Abstract
PURPOSE
We aimed to explore the value of contrast-enhanced ultrasonography (CEUS) in guidance of percutaneous biopsy of anterior mediastinal lesions.
METHODS
Ninety patients with solitary anterior mediastinal lesions (55 males, 35 females; mean age, 46±4 years) were included. Patients were randomly divided into CEUS group (n=45) and conventional ultrasonography (US) group (n=45). Real-time US-guided core needle (16 G) percutaneous biopsies were performed in all lesions. The display of internal mammary arteries, internal necrosis, and active areas were recorded and compared. Biopsy success rate and diagnostic accuracy were compared between the two groups.
RESULTS
Display rate of unenhanced internal necrosis was higher in the CEUS group than in the US group (88.9% vs. 46.7%, P = 0.041). With real-time CEUS guidance, internal mammary arteries were effectively displayed and avoided during biopsies in 68.9% of the lesions (31/45). Of the lesions, 88.9% (80/90) were histologically proven, including 13 benign lesions and 67 malignancies. There was a significant difference in the rate of successful puncture attempts between the two groups (P = 0.041). CEUS group had a higher biopsy success rate (100% vs. 95.5%, P = 0.045) and higher diagnostic accuracy (97.8% vs. 82.2%, P = 0.035) compared with the US group (P = 0.035).
CONCLUSION
CEUS guidance is a promising technique in depicting internal necrotic areas, viable areas, and internal mammary arteries during percutaneous biopsy of anterior mediastinal lesion, with satisfying safety, accuracy, and success rates.
Anterior mediastinal tumors are common in clinical settings. Common lesions include inflammatory vs. neoplastic lesions, benign vs. malignant lesions, and primary vs. metastatic lesions. Among these, lymphomas are medically treatable, thymomas are surgically treatable, and metastatic carcinomas are nonresectable (1). Primary mediastinal tumors represent only about 3% of the tumors within the chest wall. Metastatic carcinoma and non-Hodgkin’s lymphoma are among common anterior mediastinal tumors. The rates of nonsurgical tumors such as lymphoma are higher than the rates of surgical diseases such as thymomas (2). Some anterior mediastinal tumors such as nonseminomatous germ cell tumors, thymic carcinomas, seminomas, lymphomas, and thymomas are quite similar in medical imaging appearance, but are quite different in treatment strategy; early and precise histopathologic diagnosis of anterior mediastinal lesions is essential for correct therapeutic decision, and remains an interesting diagnostic challenge (3).
Several biopsy techniques and approaches have been previously described and are available to obtain specimens of anterior mediastinal lesions, including percutaneous image-guided core needle biopsy, parasternal anterior mediastinotomy, endoscopic ultrasonography (US)-guided biopsy, video-assisted thoracoscopic surgery, cervical mediastinoscopy, and open surgical procedures (1). Most of those procedures require intubation and general anesthesia. Open biopsy is associated with morbidity, chance of pleural dissemination, and poor long-term results. For these reasons, surgically strategies are not suitable for anterior mediastinum lesions (4).
In general, US-guided percutaneous core needle biopsy is the first diagnostic choice because it is a minimally invasive, safe, and cost-effective procedure. US guidance can be performed under local anesthesia, providing sufficient tissue for histopathologic diagnosis (4). Anterior mediastinal lesions are clinically approachable under US guidance. US scan permits reliable identification of major vessels that should be avoided during the biopsy, the needle tip can be clearly visualized, and the needle can be advanced precisely within the lesion, allowing real-time imaging guidance and a quicker procedure (5). Previous studies found high diagnostic success rates for US-guided biopsies sized 1.5–10 cm (71% to 100%) (6–8).
Since rapid growth of locally advanced tumor always leads to a necrotic center, false-negative biopsy results were occasionally encountered in clinical experience (9). It is known that the failure of diagnosis is mainly due to presence of necrotic tissue, insufficient tissue sample or sampling error. However, it is difficult to accurately identify necrosis within the lesion by conventional gray scale and color Doppler US. Previously, this problem has been avoided by performing multiple biopsies from the center of the lesion to the edge, having a cytopathologist present during biopsy, or performing repeated biopsies. However, the occurrence rate of complications may be increased with those methods (9, 10).
