Abstract
PURPOSE
We aimed to evaluate the therapeutic effect and safety of transarterial embolization using bleomycin-iodinated oil and polyvinyl alcohol particles for the treatment of symptomatic hepatic focal nodular hyperplasia (FNH).
METHODS
This retrospective study included 23 consecutive patients with symptomatic hepatic FNH, who underwent embolization using bleomycin-iodinated oil and polyvinyl alcohol particles between January 2005 and December 2012. Patients were followed-up with radiologic and clinical evaluation. Therapeutic effects including changes in lesion size and symptomatic improvement were evaluated after the procedure.
RESULTS
Embolization was performed for 27 lesions in 23 patients. Follow-up period ranged from three months to 89 months. The mean lesion diameters decreased significantly from 5.0±2.4 cm to 3.2±1.5 cm at 3–9 months after embolization (P < 0.001). Five lesions had complete resolution in the follow-up period. The clinical symptoms were significantly relieved in all patients. Contrast-enhanced scans at follow-up showed complete lack of residual arterial blood supply in the majority of lesions. Local recurrence was found in one treated lesion at the 54-month follow-up. There were no major complications associated with the procedure.
CONCLUSION
Transarterial embolization using bleomycin-iodinated oil and polyvinyl alcohol particles for hepatic FNH is a safe and effective alternative treatment with good long-term symptomatic control and reduction in lesion size after embolization.
Hepatic focal nodular hyperplasia (FNH) is one of the most common benign liver tumors with no recognized malignant potential. It occurs predominantly in young women 30–50 years of age (1). The majority of FNH are small and asymptomatic, thus they are usually discovered incidentally (2, 3). Considering the indolent natural history of FNH with a low risk of hemorrhage or necrosis and no potential for malignant degeneration, patients with asymptomatic definitive FNH may be managed conservatively (4, 5).
However, in up to 30% of patients FNH produces symptoms of pain or discomfort. These are attributed to the pressure of the tumor on the liver capsule or surrounding tissues (6). Surgical resection is the preferred and curative treatment for symptomatic FNH (7), but there are only limited data evaluating the benefit-risk balance after liver resections for FNH (8, 9). Transarterial embolization for symptomatic hepatic FNH has been described as a successful alternative to surgical removal (10–13). Different embolization materials used for FNH in prior studies included ethanol, lipiodol emulsions, polyvinyl alcohol (PVA) particles, gelatin sponge particles, and microspheres. Here, we report the results of 23 consecutive patients, who underwent embolization with bleomycin-lipiodol mixture and PVA particles for symptomatic hepatic FNH from a single center.
Methods
Patient selection
Between January 2005 and December 2012, a total of 23 consecutive patients (16 men, 69.6%; seven women, 30.4%) with symptomatic FNH underwent superselective embolization. The average patient age at admission was 28±12 years. Nineteen patients had a solitary lesion, four patients had two lesions, for a total of 27 treated lesions. A definitive pathologic diagnosis of FNH following percutaneous biopsies was achieved in 18 lesions, and the other lesions exhibited the characteristic imaging features for FNH.
Clinical evaluation and imaging examinations with contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI) were performed, and the size (the longest axis) of each lesion was measured before and after the procedure.
This retrospective study was approved by the institutional review board of our hospital. The procedures of transarterial embolization were explained, and written informed consent for embolization was obtained from each patient.
Equipment and procedures
As a rule, all patients received medications for analgesia and sedation before the procedure for pain alleviation during embolization.
All patients underwent interventions in a therapeutic angiography suite (Innova 4100-IQ; GE Healthcare). Intervention was performed under local anesthesia via a unilateral femoral approach. A 4 F hepatic artery catheter (Cordis) was advanced to catheterize the celiac and superior mesenteric artery. Selective digital subtraction angiography with nonionic contrast agent (Visipaque; GE Healthcare) was performed to visualize the blood supply to the lesions and assess the patency of the port vein. Branches supplying the lesions were selectively catheterized using a 2.7 F coaxial microcatheter (Progreat) under roadmap guidance. The tip of the microcatheter was placed into the arterial branches supplying the lesion to perform selective embolization and avoid nontarget embolization. Subsequently, bleomycin-iodinated oil mixture of 15 mg of bleomycin hydrochloride (Hisun Pfizer) mixed with 15 mL of iodinated oil (Guerbet) was injected through the microcatheter until the mixture had accumulated thickly at the lesion periphery. The dosage of iodinated oil used varied depending on the size of the lesion. The embolic particles of 300–500 μm nonspherical PVA particles (Cook Medical) were injected until nearly complete stasis flow was achieved in the feeding arteries (Fig. 1). If the lesion was supplied by multiple feeding vessels, all feeding vessels underwent angiographic embolization whenever technically feasible. Embolization was performed until final arteriography demonstrated no further filling of the lesion.
