Abstract
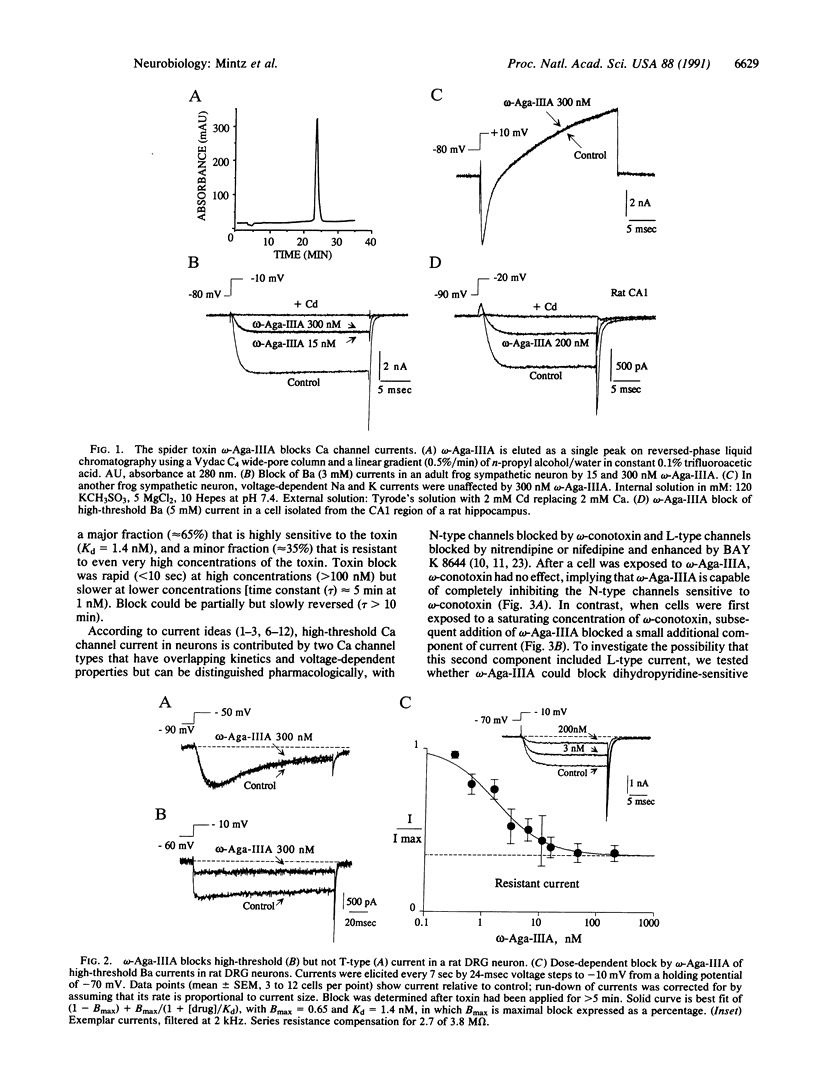
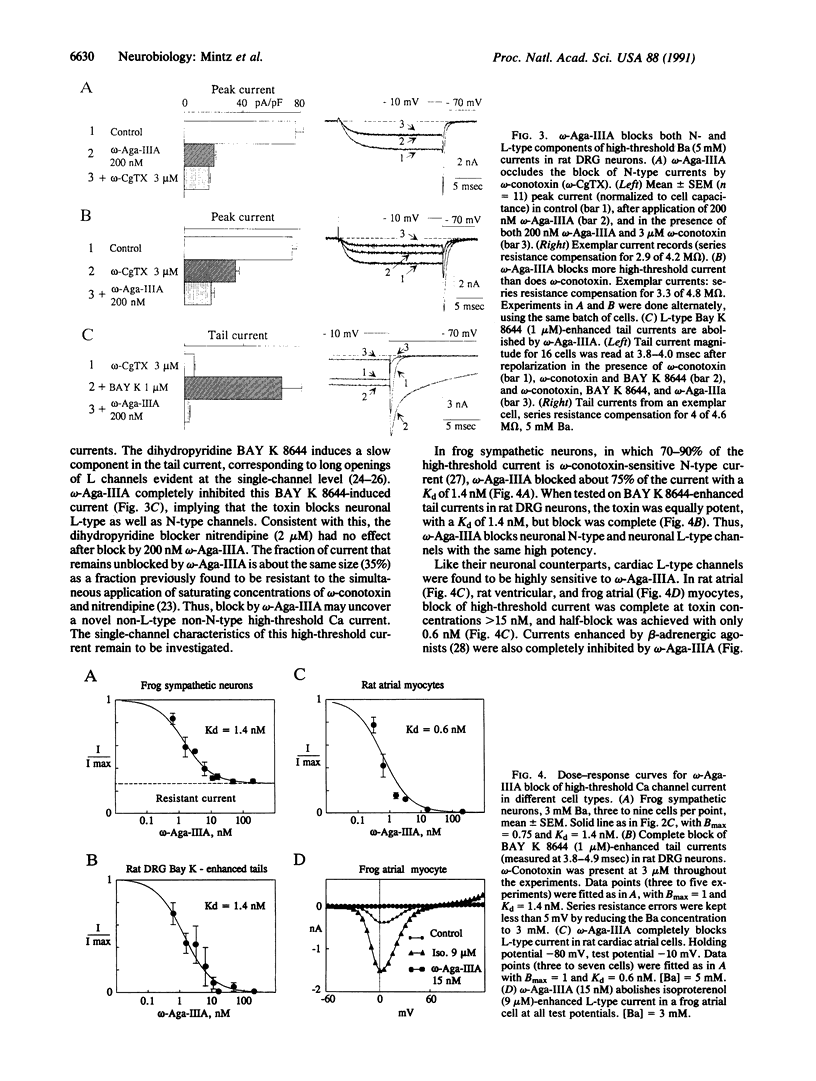
omega-Aga-IIIA, an 8.5-kDa peptide toxin isolated from the venom of Agelenopsis aperta, was found to be a highly potent inhibitor of Ca channels in cardiac muscle and in peripheral and central neurons of rats and frogs. Cardiac L-type Ca channels were completely (Kd approximately 0.6 nM) blocked by omega-Aga-IIIA. In sensory neurons, the toxin inhibited most high-threshold Ca current but not T-type Ca current. omega-Aga-IIIA blocked with similar potency (Kd approximately 1.5 nM) both omega-conotoxin GVIA-sensitive and dihydropyridine-sensitive current components but left a fraction (approximately 35%) of high-threshold current that was also resistant to omega-conotoxin and dihydropyridines. The toxin blocks N- and L-type channels with equal potency and therefore may identify a high-affinity binding site common to these two Ca channel types.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aosaki T., Kasai H. Characterization of two kinds of high-voltage-activated Ca-channel currents in chick sensory neurons. Differential sensitivity to dihydropyridines and omega-conotoxin GVIA. Pflugers Arch. 1989 Jun;414(2):150–156. doi: 10.1007/BF00580957. [DOI] [PubMed] [Google Scholar]
- Bean B. P. ATP-activated channels in rat and bullfrog sensory neurons: concentration dependence and kinetics. J Neurosci. 1990 Jan;10(1):1–10. doi: 10.1523/JNEUROSCI.10-01-00001.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean B. P. Classes of calcium channels in vertebrate cells. Annu Rev Physiol. 1989;51:367–384. doi: 10.1146/annurev.ph.51.030189.002055. [DOI] [PubMed] [Google Scholar]
- Bindokas V. P., Adams M. E. omega-Aga-I: a presynaptic calcium channel antagonist from venom of the funnel web spider, Agelenopsis aperta. J Neurobiol. 1989 Jun;20(4):171–188. doi: 10.1002/neu.480200402. [DOI] [PubMed] [Google Scholar]
- Bowers C. W., Phillips H. S., Lee P., Jan Y. N., Jan L. Y. Identification and purification of an irreversible presynaptic neurotoxin from the venom of the spider Hololena curta. Proc Natl Acad Sci U S A. 1987 May;84(10):3506–3510. doi: 10.1073/pnas.84.10.3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branton W. D., Kolton L., Jan Y. N., Jan L. Y. Neurotoxins from Plectreurys spider venom are potent presynaptic blockers in Drosophila. J Neurosci. 1987 Dec;7(12):4195–4200. doi: 10.1523/JNEUROSCI.07-12-04195.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. M., Kunze D. L., Yatani A. The agonist effect of dihydropyridines on Ca channels. Nature. 1984 Oct 11;311(5986):570–572. doi: 10.1038/311570a0. [DOI] [PubMed] [Google Scholar]