The key to improve the diagnostic accuracy of core needle biopsy is to identify tumor tissues and necrotic tissues, and to obtain adequate representative specimens (11). Contrast-enhanced ultrasonography (CEUS) provides information on microvascularity and blood flow in normal and pathologic tissues (12–14). A CEUS-guided intervention can be performed in almost the same way as routine US-guided procedure. Tumor tissue, identified by the presence of vascularity, can be reliably visualized under CEUS. CEUS-guided biopsy increases the diagnostic rate by 10% and decreases the false negative rate, particularly in large tumors with areas of necrosis (10, 12, 15).
The purpose of our current study was to explore the clinical application of CEUS in guidance of biopsy of anterior mediastinal lesions and to determine whether CEUS before biopsy could improve the diagnostic efficacy of core needle biopsy.
Methods
Patients
This study was approved by the Ethics Committee of our institution. Written informed consent was obtained from each patient before CEUS and biopsy procedures. Between April 2012 and March 2015, 90 consecutive patients (35 women, 55 men; mean age, 46±4 years) with solitary anterior mediastinal lesions were included in our study. Out of 90 patients, 56 presented with clinical features such as chest pain, cough, dyspnea, weight loss, and hoarseness of voice. Anterior mediastinal lesions were detected on routine physical examination in the remaining 34 patients.
The inclusion criteria were as follows: patients referred to our hospital for US-guided percutaneous biopsy of anterior mediastinum lesions; solitary lesions detected on contrast-enhanced chest CT within one month; lesions larger than 2.5 cm in diameter that are accessible via a percutaneous approach with conventional US. Exclusion criteria were a contraindication to core needle biopsy (international normalized ratio >1.7, prothrombin activity <40%, or platelet count <40 000/mL, bleeding tendency), or a contraindication to the ultrasound contrast agent SonoVue® (Bracco Imaging Spa) (e.g., severe allergic reactions or cardiopulmonary dysfunction).
All patients were randomized into two groups: those who underwent CEUS-guided biopsy (CEUS group, n=45) and those who underwent conventional US guidance (US group, n=45).
Ultrasonography examination
With reference to the contrast-enhanced chest CT, conventional US scanning was performed in all lesions to detect the lesion, to observe the lesion’s location, size, and sonographic features. Patients were examined in the supine position with their arms lifted above the head to expand the intercostal spaces. We observed from the intercostal approach, and chose the optimal acoustic window for further biopsy route or CEUS examination (CEUS group).
In the US group, color Doppler flow scans were used to detect the large vessels within lesions, to avoid injuries during biopsy. The pulse repetition frequency and wall filters were adjusted to enable a better display of intralesion vessels and to avoid “blooming” artifacts. In the US group, “internal necrosis” was defined as an anechoic area without blood flow signal and “active area” were defined as echoic areas with relatively rich blood flow signal.
Each CEUS examination was performed after a quick bolus injection of 2.4 mL SonoVue® via a 20 G intravenous catheter in the cubital vein. The enhancement patterns (homogeneous or inhomogeneous), internal necrosis or active areas were recorded. Active areas were defined as the most obviously enhanced regions of lesions. Necrosis was defined as anechoic regions without enhancement. For the anterior mediastinal lesions extending to the parasternal region, the display rate of internal mammary artery was also recorded.
Conventional US, CEUS and further US-guided biopsy procedures were conducted by a HD15 unit (Philips Medical) equipped with a C5-2 broadband curved transducer. All US examinations were performed by a physician of 10 years of experience in CEUS examination. The clips were recorded, stored, and reviewed by two readers, who reached a consensus with emphasis on the presence of necrotic and active areas. The display rate of internal necrosis, active areas, and internal mammary arteries were recorded and compared between the two groups.
Core needle biopsy procedure
Core needle biopsies of anterior mediastinum lesions were performed after optimal biopsy routes and sampling sites were selected, which included the active area, avoiding internal necrosis and large vessels.