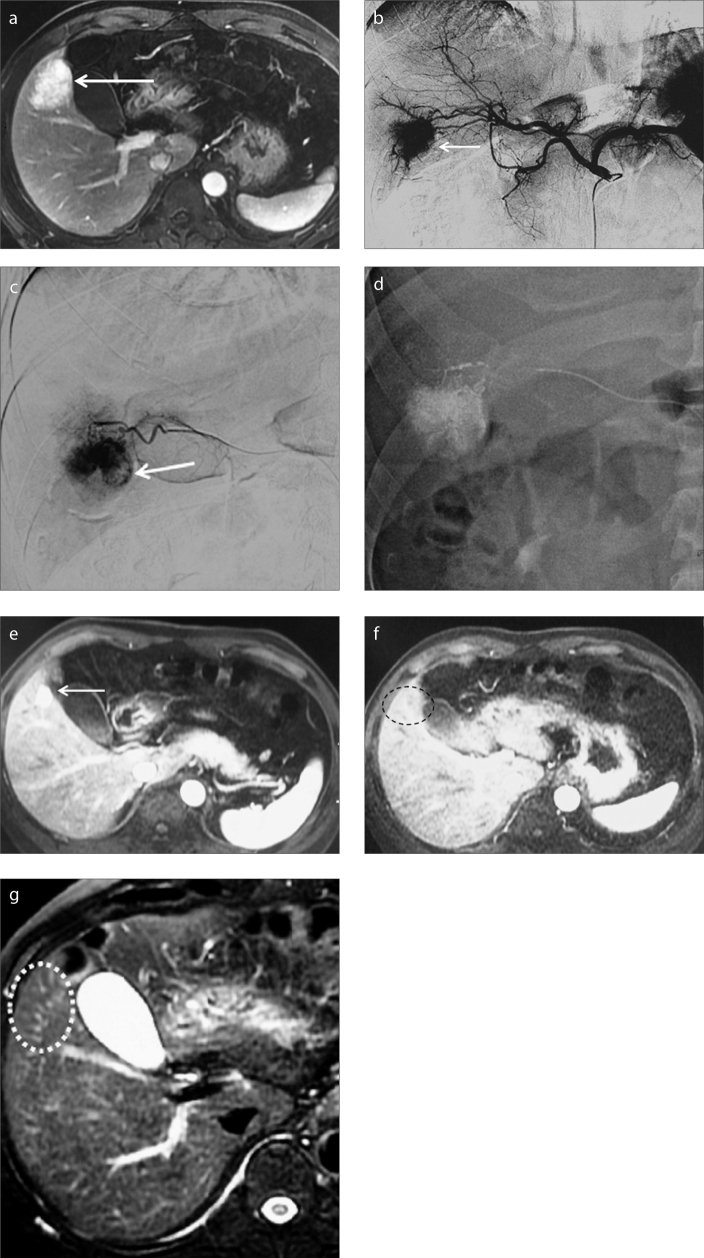
Figure 1.
a–g. A 37-year-old male patient suffering from a definitive diagnosis of symptomatic FNH of liver. Preprocedural arterial phase contrast-enhanced MRI (a) demonstrates a hypervascular mass (arrow) located in segment V. Celiac arteriogram (b) demonstrates the hypervascular lesion (arrow) supplied by branches of the right hepatic artery. Selective angiography of the feeding arterial branch with a microcatheter confirms the hypervascular mass (arrow) in the late arterial phase (c). Panel (d) shows selective embolization of the target artery with a microcatheter. Contrast-enhanced MRI (e) performed at 21-month follow-up demonstrates minimal residual lesion (arrow) at the site of previous embolization. Contrast-enhanced MRI in the arterial phase (f) and T2-weighted MRI (g) obtained at 45-month follow-up show no signs of the treated mass at the site of previous embolization (dotted circle), indicating complete resolution of the lesion.
After the procedure, prophylactic antibiotics were routinely administered intravenously and appropriate hydration was performed for 2–3 days. Analgesics and antiemetics were administered for symptoms occurring immediately postprocedure. Hepatic function (e.g., aspartate amino transferase, alanine amino transferase) and complete blood cell count were monitored before the embolization and on day 1 after the procedure.
