‐transient AEs 40, 41.
Conclusion
The blinding was strongly sustained throughout the RCT, AEs were few and mild, and the effect in the CSMT and placebo group was probably a placebo response. Because some migraineurs do not tolerate medication because of AEs or co‐morbid disorders, CSMT might be considered in situations where other therapeutic options are ineffective or poorly tolerated.
Disclosure of conflicts of interest
All authors have completed the International Committee of Medical Journal Editors uniform disclosure form and declare no financial or other conflicts of interest.
Supporting information
Appendix S1. CONSORT checklist.
Acknowledgements
The authors want to express their sincere gratitude to Akershus University Hospital, which kindly provided the research facilities, and Chiropractor Clinic 1, Oslo, Norway, which performed all x‐ray assessments. This study was supported by grants from Extrastiftelsen, the Norwegian Chiropractic Association, Akershus University Hospital and University of Oslo in Norway.
References
- 1. Tfelt‐Hansen P, Block G, Dahlof C, et al International Headache Society Clinical Trial Subcommittee. Guidelines for controlled trials of drugs in migraine: second edition. Cephalalgia 2000; 20: 765–786. [DOI] [PubMed] [Google Scholar]
- 2. Headache Classification Subcommittee of the International Headache Society . The International Classification of Headache Disorders: 2nd edition. Cephalalgia 2004; 24(Suppl. 1): 9–160. [DOI] [PubMed] [Google Scholar]
- 3. Vos T, Flaxman AD, Naghavi M, et al Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012; 380: 2163–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Diener HC, Charles A, Goadsby PJ, Holle D. New therapeutic approaches for the prevention and treatment of migraine. Lancet Neurol 2015; 14: 1010–1022. [DOI] [PubMed] [Google Scholar]
- 5. McLain RF, Pickar JG. Mechanoreceptor endings in human thoracic and lumbar facet joints. Spine (Phila Pa 1976) 1998; 23: 168–173. [DOI] [PubMed] [Google Scholar]
- 6. Vernon H. Qualitative review of studies of manipulation‐induced hypoalgesia. J Manipulative Physiol Ther 2000; 23: 134–138. [DOI] [PubMed] [Google Scholar]
- 7. Vicenzino B, Paungmali A, Buratowski S, Wright A. Specific manipulative therapy treatment for chronic lateral epicondylalgia produces uniquely characteristic hypoalgesia. Man Ther 2001; 6: 205–212. [DOI] [PubMed] [Google Scholar]
- 8. Boal RW, Gillette RG. Central neuronal plasticity, low back pain and spinal manipulative therapy. J Manipulative Physiol Ther 2004; 27: 314–326. [DOI] [PubMed] [Google Scholar]
- 9. Bialosky JE, Bishop MD, Price DD, Robinson ME, George SZ. The mechanisms of manual therapy in the treatment of musculoskeletal pain: a comprehensive model. Man Ther 2009; 14: 531–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. De Camargo VM, Alburquerque‐Sendin F, Berzin F, Stefanelli VC, de Souza DP, Fernandez‐de‐las‐Penas C. Immediate effects on electromyographic activity and pressure pain thresholds after a cervical manipulation in mechanical neck pain: a randomized controlled trial. J Manipulative Physiol Ther 2011; 34: 211–220. [DOI] [PubMed] [Google Scholar]
- 11. Hancock MJ, Maher CG, Latimer J, McAuley JH. Selecting an appropriate placebo for a trial of spinal manipulative therapy. Aust J Physiother 2006; 52: 135–138. [DOI] [PubMed] [Google Scholar]
- 12. Chaibi A, Tuchin PJ, Russell MB. Manual therapies for migraine: a systematic review. J Headache Pain 2011; 12: 127–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chaibi A, Russell MB. Manual therapies for primary chronic headaches: a systematic review of randomized controlled trials. J Headache Pain 2014; 15: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chaibi A, Saltyte Benth J, Bjorn Russell M. Validation of placebo in a manual therapy randomized controlled trial. Sci Rep 2015; 5: 11774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Silberstein S, Tfelt‐Hansen P, Dodick DW, et al Task force of the International Headache Society Clinical Trial Subcommittee. Guidelines for controlled trials of prophylactic treatment of chronic migraine in adults. Cephalalgia 2008; 28: 484–495. [DOI] [PubMed] [Google Scholar]
- 16. Moher D, Hopewell S, Schulz KF, et al CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ 2010; 340: c869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chaibi A, Saltyte Benth J, Tuchin PJ, Russell MB. Chiropractic spinal manipulative therapy for migraine: a study protocol of a single‐blinded placebo‐controlled randomised clinical trial. BMJ Open 2015; 5: e008095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. French HP, Brennan A, White B, Cusack T. Manual therapy for osteoarthritis of the hip or knee ‐ a systematic review. Man Ther 2011; 16: 109–117. [DOI] [PubMed] [Google Scholar]