Skin was sterilized, local anesthetic (2% lidocaine) was applied, and biopsy was performed under US guidance, using 16 G core tissue biopsy needle and Bard® Magnum® biopsy instrument (Bard Peripheral Vascular Inc.). The needle was advanced into the target area under real-time US guidance, while patients were instructed to suspend respiration.
For each patient, two 15 mm long core specimens were obtained. The specimen was placed on a small piece of filter paper before being immersed in 10% formalin and was checked by the operator to evaluate whether the specimen was adequate or the biopsy was successful. The specimens were later sent for histologic examination.
After the biopsy procedure, patients were closely monitored for 3–4 hours. US examinations were performed to evaluate whether any complications such as localized hematoma or pneumothorax developed.
Histologic examination
All histopathologic slides were reviewed by a pathologist of 10 years of experience, who was blinded to US and CT findings. The final histopathologic diagnosis was based on hematoxylin-eosin stained sections and immunohistochemical staining results.
Final diagnosis
The patients were clinically followed up for six months. Diagnosis was considered true-positive if histologic result of the biopsy specimen held true in subsequent analysis. Malignancies were confirmed by surgical specimens or re-biopsy specimens from remote areas obtained later. Benign diagnoses were considered to be true-positive if chest CT scans during six months of follow-up showed disappearance or shrinkage of the lesion after certain treatment.
False-negative diagnoses were defined as biopsy specimens that were deemed negative for malignancy on histology, while subsequent histologic examination by surgery or re-biopsy confirmed malignancy; inadequate biopsy specimens; or no definite benign diagnosis such as chronic inflammation and necrosis. The diagnostic accuracy of biopsy was defined as the percentage of lesions that had true-positive results at the initial histologic biopsy.
Statistical analysis
All data were expressed as mean±standard deviation. The difference in diagnostic accuracy between the two groups was analyzed by using the independent samples t test. Inter-reader agreement between the two observers was compared by Kappa test. All statistical analyses in our study were performed with SPSS 18.0 software package (SPSS Inc.). P < 0.05 was considered statistically significant.
Results
There was no statistically significant difference in patients’ ages, male to female ratio, size of lesion, or malignant to benign ratio (according to final diagnoses) between the US and CEUS groups (Table 1).
Table 1.
Baseline characteristics of patients included in our study
| US group (n=45) | CEUS group (n=45) | P | |
|---|---|---|---|
| Age (year), mean±SD | 37±21 (18–72) | 39±18 (14–74) | 0.415 |
|
| |||
| Male/female, n | 27/18 | 32/13 | 0.792 |
|
| |||
| Size of lesion (mm), mean±SD | 67.7±27.6 (41–153) | 80.4±23.9 (38–143) | 0.571 |
|
| |||
| Final diagnoses, n | |||
| Malignant | 40 | 37 | |
| Benign | 5 | 8 | |
| Malignant/benign ratio | 40/5 | 37/8 | 0.168 |
US, conventional ultrasonography; CEUS, contrast-enhanced ultrasonography; SD, standard deviation.
All 90 anterior mediastinal lesions were inhomogeneous hypoechoic on gray-scale US. Color flow imaging detected branched intralesion vessels in 78.9% of the lesions (71/90); the mean value of resistive index in Doppler spectrum was 0.47±0.23. Internal necrosis defined as an anechoic area without blood flow signal was found in 21 patients in the US group (46.7%).
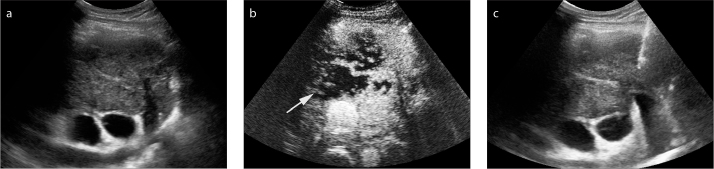
CEUS examinations were successfully performed in all 45 patients in the CEUS group, and no adverse reaction of SonoVue® was observed. After injection of contrast agents, all 45 anterior mediastinal lesions showed inhomogeneous hyperenhancement. Unenhanced internal necrosis was clearly displayed in 88.9% of the lesions (40/45), at a significantly higher rate than that in the US group (88.9% vs. 46.7%, P = 0.041) (Fig. 1).
