Abstract
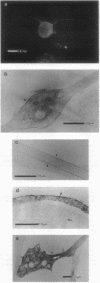
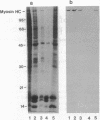
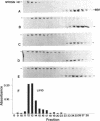
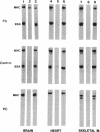
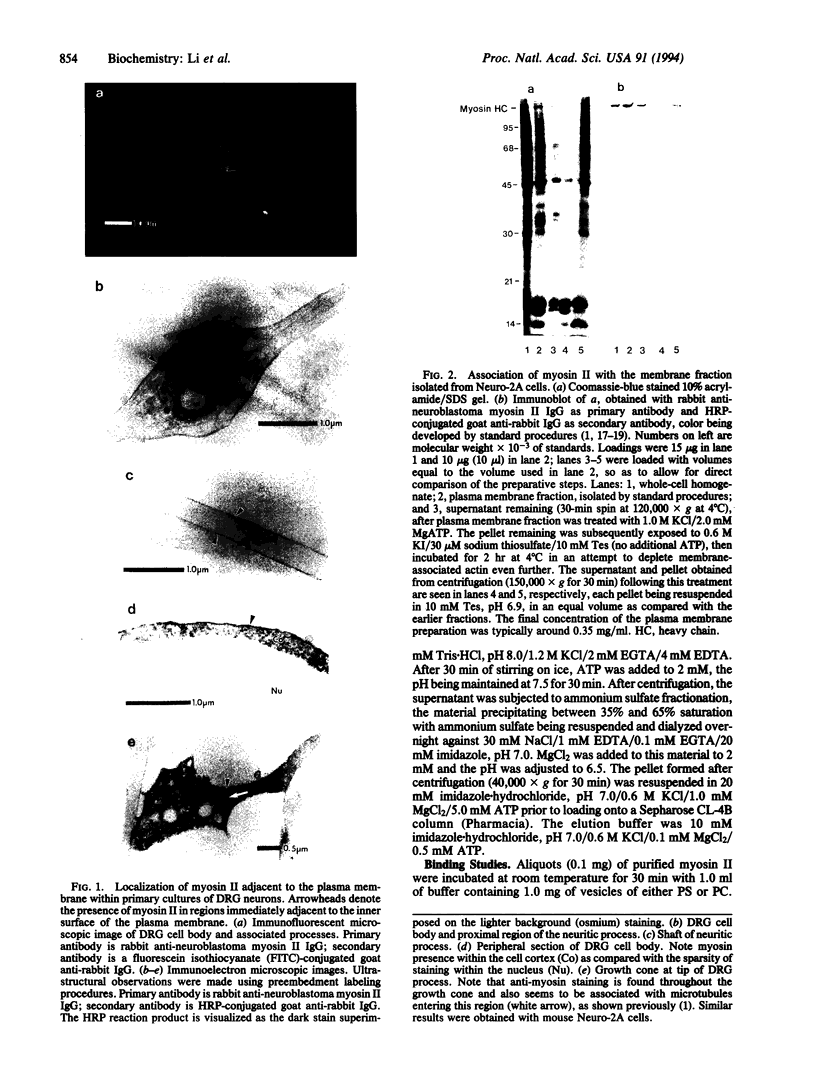
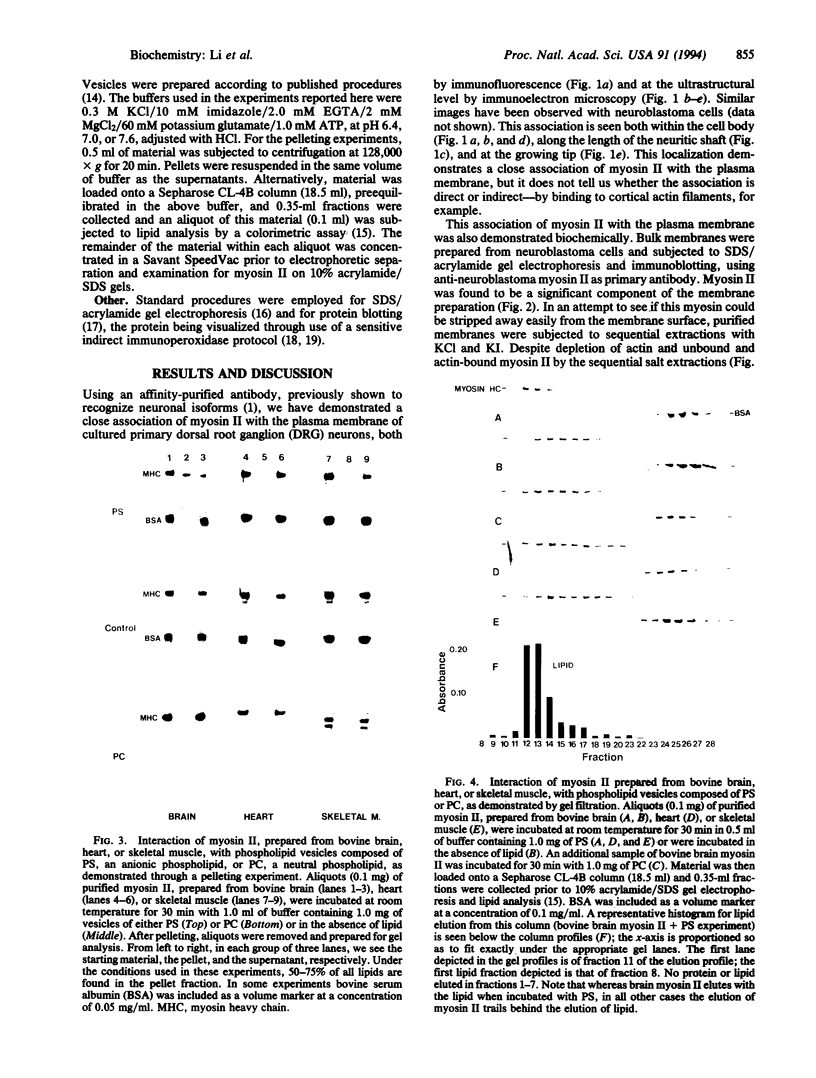
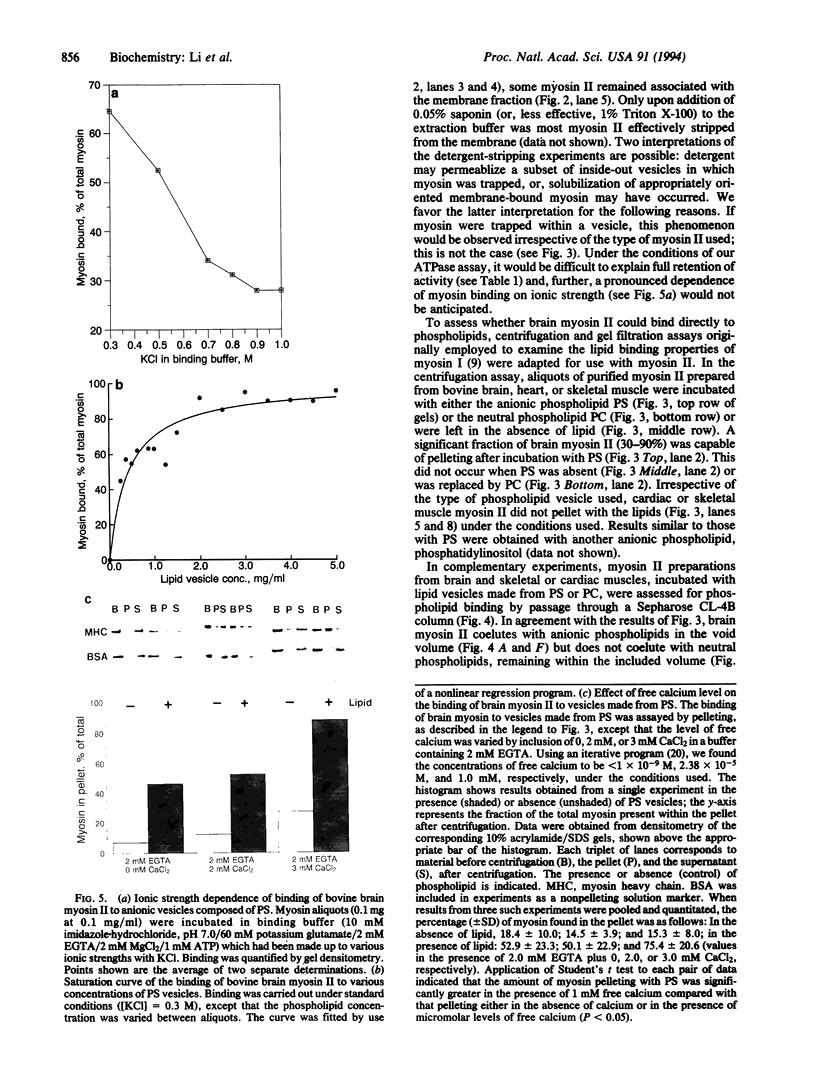
Myosin II has been observed in close proximity to the neuronal plasma membrane, suggesting the possibility that at least one isoform of neuronal myosin II may be capable of direct association. Here, we demonstrate that a significant fraction (> 30%, saturable around 90%) of brain myosin II, but not myosins from skeletal or cardiac muscle, can bind to lipid vesicles composed of the anionic phospholipid L-alpha-phosphatidyl-L-serine but not with vesicles made from the neutral phospholipid L-alpha-phosphatidylcholine. Binding to lipid vesicles made from L-alpha-phosphatidyl-L-serine is enhanced in the presence of millimolar amounts of free calcium. ATPase activity remains unimpaired after vesicle association. Myosin II was also shown to remain in tight association with purified plasma membranes, even after depletion of actin. The above observations suggest that mechanisms involving membrane-bound myosin II are required to facilitate metazoan cell motility.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams R. J., Pollard T. D. Binding of myosin I to membrane lipids. Nature. 1989 Aug 17;340(6234):565–568. doi: 10.1038/340565a0. [DOI] [PubMed] [Google Scholar]