- 19. Cooperstein R. Gonstead chiropractic technique (GCT). J Chiropr Med 2003; 2: 16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Russell MB, Rasmussen BK, Brennum J, Iversen HK, Jensen RA, Olesen J. Presentation of a new instrument: the diagnostic headache diary. Cephalalgia 1992; 12: 369–374. [DOI] [PubMed] [Google Scholar]
- 21. Tfelt‐Hansen P, Pascual J, Ramadan N, et al Guidelines for controlled trials of drugs in migraine: third edition. A guide for investigators. Cephalalgia 2012; 32: 6–38. [DOI] [PubMed] [Google Scholar]
- 22. Headache Classification Subcommittee of the International Headache Society . The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia 2013; 33: 629–808. [DOI] [PubMed] [Google Scholar]
- 23. Tfelt‐Hansen P, Bjarnason NH, Dahlof C, Derry S, Loder E, Massiou H. Evaluation and registration of adverse events in clinical drug trials in migraine. Cephalalgia 2008; 28: 683–688. [DOI] [PubMed] [Google Scholar]
- 24. Silberstein SD, Neto W, Schmitt J, Jacobs D. Topiramate in migraine prevention: results of a large controlled trial. Arch Neurol 2004; 61: 490–495. [DOI] [PubMed] [Google Scholar]
- 25. Dixon JR. The International Conference on Harmonization Good Clinical Practice guideline. Qual Assur 1998; 6: 65–74. [DOI] [PubMed] [Google Scholar]
- 26. Ioannidis JP, Evans SJ, Gotzsche PC, et al Better reporting of harms in randomized trials: an extension of the CONSORT statement. Ann Intern Med 2004; 141: 781–788. [DOI] [PubMed] [Google Scholar]
- 27. Scholten‐Peeters GG, Thoomes E, Konings S, et al Is manipulative therapy more effective than sham manipulation in adults: a systematic review and meta‐analysis. Chiropr Man Therap 2013; 21: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Meissner K, Fassler M, Rucker G, et al Differential effectiveness of placebo treatments: a systematic review of migraine prophylaxis. JAMA Intern Med 2013; 173: 10. [DOI] [PubMed] [Google Scholar]
- 29. Ashina S, Bendtsen L, Lyngberg AC, Lipton RB, Hajiyeva N, Jensen R. Prevalence of neck pain in migraine and tension‐type headache: a population study. Cephalalgia 2015; 35: 211–219. [DOI] [PubMed] [Google Scholar]
- 30. Parker GB, Tupling H, Pryor DS. A controlled trial of cervical manipulation of migraine. Aust NZ J Med 1978; 8: 589–593. [DOI] [PubMed] [Google Scholar]
- 31. Nelson CF, Bronfort G, Evans R, Boline P, Goldsmith C, Anderson AV. The efficacy of spinal manipulation, amitriptyline and the combination of both therapies for the prophylaxis of migraine headache. J Manipulative Physiol Ther 1998; 21: 511–519. [PubMed] [Google Scholar]
- 32. Tuchin PJ, Pollard H, Bonello R. A randomized controlled trial of chiropractic spinal manipulative therapy for migraine. J Manipulative Physiol Ther 2000; 23: 91–95. [PubMed] [Google Scholar]
- 33. Cagnie B, Vinck E, Beernaert A, Cambier D. How common are side effects of spinal manipulation and can these side effects be predicted? Man Ther 2004; 9: 151–156. [DOI] [PubMed] [Google Scholar]
- 34. Hurwitz EL, Morgenstern H, Vassilaki M, Chiang LM. Adverse reactions to chiropractic treatment and their effects on satisfaction and clinical outcomes among patients enrolled in the UCLA Neck Pain Study. J Manipulative Physiol Ther 2004; 27: 16–25. [DOI] [PubMed] [Google Scholar]
- 35. Thiel HW, Bolton JE, Docherty S, Portlock JC. Safety of chiropractic manipulation of the cervical spine: a prospective national survey. Spine (Phila Pa 1976) 2007; 32: 2375–2378. [DOI] [PubMed] [Google Scholar]
- 36. Rubinstein SM, Leboeuf‐Yde C, Knol DL, de Koekkoek TE, Pfeifle CE, van Tulder MW. The benefits outweigh the risks for patients undergoing chiropractic care for neck pain: a prospective, multicenter, cohort study. J Manipulative Physiol Ther 2007; 30: 408–418. [DOI] [PubMed] [Google Scholar]
- 37. Eriksen K, Rochester RP, Hurwitz EL. Symptomatic reactions, clinical outcomes and patient satisfaction associated with upper cervical chiropractic care: a prospective, multicenter, cohort study. BMC Musculoskelet Disord 2011; 12: 219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Walker BF, Hebert JJ, Stomski NJ, et al Outcomes of usual chiropractic. The OUCH randomized controlled trial of adverse events. Spine 2013; 38: 1723–1729. [DOI] [PubMed] [Google Scholar]
- 39. Maiers M, Evans R, Hartvigsen J, Schulz C, Bronfort G. Adverse events among seniors receiving spinal manipulation and exercise in a randomized clinical trial. Man Ther 2015; 20: 335–341. [DOI] [PubMed] [Google Scholar]
- 40. Jackson JL, Cogbill E, Santana‐Davila R, et al A comparative effectiveness meta‐analysis of drugs for the prophylaxis of migraine headache. PLoS One 2015; 10: e0130733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ferrari MD, Roon KI, Lipton RB, Goadsby PJ. Oral triptans (serotonin 5‐HT(1B/1D) agonists) in acute migraine treatment: a meta‐analysis of 53 trials. Lancet 2001; 358: 1668–1675. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. CONSORT checklist.