Figure 1.
a–c. Contrast-enhanced ultrasonography (CEUS) showed necrosis before percutaneous biopsy of anterior mediastinum lesion in a 46-year male. Gray-scale ultrasonography (US) detected a heterogeneous hypoechoic anterior mediastinum lesion, no necrotic area was shown with conventional US (a). The lesion showed rapid and heterogeneous hyperenhancement in the arterial phase (20 s after SonoVue injection), a large and irregular necrotic area (arrow) was displayed inside (b). After CEUS, 16 G coarse needle percutaneous biopsy (arrow) was performed successfully in the active areas of the lesion (c).
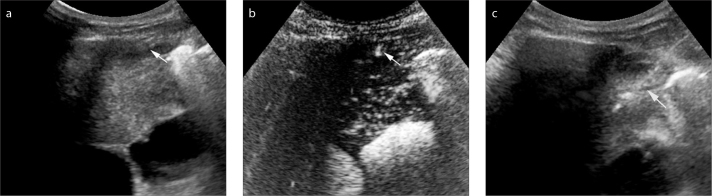
With reference to the chest CT scan results, we paid special attention to the internal mammary artery. During the early arterial phase of CEUS enhancement (5–10 s after injection of contrast agents), real-time CEUS showed rapid and obviously hyperenhanced internal mammary artery in 68.9% of patients in the CEUS group (Fig. 2a, 2b), which were effectively avoided during further biopsy (Fig. 2c). In all cases, good inter-reader agreement (κ=0.785) was achieved in interpretation of the CEUS clips.
Figure 2.
a–c. CEUS depicted internal mammary artery before percutaneous biopsy of an anterior mediastinum lesion in a 35-year male. Gray-scale US detected a homogenous hypoechoic anterior mediastinum lesion, internal mammary artery (arrow) was suspected in the peripheral part of the lesion (a). After injection of SonoVue®, the internal mammary artery was rapidly and obviously enhanced (arrow). The enhancement began approximately at 11 s (b). After CEUS, 16 G coarse needle percutaneous biopsy was performed successfully in the active areas of the lesion (arrow). The internal mammary artery was avoided during the real-time US guidance (c).
In the US group, percutaneous biopsies with 16 G core needles were successfully performed in 43 of 45 anterior mediastinal lesions. In two cases, the core needle biopsy specimen was not adequate for histologic evaluation.
In the CEUS group, we arranged the safe biopsy routes targeting the obviously hyperenhanced active areas of the lesions. Also, we avoided internal mammary artery and large vessels during CEUS-guided biopsy. In anterior mediastinal lesions with unenhanced necrotic areas, biopsies were performed avoiding the necrosis. Biopsy with CEUS guidance was 100% successful in the active areas of all 45 anterior mediastinal lesions.
A significant difference between the CEUS group and the US group was found in the rate of successful puncture attempts (Table 2). None of the patients had adverse reactions or biopsy complications.
Table 2.
Comparison of biopsy success rate between US and CEUS groups
| US group (n=45) | CEUS group (n=45) | P | |
|---|---|---|---|
| Internal necrosis | |||
| Number, n (%) | 21 (46.7) | 40 (88.9) | 0.041 |
| Size (mm)* | 17.1±14.9 | 29.6±17.3 | 0.039 |
|
| |||
| Display of large vessels, n (%) | 15 (33.3) | 37 (82.2) | 0.030 |
|
| |||
| Display of internal mammary arteries, n (%) | 2 (4.4) | 31 (68.9) | 0.021 |
|
| |||
| Number of puncture attempts* | 2.65±0.45 | 2.02±0.05 | 0.041 |
|
| |||
| Biopsy success, n (%) | 43 (95.5) | 45 (100) | 0.045 |
US, conventional ultrasonography; CEUS, contrast-enhanced ultrasonography.
Data are presented as mean±standard deviation.