- Barylko B., Tooth P., Kendrick-Jones J. Proteolytic fragmentation of brain myosin and localisation of the heavy-chain phosphorylation site. Eur J Biochem. 1986 Jul 15;158(2):271–282. doi: 10.1111/j.1432-1033.1986.tb09747.x. [DOI] [PubMed] [Google Scholar]
- Bower S. M., Chantler P. D. The importance of choice of visualization technique in the use of indirect immunodetection methods: specific reference to the detection of light chain movement on a regulatory myosin. Biotech Histochem. 1991;1(1):37–43. doi: 10.3109/10520299109110548. [DOI] [PubMed] [Google Scholar]
- Chantler P. D., Szent-Györgyi A. G. Regulatory light-chains and scallop myosin. Full dissociation, reversibility and co-operative effects. J Mol Biol. 1980 Apr 15;138(3):473–492. doi: 10.1016/s0022-2836(80)80013-1. [DOI] [PubMed] [Google Scholar]
- Clarke B. J., Hohman T. C., Bowers B. Purification of plasma membrane from Acanthamoeba castellanii. J Protozool. 1988 Aug;35(3):408–413. doi: 10.1111/j.1550-7408.1988.tb04118.x. [DOI] [PubMed] [Google Scholar]
- De Lozanne A., Spudich J. A. Disruption of the Dictyostelium myosin heavy chain gene by homologous recombination. Science. 1987 May 29;236(4805):1086–1091. doi: 10.1126/science.3576222. [DOI] [PubMed] [Google Scholar]
- Fukui Y., Lynch T. J., Brzeska H., Korn E. D. Myosin I is located at the leading edges of locomoting Dictyostelium amoebae. Nature. 1989 Sep 28;341(6240):328–331. doi: 10.1038/341328a0. [DOI] [PubMed] [Google Scholar]
- Gadasi H., Korn E. D. Evidence for differential intracellular localization of the Acanthamoeba myosin isoenzymes. Nature. 1980 Jul 31;286(5772):452–456. doi: 10.1038/286452a0. [DOI] [PubMed] [Google Scholar]