Follow-up
Initial follow-up imaging and clinical evaluation was achieved in all patients 3–9 months after the procedure. Radiologic examination was performed with contrast-enhanced CT or MRI. The patients were followed radiologically and clinically for 3–89 months after the procedure by two interventional radiologists and a hepatobiliary surgeon. Recurrence was defined as lesion enlargement in the treated area with or without still-enhancing areas within or near the treated area. The imaging acquisitions were interpreted by two radiologists with more than 10 years of experience in abdominal imaging.
Statistical analysis
Categorical data were expressed as the number and percentage. Continuous data were expressed as mean±SD, and compared using Student’s t test when appropriate. Statistical analysis was performed using statistical software SPSS 16.0 (SPSS Inc.). Significance was established as P values less than 0.05.
Results
All patients included were symptomatic. FNH-associated abdominal or back pain was the most common symptom. Embolization was performed for a total of 27 lesions in 23 patients. The mean dose of bleomycin used was 10±4 mg per session.
A hypervascular mass with a dominant feeding artery radiating in a spinning wheel pattern from the periphery into the mass, without arteriovenous shunt or portal vein invasion, was the characteristic angiographic feature of FNH in the majority of cases (Fig. 2b).
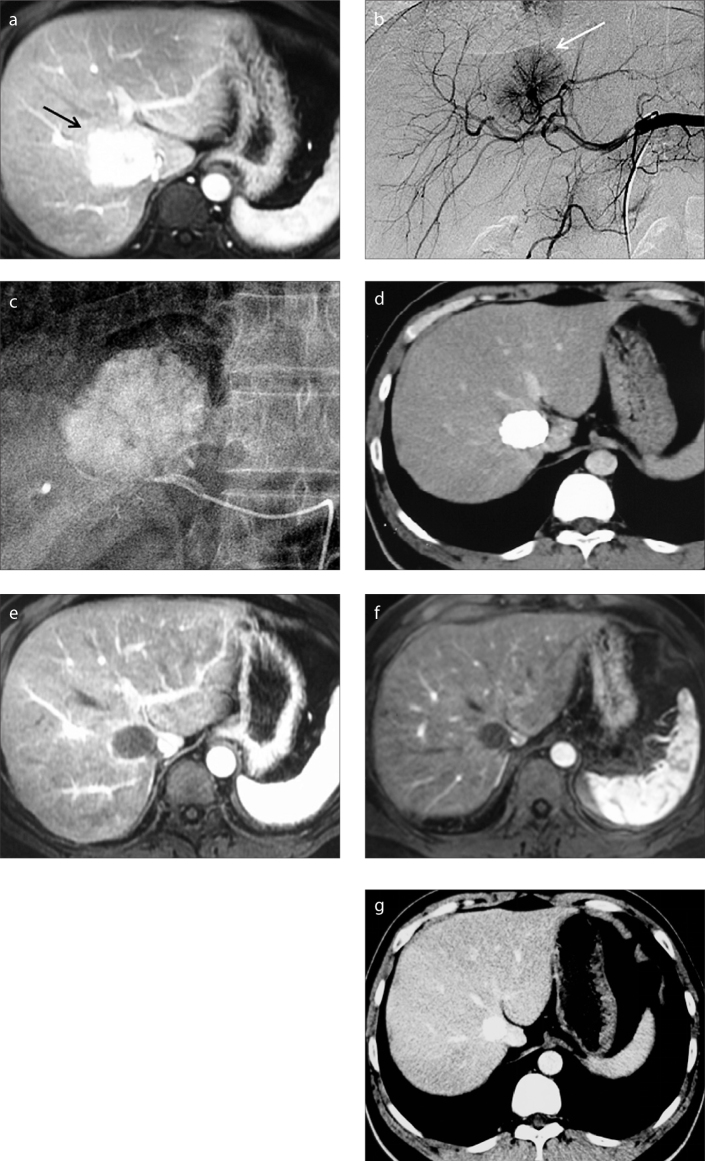
Figure 2.
a–g. A 34-year-old male patient suffering from symptomatic FNH of the liver. Preprocedural enhanced MRI in the arterial phase (a) demonstrates an enhancing mass (arrow) located in segment VIII. This mass increased in size compared with an earlier examination. Celiac arteriogram (b) demonstrates a hypervascular mass (arrow) that is supplied by a dominant feeding artery radiating in a spinning wheel pattern from the periphery into the mass. (c) The feeding artery was selectively catheterized and embolized with a microcatheter. CT imaging (d) performed at three-month follow-up demonstrates significant reduction in lesion size and good uptake of iodized oil in the lesion. Contrast-enhanced MRI exams performed at six-month (e) and 24-month (f) follow-ups show no residual enhancement of the mass, which has slightly decreased in size. Arterial phase CT image (g) obtained at 36-month follow-up demonstrates further slight decrease in size.