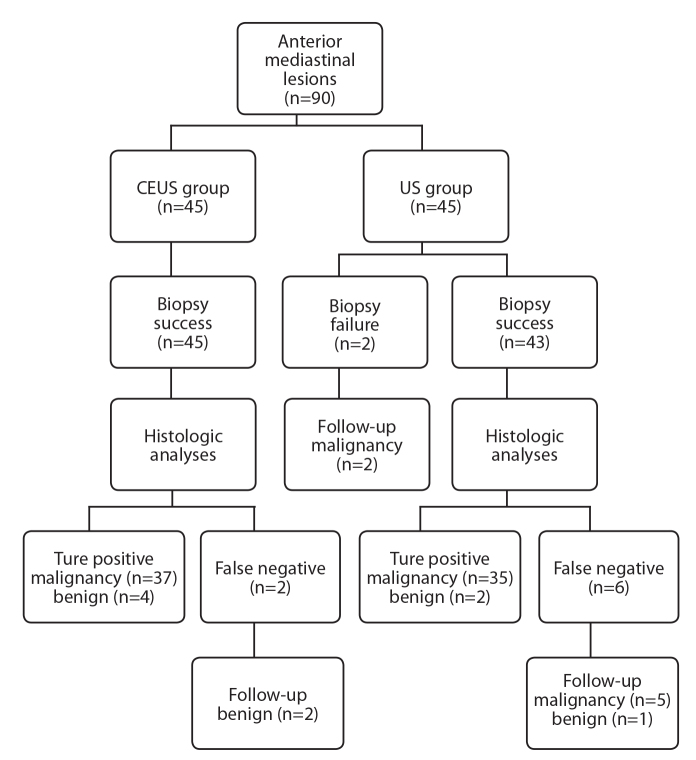
Histologic diagnoses were achieved in 80 of all 90 anterior mediastinal lesions, including 13 benign lesions and 67 malignancies (Table 3).
Table 3.
Histologic diagnoses of biopsies in US and CEUS groups
| US group (n=37 lesions) | CEUS group (n=43 lesions) | |
|---|---|---|
| Malignant | 34 | 37 |
| Malignant thymoma | 5 | 8 |
| Lymphoma | 4 | 7 |
| Sarcomas | 4 | 5 |
| Seminoma | 5 | 7 |
| Squamous cell carcinomas | 2 | 1 |
| Adenocarcinoma | 3 | 1 |
| Metastatic carcinoma | 4 | 5 |
| Germ cell tumor | 1 | 1 |
| Carcinoid tumor | 2 | 2 |
| Neuroendocrine carcinoma | 4 | 3 |
| Benign | 3 | 6 |
| Tuberculous granuloma | 1 | 1 |
| Thymoma | 2 | 4 |
| Solitary fibrous tumor | 0 | 1 |
US, conventional ultrasonography; CEUS, contrast-enhanced ultrasonography.
In the CEUS group, the initial biopsy led to a correct diagnosis in 43 of 45 lesions (95.5%). There were two false-negative diagnoses including one tuberculous granuloma and one thymoma, which had no definite benign diagnosis.
In the US group, the initial biopsy led to a correct diagnosis in 37 of 45 lesions (82.2%). False-negative diagnoses were obtained in four patients because of necrosis of the biopsy specimen; in three patients because of inadequate specimen; in one patient because of no definite benign diagnosis (thymoma).
Diagnostic accuracy was significantly different between the CEUS and the US groups (P = 0.035). No false-positive diagnosis occurred in this study (Fig. 3).
Figure 3.
Flowchart shows algorithm for final diagnosis of 90 anterior mediastinum lesions.
Discussion
Real-time CEUS guidance is an updated effective technology to be used during core needle biopsy of anterior mediastinal lesions. In this study, we found that successful puncture attempts, biopsy success and diagnostic accuracy were significantly higher with CEUS guidance than with conventional US guidance.
Containing various important anatomic structures, anterior mediastinum is an extremely important and complex part of the thorax. With increasing frequency of both benign and malignant lesions, a precise histologic diagnosis plays an important role in determining the appropriate treatment clinically (16).