- Hawkes R., Niday E., Gordon J. A dot-immunobinding assay for monoclonal and other antibodies. Anal Biochem. 1982 Jan 1;119(1):142–147. doi: 10.1016/0003-2697(82)90677-7. [DOI] [PubMed] [Google Scholar]
- Hayden S. M., Wolenski J. S., Mooseker M. S. Binding of brush border myosin I to phospholipid vesicles. J Cell Biol. 1990 Aug;111(2):443–451. doi: 10.1083/jcb.111.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knecht D. A., Loomis W. F. Antisense RNA inactivation of myosin heavy chain gene expression in Dictyostelium discoideum. Science. 1987 May 29;236(4805):1081–1086. doi: 10.1126/science.3576221. [DOI] [PubMed] [Google Scholar]
- Marsh J. B., Weinstein D. B. Simple charring method for determination of lipids. J Lipid Res. 1966 Jul;7(4):574–576. [PubMed] [Google Scholar]
- Matsudaira P. T., Burgess D. R. SDS microslab linear gradient polyacrylamide gel electrophoresis. Anal Biochem. 1978 Jul 1;87(2):386–396. doi: 10.1016/0003-2697(78)90688-7. [DOI] [PubMed] [Google Scholar]
- Matsumura S., Takashima T., Ohmori H., Kumon A. The effects of phosphorylation and dephosphorylation of brain myosin on its actin-activated Mg2+-ATPase and contractile activities. J Biochem. 1988 Feb;103(2):237–246. doi: 10.1093/oxfordjournals.jbchem.a122254. [DOI] [PubMed] [Google Scholar]
- Miller M., Bower E., Levitt P., Li D., Chantler P. D. Myosin II distribution in neurons is consistent with a role in growth cone motility but not synaptic vesicle mobilization. Neuron. 1992 Jan;8(1):25–44. doi: 10.1016/0896-6273(92)90106-n. [DOI] [PubMed] [Google Scholar]