The mean hospital stay was 5±2 days. Embolization was well tolerated. After the procedure, 17 of the 23 patients (74%) had postembolization syndrome characterized by pyrexia, loss of appetite, abdominal pain, low-grade fever, or nausea. These symptoms were transient and self-limited. No clinical complications or adverse events associated with the procedure were noted throughout the follow-up period. Transient impairment of liver function was found in eight patients, in whom liver transaminase level increased to twice the normal baseline value on day 1 after the procedure, but levels returned to normal in two weeks in all patients. Major complications related to bleomycin, such as sclerosing cholangitis, interstitial pneumonia, or pulmonary fibrosis, were not noted in this study.
All 23 patients underwent an initial follow-up with imaging and clinical evaluation 3–9 months after embolization. Eleven patients were reevaluated at 12–38 months (mean, 25.9±7.6 months), and eight patients were reevaluated at 45–89 months (60.3±15.4 months).
Radiologic follow-up demonstrated significant size reduction in the treated lesions. The mean lesion diameter of 23 patients was 5.0±2.4 cm before the intervention and 3.2±1.5 cm on initial follow-up imaging performed 3–9 months after the intervention (Fig. 2). One of 27 lesions could no longer be visualized on MRI at the seven-month follow-up, which indicated that the lesion had complete resolution. The difference between pre- and postembolization diameters was statistically significant (Paired t test, P < 0.001) (Table). Contrast-enhanced images showed complete lack of residual arterial blood supply in the majority of the lesions. Initial follow-up CT performed after embolization demonstrated good uptake of iodized oil in the majority of treated lesions evaluated by abdominal CT (Fig. 2d).
Table.
Changes in mean tumor size pre-embolization and postembolization
| Pre-embolization (n=23) | 3–9 months postembolization (n=23) | 25.9±7.6 months postembolization (n=11) | P | |
|---|---|---|---|---|
| Tumor size (cm), mean±SD | 5.0±2.4 | 3.2±1.5 | 2.4±1.2 | < 0.001 |
The mean lesion diameter of the 11 patients reevaluated at 12–38 months decreased from 3.4±1.6 cm at the initial follow-up to 2.4±1.2 cm at the second follow-up, indicating that the treated lesions continued to significantly shrink in size (P < 0.001).
Four of 10 lesions of eight patients, who had their second follow-up at 45–89 months, showed complete resolution at 45, 48, 62, and 89 months, respectively (Fig. 1). Unfortunately, recurrence was found in one lesion. CT examinations at 54-month follow-up of this patient showed arterial enhancing within the treated area on arterial phase imaging and enlargement in lesion size. The patient presented no recurrence of clinical symptoms. The recurrence was treated with a second embolization session and is still being followed. No recurrence was found in the remaining lesions throughout the observation period.
Clinically, improvement in symptoms of abdominal or back pain was found in most cases after embolization. Sixteen patients (70%) had complete resolution of symptoms 3–9 months after the procedure. No recurrence of clinical symptoms was seen in any patients throughout the whole observation period.
Discussion
In the present study, embolization treatment of hepatic FNH using bleomycin-iodinated oil and PVA demonstrated improvement or even complete resolution of symptoms and significant reduction in size of treated lesions.
Abdominal pain or discomfort is the most frequent indication for treatment of hepatic FNH. The treatments of hepatic FNH such as surgical approaches and conservative management has changed over time and is still evolving. The indications for hepatic resection include persistent pain with a definitive diagnosis and lesions with diagnostic uncertainty (14, 15). However, the procedure of hepatic resection is associated with a significant morbidity rate and complete excision is usually difficult or even impossible.
Transarterial embolization for symptomatic FNH was shown to be an effective technique in the published literature. In previous studies, the main indications for embolization were patients with symptomatic lesion, increasing lesion size, and symptomatic patients who declined surgical resection (10–13).