Previously, conventional US was an effective modality for real-time guidance of core needle biopsy (11). With the real-time monitoring of US, the tip of biopsy needle can be monitored throughout the procedure, the depth of penetration can be determined, and the biopsy route can be adjusted to avoid puncturing the heart or any of the great vessels (5). Compared with CT, US-guided biopsy offers a number of advantages, including lower cost, bedside capabilities, lack of radiation exposure, time savings, and real-time monitoring (17). Also, free-hand US-guidance is more flexible during the biopsy of anterior mediastinum lesions, as it can approach the lesion from any direction, and biopsy can be performed in respiration phase, which renders the lesion most accessible (12).
As the blood supply of tumor vasculature cannot meet the need of fast tumor growth, necrosis can be easily found in malignant lesions (4), which should be avoided during biopsy on the assumption that they represent zones of nonvital tissue. With conventional gray-scale US, the internal necrosis was defined as anechoic areas without color flow signal. As the judgment of gray-scale echogenicity is operator-dependent and color Doppler flow scanning has various artifacts caused by breathing and heartbeat, necrotic areas can be missed. In clinical practice, a small percentage of specimens were inadequate for diagnosis because of their inner necrosis. False-negative diagnoses were obtained in four patients (8.9%) in the US group because of necrosis of the biopsy specimen, and in three patients (6.7%) because of inadequate specimen.
Recently, several investigators showed that CEUS allowed clinicians to differentiate necrosis from active tumor tissue with great confidence (11, 18, 19). CEUS-guided biopsy can be performed in the same way as conventional US-guided procedure (12, 20, 21). In our present study, CEUS could delineate lesion necrosis as unenhanced regions in 88.9% of lesions, which was significantly higher than the rate of necrosis detected in the US group (46.7%). We biopsied from hyperenhanced active areas, where sampling specimens were thought to be more reliable for histologic diagnosis. With CEUS guidance, biopsy was 100% successful in the active areas and 95.5% of patients in the CEUS group had sufficient specimens for histologic diagnoses. Compared with the conventional US group, CEUS could accurately distinguish necrosis and active areas in the anterior mediastinum lesion, significantly improve the diagnostic accuracy of initial biopsy, and avoid multiple biopsies.
For parasternal anterior mediastinal lesions, the internal mammary artery should be identified and avoided during biopsy. For larger anterior mediastinal lesions, the pulmonary artery, aorta and superior vena cava should also be identified and avoided (22, 23). In our practice, we paid special attention to the internal mammary artery displayed on chest CT. During the early arterial phase of CEUS enhancement, real time CEUS could distinctively and dynamically detect internal mammary artery in 68.9% of patients in the CEUS group, which was effectively avoided during further biopsy. Also during the arterial phase of hyperenhancement, real-time CEUS may be convenient and sensitive to detect vascularization and large vessels inside the anterior mediastinal lesions, which can be avoided during the following biopsy. In this way, severe complications such as bleeding and pneumothorax can be avoided by choosing the safe biopsy route, advancing the needle to a safe depth, and fine adjusting needle tip flexibly during real-time US guidance.
Our current study has several limitations. First, CEUS is highly operator-dependent. Second, as the numbers of patients enrolled in our study were limited, benign anterior mediastinal lesions were detected in only 14.4% of the patients.
In conclusion, real-time CEUS guidance can effectively depict internal mammary arteries, internal necrosis, and viable areas within tumors during core needle biopsy of anterior mediastinal lesions. CEUS can be of help in guaranteeing safety, accuracy, and success of core needle biopsy.
Main points.
Real-time CEUS guidance can effectively differentiate necrosis and viable tumor areas during core needle biopsy of anterior mediastinal lesions.
Real-time CEUS guidance can effectively depict and avoid internal mammary arteries during core needle biopsy of anterior mediastinal lesions.
CEUS can help to guarantee the safety, accuracy, and success of core needle biopsy.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (NSFC Grant 81371577, NSFC Grant 81571676 and NSFC Grant 81501471).
The foundation had no involvement in study design, in the collection, analysis or interpretation of data, nor in the writing of the manuscript or in the decision to submit the paper for publication.