- Miyata H., Bowers B., Korn E. D. Plasma membrane association of Acanthamoeba myosin I. J Cell Biol. 1989 Oct;109(4 Pt 1):1519–1528. doi: 10.1083/jcb.109.4.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternak C., Spudich J. A., Elson E. L. Capping of surface receptors and concomitant cortical tension are generated by conventional myosin. Nature. 1989 Oct 12;341(6242):549–551. doi: 10.1038/341549a0. [DOI] [PubMed] [Google Scholar]
- Sellers J. R., Kachar B. Polarity and velocity of sliding filaments: control of direction by actin and of speed by myosin. Science. 1990 Jul 27;249(4967):406–408. doi: 10.1126/science.2377894. [DOI] [PubMed] [Google Scholar]
- Sinard J. H., Pollard T. D. Microinjection into Acanthamoeba castellanii of monoclonal antibodies to myosin-II slows but does not stop cell locomotion. Cell Motil Cytoskeleton. 1989;12(1):42–52. doi: 10.1002/cm.970120106. [DOI] [PubMed] [Google Scholar]
- Smith S. J. Neuronal cytomechanics: the actin-based motility of growth cones. Science. 1988 Nov 4;242(4879):708–715. doi: 10.1126/science.3055292. [DOI] [PubMed] [Google Scholar]
- Sun W. D., Chantler P. D. A unique cellular myosin II exhibiting differential expression in the cerebral cortex. Biochem Biophys Res Commun. 1991 Feb 28;175(1):244–249. doi: 10.1016/s0006-291x(05)81226-4. [DOI] [PubMed] [Google Scholar]
- Sun W., Chantler P. D. Cloning of the cDNA encoding a neuronal myosin heavy chain from mammalian brain and its differential expression within the central nervous system. J Mol Biol. 1992 Apr 20;224(4):1185–1193. doi: 10.1016/0022-2836(92)90482-y. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]