Various embolic materials were used in previous studies. Theoretically, embolization with bleomycin-iodinated oil and PVA is more effective than embolization with a single embolic agent. The mechanism involves the embolic effect of iodinated oil combined with PVA and sclerosing effect of bleomycin (16). Compared with previous studies, follow-up radiologic examinations in the present study showed scarcely residual enhancing FNH tissue after embolization in the majority of the cases.
Histologically, the FNH lesion is composed primarily of Kupffer cells and normal hepatocytes, and is believed to occur in response to a vascular abnormality (17). Bleomycin has been used as a sclerosing agent in the treatment of vascular malformation and hemangiomas. In hepatic FNH treatment, bleomycin is thought act as a local sclerosing effect on the vascular endothelium inducing secondary formation of intraluminal microthrombi and resulting in the destruction of the feeding arteries of the tumor (18).
Previous studies have shown that bleomycin-lipiodol mixture is a safe embolic mixture. The mean dose of bleomycin used in the present study was 10±4 mg (range, 5–17mg), which was much lower than the bleomycin-induced toxicity levels (cumulative doses of 100–450mg) (19). Major complications related to bleomycin, such as sclerosing cholangitis or pulmonary fibrosis were not noted in this study.
Our study has three limitations. First, due to the retrospective nature of this study, detailed clinical data collection of patients pre-embolization was unavailable, and no additional radiologic follow-up was performed on the basis of lesion dimensions postembolization. Second, measures of pain reduction to formally evaluate the changes in patient’s symptoms were absent. Finally, the number of patients followed and the duration of follow-up were not uniform or standardized.
In conclusion, bleomycin-iodinated oil and PVA embolization of hepatic FNH improved or even completely resolved symptoms and significantly decreased the lesion size. Embolization using bleomycin-iodinated oil and PVA may be considered as an effective and minimally invasive alternative for the treatment of symptomatic FNH.
Main points.
Abdominal pain or discomfort is the most frequent indication for treatment of hepatic focal nodular hyperplasia (FNH).
Transcatheter arterial embolization (TAE) using bleomycin-iodinated oil and polyvinyl alcohol particles for FNH is a safe and effective alternative treatment to surgical resection.
Bleomycin mixed with iodinated oil acts not only as an embolizing agent for the treatment of FNH but also a sclerosing agent, which results in the destruction of the feeding arteries of the lesion.
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Trotter JF, Everson GT. Liver tumors benign focal lesions of the liver. Clin Liver Dis. 2001;5:17–42. doi: 10.1016/s1089-3261(05)70152-5. https://doi.org/10.1016/S1089-3261(05)70152-5. [DOI] [PubMed] [Google Scholar]
- 2.Bioulac-Sage P, Balabaud C, Wanless IR. Diagnosis of focal nodular hyperplasia: not so easy. Am J Surg Pathol. 2001;25:1322–1325. doi: 10.1097/00000478-200110000-00015. https://doi.org/10.1097/00000478-200110000-00015. [DOI] [PubMed] [Google Scholar]
- 3.Kerlin P, Davis GL, McGill DB, Weisland LG, Adison MA, Sheedy PF. Hepatic adenoma and focal nodular hyperplasia: clinical, pathological and radiographic features. Gastroenterology. 1983;84:994–1002. [PubMed] [Google Scholar]
- 4.Terkivatan T, Hussain SM, Lameris JS, Ijzermans JN. Transcatheter arterial embolization as a safe and effective treatment for focal nodular hyperplasia of the liver. Cardiovasc Intervent Radiol. 2002;25:450–453. doi: 10.1007/s00270-002-1929-6. https://doi.org/10.1007/s00270-002-1929-6. [DOI] [PubMed] [Google Scholar]
- 5.Nagorney DM. Benign hepatic tumors: focal nodular hyperplasia and hepatocellular adenoma. World J Surg. 1995;19:13–18. doi: 10.1007/BF00316973. https://doi.org/10.1007/BF00316973. [DOI] [PubMed] [Google Scholar]