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Shrivastava CP, Devgarha S, Ahlawat V. Mediastinal tumors: a clinicopathological analysis. Asian Cardiovasc Thorac Ann. 2006;14:102–104. doi: 10.1177/021849230601400204. https://doi.org/10.1177/021849230601400204. [DOI] [PubMed] [Google Scholar]
- 2.Karki S, Chalise S. Analysis of mediastinal lesions: A study of 27 cases. J Pathol Nepal. 2011;1:114–117. https://doi.org/10.3126/jpn.v1i2.5404. [Google Scholar]
- 3.Vaziri M, Pazooki M, Zahedi-Shoolami L. Mediastinal Masses: Review of 105 Cases. Acta Med Iran. 2009;47:297–300. [Google Scholar]
- 4.Nasit JG, Patel M, Parikh B, Shah M, Davara K. Anterior mediastinal masses: A study of 50 cases by fine needle aspiration cytology andcore needle biopsy as a diagnostic procedure. South Asian J Cancer. 2013;2:7–13. doi: 10.4103/2278-330X.105872. https://doi.org/10.4103/2278-330X.105872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liao WY, Chen MZ, Chang YL, et al. US-guided transthoracic cutting biopsy for peripheral thoracic lesions less than 3 cm indiameter. Radiology. 2000;217:685–691. doi: 10.1148/radiology.217.3.r00dc21685. https://doi.org/10.1148/radiology.217.3.r00dc21685. [DOI] [PubMed] [Google Scholar]
- 6.Yang PC, Chang DB, Lee YC, Yu CJ, Kuo SH, Luh KT. Mediastinal malignancy: ultrasound guided biopsy through the supraclavicular approach. Thorax. 1992;47:377–380. doi: 10.1136/thx.47.5.377. https://doi.org/10.1136/thx.47.5.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sawhney S, Jain R, Berry M. Tru-Cut biopsy of mediastinal masses guided by real-time sonography. Clin Radiol. 1991;44:16–9. doi: 10.1016/s0009-9260(05)80219-3. https://doi.org/10.1016/S0009-9260(05)80219-3. [DOI] [PubMed] [Google Scholar]
- 8.Andersson T, Lindgren PG, Elvin A. Ultrasound guided tumour biopsy in the anterior mediastinum. An alternative to thoracotomy and mediastinoscopy. Acta Radiol. 1992;33:423–426. https://doi.org/10.3109/02841859209172026. [PubMed] [Google Scholar]
- 9.Sartori S, Nielsen I, Trevisani L, Tombesi P, Ceccotti P, Abbasciano V. Contrast-enhanced sonography as guidance for transthoracic biopsy of a peripheral lung lesion with large necrotic areas. J Ultrasound Med. 2004;23:133–136. doi: 10.7863/jum.2004.23.1.133. [DOI] [PubMed] [Google Scholar]
- 10.Bang N, Bachmann Nielsen M, Vejborg I, Mellon Mogensen A. Clinical report: contrast enhancement of tumor perfusion as a guidance for biopsy. Eur J Ultrasound. 2000;12:159–161. doi: 10.1016/s0929-8266(00)00108-7. https://doi.org/10.1016/S0929-8266(00)00108-7. [DOI] [PubMed] [Google Scholar]
- 11.Cao BS, Wu JH, Li XL, Deng J, Liao GQ. Sonographically guided transthoracic biopsy of peripheral lung and mediastinal lesions: role of contrast-enhanced sonography. J Ultrasound Med. 2011;30:1479–1490. doi: 10.7863/jum.2011.30.11.1479. [DOI] [PubMed] [Google Scholar]
- 12.Lorentzen T, Nolsøe CP, Ewertsen C, et al. EFSUMB Guidelines on Interventional Ultrasound (INVUS), Part I. Ultraschall Med. 2015;36:464–472. doi: 10.1055/s-0035-1553601. https://doi.org/10.1055/s-0035-1553601. [DOI] [PubMed] [Google Scholar]