- Carbone E., Sher E., Clementi F. Ca currents in human neuroblastoma IMR32 cells: kinetics, permeability and pharmacology. Pflugers Arch. 1990 Apr;416(1-2):170–179. doi: 10.1007/BF00370239. [DOI] [PubMed] [Google Scholar]
- Fox A. P., Nowycky M. C., Tsien R. W. Kinetic and pharmacological properties distinguishing three types of calcium currents in chick sensory neurones. J Physiol. 1987 Dec;394:149–172. doi: 10.1113/jphysiol.1987.sp016864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox A. P., Nowycky M. C., Tsien R. W. Single-channel recordings of three types of calcium channels in chick sensory neurones. J Physiol. 1987 Dec;394:173–200. doi: 10.1113/jphysiol.1987.sp016865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glossmann H., Striessnig J. Molecular properties of calcium channels. Rev Physiol Biochem Pharmacol. 1990;114:1–105. doi: 10.1007/BFb0031018. [DOI] [PubMed] [Google Scholar]
- Hess P. Calcium channels in vertebrate cells. Annu Rev Neurosci. 1990;13:337–356. doi: 10.1146/annurev.ne.13.030190.002005. [DOI] [PubMed] [Google Scholar]
- Hess P., Lansman J. B., Tsien R. W. Different modes of Ca channel gating behaviour favoured by dihydropyridine Ca agonists and antagonists. Nature. 1984 Oct 11;311(5986):538–544. doi: 10.1038/311538a0. [DOI] [PubMed] [Google Scholar]
- Huettner J. E., Baughman R. W. Primary culture of identified neurons from the visual cortex of postnatal rats. J Neurosci. 1986 Oct;6(10):3044–3060. doi: 10.1523/JNEUROSCI.06-10-03044.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson H., Parks T. N. Spider toxins: recent applications in neurobiology. Annu Rev Neurosci. 1989;12:405–414. doi: 10.1146/annurev.ne.12.030189.002201. [DOI] [PubMed] [Google Scholar]
- Jones S. W., Marks T. N. Calcium currents in bullfrog sympathetic neurons. I. Activation kinetics and pharmacology. J Gen Physiol. 1989 Jul;94(1):151–167. doi: 10.1085/jgp.94.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung H. T., Branton W. D., Phillips H. S., Jan L., Byerly L. Spider toxins selectively block calcium currents in Drosophila. Neuron. 1989 Dec;3(6):767–772. doi: 10.1016/0896-6273(89)90245-6. [DOI] [PubMed] [Google Scholar]