- 6.de Rave S, Hussain SM. A liver tumor as an incidental finding: differential diagnosis and treatment. Scand J Gastroenterol Suppl. 2002;236:81–86. doi: 10.1080/003655202320621517. https://doi.org/10.1080/003655202320621517. [DOI] [PubMed] [Google Scholar]
- 7.Cherqui D, Rahmouni A, Charlotte F, et al. Management of focal nodular hyperplasia and hepatocellular adenoma in young women: a series of 41 patients with clinical, radiological and pathological correlations. Hepatology. 1995;22:1674–1681. https://doi.org/10.1002/hep.1840220610. [PubMed] [Google Scholar]
- 8.Kamphues C, Engel S, Denecke T, et al. Safety of liver resection and effect on quality of life in patients with benign hepatic disease: single center experience. BMC Surg. 2011;11:16–21. doi: 10.1186/1471-2482-11-16. https://doi.org/10.1186/1471-2482-11-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kneuertz PJ, Marsh JW, de Jong MC, et al. Improvements in quality of life after surgery for benign hepatic tumors: results from a dual center analysis. Surgery. 2012;152:193–201. doi: 10.1016/j.surg.2012.05.004. https://doi.org/10.1016/j.surg.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 10.Soucy P, Rasuli P, Chou S, Carpenter B. Definitive treatment of focal nodular hyperplasia of the liver by ethanol embolization. J Pediatr Surg. 1989;24:1095–1097. doi: 10.1016/s0022-3468(89)80226-x. https://doi.org/10.1016/S0022-3468(89)80232-5. [DOI] [PubMed] [Google Scholar]
- 11.Terkivatan T, Hussain SM, Lameris JS, Ijzermans JNM. Transcatheter arterial embolization as a safe and effective treatment for focal nodular hyperplasia of the liver. Cardiovasc Intervent Radiol. 2002;25:450–453. doi: 10.1007/s00270-002-1929-6. https://doi.org/10.1007/s00270-002-1929-6. [DOI] [PubMed] [Google Scholar]
- 12.Geschwind JFH, Degli MS, Morris JM, Choti MA. Treatment of focal nodular hyperplasia with selective transcatheter arterial embolization using iodized oil and polyvinyl alcohol. Cardiovasc Intervent Radiol. 2002;25:340–341. doi: 10.1007/s00270-001-0068-9. https://doi.org/10.1007/s00270-001-0068-9. [DOI] [PubMed] [Google Scholar]
- 13.Gussick SD, Quebbeman EJ, Rilling WS. Bland embolization of telangiectatic subtype of hepatic focal nodular hyperplasia. J Vasc Interv Radiol. 2005;16:1535–1538. doi: 10.1097/01.RVI.0000182174.50423.00. https://doi.org/10.1097/01.RVI.0000182174.50423.00. [DOI] [PubMed] [Google Scholar]
- 14.Clarke DL, Currie EJ, Madhavan KK, Parks RW, Garden OJ. Hepatic resection for benign non-cystic liver lesions. HPB. 2004;6:115–119. doi: 10.1080/13651820410026326. https://doi.org/10.1080/13651820410026326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Navarro AP, Gomez D, Lamb CM, Brooks A, Cameron IC. Focal nodular hyperplasia: a review of current indications for and outcomes of hepatic resection. HPB (Oxford) 2014;16:503–511. doi: 10.1111/hpb.12169. https://doi.org/10.1111/hpb.12169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bozkaya H, Cinar C, Besir FH, Parıldar M, Oran I. Minimally invasive treatment of giant haemangiomas of the liver: embolisation with bleomycin. Cardiovasc Intervent Radio. 2014;37:101–107. doi: 10.1007/s00270-013-0618-y. https://doi.org/10.1007/s00270-013-0618-y. [DOI] [PubMed] [Google Scholar]
- 17.Mathieu D, Bruneton JN, Drouillard J, Pointreau CC, Vasile N. Hepatic adenomas and focal nodular hyperplasia: dynamic CT study. Radiology. 1986;160:53–58. doi: 10.1148/radiology.160.1.3520655. https://doi.org/10.1148/radiology.160.1.3520655. [DOI] [PubMed] [Google Scholar]
- 18.Zeng Q, Li Y, Chen Y, Ouyang Y, He X, Zhang H. Gigantic cavernous hemangioma of the liver treated by intra-arterial embolization with pingyangmycin-lipiodol emulsion: a multi-center study. Cardiovasc Intervent Radiol. 2004;27:481–482. doi: 10.1007/s00270-003-2754-2. https://doi.org/10.1007/s00270-003-2754-2. [DOI] [PubMed] [Google Scholar]
- 19.Bennett JM, Reich SD. Bleomycin. Ann Intern Med. 1979;90:945–948. doi: 10.7326/0003-4819-90-6-945. https://doi.org/10.7326/0003-4819-90-6-945. [DOI] [PubMed] [Google Scholar]