- 13.Claudon M, Dietrich CF, Choi BI, et al. Guidelines and good clinical practice recommendations for Contrast Enhanced Ultrasound (CEUS) in the liver-update 2012: A WFUMB-EFSUMB initiative in cooperation with representatives of AFSUMB, AIUM, ASUM, FLAUS and ICUS. Ultrasound Med Biol. 2013;39:187–210. doi: 10.1016/j.ultrasmedbio.2012.09.002. https://doi.org/10.1016/j.ultrasmedbio.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 14.Claudon M, Dietrich CF, Choi BI, et al. Guidelines and Good Clinical Practice Recommendations for Contrast Enhanced Ultrasound (CEUS) in the Liver-Update 2012 A WFUMB-EFSUMB Initiative in Cooperation With Representatives of AFSUMB, AIUM, ASUM, FLAUS and ICUS. Ultraschall Med. 2013;34:11–29. doi: 10.1055/s-0032-1325499. [DOI] [PubMed] [Google Scholar]
- 15.Wu W, Chen MH, Yin SS, et al. The role of contrast-enhanced sonography of focal liver lesions before percutaneous biopsy. AJR Am J Roentgenol. 2006;187:752–761. doi: 10.2214/AJR.05.0535. https://doi.org/10.2214/AJR.05.0535. [DOI] [PubMed] [Google Scholar]
- 16.Date H. Diagnostic strategies for mediastinal tumors and cysts. Thorac Surg Clin. 2009;19:29–35. doi: 10.1016/j.thorsurg.2008.09.001. https://doi.org/10.1016/j.thorsurg.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 17.Chen F, Jiang TA, Zhao QY. Percutaneous biopsy of anterior mediastinal mass guided by real-time US fused with CT. J Clin Ultrasound. 2011;39:38–40. doi: 10.1002/jcu.20744. https://doi.org/10.1002/jcu.20744. [DOI] [PubMed] [Google Scholar]
- 18.Wang S, Yang W, Zhang H, Xu Q, Yan K. The role of contrast-enhanced ultrasound in selection indication and improving diagnosis for transthoracic biopsy in peripheral pulmonary and mediastinal lesions. Biomed Res Int. 2015;2015:231782. doi: 10.1155/2015/231782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dong Y, Mao F, Wang WP, Ji ZB, Fan PL. Value of contrast-enhanced ultrasound in guidance of percutaneous biopsy in peripheral pulmonary lesions. Biomed Res Int. 2015;2015:531507. doi: 10.1155/2015/531507. https://doi.org/10.1155/2015/531507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dietrich CF, Annema JT, Clementsen P, Cui XW, Borst MM, Jenssen C. Ultrasound techniques in the evaluation of the mediastinum, part I: endoscopic ultrasound (EUS), endobronchial ultrasound (EBUS) and transcutaneous mediastinal ultrasound (TMUS), introduction into ultrasound techniques. J Thorac Dis. 2015;7:E311–E325. doi: 10.3978/j.issn.2072-1439.2015.09.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jenssen C, Annema JT, Clementsen P, Cui XW, Borst MM, Dietrich CF. Ultrasound techniques in the evaluation of the mediastinum, part 2: mediastinal lymph node anatomy and diagnostic reach of ultrasound techniques, clinical work up of neoplastic and inflammatory mediastinal lymphadenopathy using ultrasound techniques and how to learn mediastinal endosonography. J Thorac Dis. 2015;7:E439–E458. doi: 10.3978/j.issn.2072-1439.2015.10.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Middleton WD, Teefey SA, Dahiya N. Ultrasound-guided chest biopsies. Ultrasound Q. 2006;22:241–252. doi: 10.1097/01.ruq.0000237258.48756.94. https://doi.org/10.1097/01.ruq.0000237258.48756.94. [DOI] [PubMed] [Google Scholar]
- 23.Dietrich CF, Mathis G, Cui XW, Ignee A, Hocke M, Hirche TO. Ultrasound of the pleurae and lungs. Ultrasound Med Biol. 2015;41:351–365. doi: 10.1016/j.ultrasmedbio.2014.10.002. https://doi.org/10.1016/j.ultrasmedbio.2014.10.002. [DOI] [PubMed] [Google Scholar]