- Lin J. W., Rudy B., Llinás R. Funnel-web spider venom and a toxin fraction block calcium current expressed from rat brain mRNA in Xenopus oocytes. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4538–4542. doi: 10.1073/pnas.87.12.4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Sugimori M., Lin J. W., Cherksey B. Blocking and isolation of a calcium channel from neurons in mammals and cephalopods utilizing a toxin fraction (FTX) from funnel-web spider poison. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1689–1693. doi: 10.1073/pnas.86.5.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCleskey E. W., Fox A. P., Feldman D. H., Cruz L. J., Olivera B. M., Tsien R. W., Yoshikami D. Omega-conotoxin: direct and persistent blockade of specific types of calcium channels in neurons but not muscle. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4327–4331. doi: 10.1073/pnas.84.12.4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra R., Morad M. A uniform enzymatic method for dissociation of myocytes from hearts and stomachs of vertebrates. Am J Physiol. 1985 Nov;249(5 Pt 2):H1056–H1060. doi: 10.1152/ajpheart.1985.249.5.H1056. [DOI] [PubMed] [Google Scholar]
- Nowycky M. C., Fox A. P., Tsien R. W. Long-opening mode of gating of neuronal calcium channels and its promotion by the dihydropyridine calcium agonist Bay K 8644. Proc Natl Acad Sci U S A. 1985 Apr;82(7):2178–2182. doi: 10.1073/pnas.82.7.2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivera B. M., Gray W. R., Zeikus R., McIntosh J. M., Varga J., Rivier J., de Santos V., Cruz L. J. Peptide neurotoxins from fish-hunting cone snails. Science. 1985 Dec 20;230(4732):1338–1343. doi: 10.1126/science.4071055. [DOI] [PubMed] [Google Scholar]
- Plummer M. R., Logothetis D. E., Hess P. Elementary properties and pharmacological sensitivities of calcium channels in mammalian peripheral neurons. Neuron. 1989 May;2(5):1453–1463. doi: 10.1016/0896-6273(89)90191-8. [DOI] [PubMed] [Google Scholar]
- Regan L. J., Sah D. W., Bean B. P. Ca2+ channels in rat central and peripheral neurons: high-threshold current resistant to dihydropyridine blockers and omega-conotoxin. Neuron. 1991 Feb;6(2):269–280. doi: 10.1016/0896-6273(91)90362-4. [DOI] [PubMed] [Google Scholar]
- Reuter H. The dependence of slow inward current in Purkinje fibres on the extracellular calcium-concentration. J Physiol. 1967 Sep;192(2):479–492. doi: 10.1113/jphysiol.1967.sp008310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien R. W., Lipscombe D., Madison D. V., Bley K. R., Fox A. P. Multiple types of neuronal calcium channels and their selective modulation. Trends Neurosci. 1988 Oct;11(10):431–438. doi: 10.1016/0166-2236(88)90194-4. [DOI] [PubMed] [Google Scholar]




