Abstract
We hypothesized that dietary walnut would prevent high-fat-diet (HFD)-induced hepatic apoptosis based on its antioxidant properties. Male C57BL/6J mice were fed a rodent chow or HFD (45% energy-derived) ± walnuts (21.5% energy-derived) for 6 weeks. Liver histological and biochemical analyses revealed significantly elevated fat accumulation in mice fed HFD compared to mice fed the chow or HFD+walnuts. Walnut supplementation prevented HFD-mediated alteration of the levels of key proteins in lipid homeostasis such as Sirt1, AMPK and FAS, leading to decreased fat accumulation. In addition, walnut supplementation to HFD significantly decreased the hepatic levels of cytochrome P450-2E1, nitrated proteins and lipid peroxidation. Furthermore, walnut supplementation decreased the activated cell death-associated p-JNK and p-p38K accompanied with increased hepatocyte apoptosis in HFD group. The beneficial effects of dietary walnut likely result, at least partially, from its antioxidant ingredients and attenuating HFD-induced hepatic steatosis, nitroxidative stress and apoptosis.
Keywords: Walnut, High-fat diet, Liver, CYP2E1, oxidative stress, JNK, Apoptosis
Graphical abstract
1. Introduction
Non-alcoholic fatty liver disease (NAFLD), a condition regarded as the hepatic manifestation of the metabolic syndrome, is one of the most common causes of chronic liver diseases [1]. The dramatic increase in prevalence of metabolic syndrome and NAFLD has been linked to the global spreading of the Western diet coupled with a more sedentary lifestyle [2, 3]. The molecular pathogenesis mechanisms of NAFLD have not well understood, but one of the well-established pathophysiological mechanisms is the “two-hit” hypothesis [4]. In this proposal, fat accumulation is considered the first hit, that primes the liver for more severe forms of NAFLD especially in the presence of increased oxidative stress being the “second hit” [5–7]. Indeed, emerging data recently have shown that increased liver triglycerides lead to increased oxidative stress in the hepatocytes of both animals and humans [8–12]. It has been proposed that fat accumulation in hepatocytes is coupled with mitochondrial dysfunction, which may be manifested by increased nitroxidative stress arising from enhanced generation of reactive oxygen species (ROS) and reactive nitrogen species (RNS) [13].
Nitroxidative stress occurs when cellular levels of ROS exceed the neutralizing capabilities of cellular nonenzymatic and enzymatic antioxidants. Sources of excessive ROS include intracellular enzymes such as nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, inducible nitric oxide synthase (iNOS) and cytochrome P450-2E1 (CYP2E1) [14]. The increased production of ROS is also known to cause lipid peroxidation, followed by an inflammatory response, and activation of stellate cells, leading to fibrogenesis [11, 15, 16]. On the other hands, nitric oxide (NO) and peroxynitrite (ONOO−), formed in combination with superoxide that might be increased by CYP2E1 or from mitochondrial sources, are major sources of RNS in biological systems [17]. Peroxynitrite can induce post-translational protein modifications of many cellular proteins including tyrosine and cysteine residues, to form 3-nitrotyrosine (3-NT) and S-nitrosylation of cysteines, respectively, usually contributing to inactivation and/or degradation of many functional proteins, some of which might be essential for cell signaling and survival [18, 19]. Furthermore, recent studies have revealed that excessive levels of ROS/RNS can lead to cellular injury and death by altering cellular macromolecules other than proteins including DNA and lipids [20]. The association between increased oxidative stress and a high rate of cellular apoptosis has been reported in hepatocytes [21]. Hepatocellular apoptosis is emerging as an important early mechanism for the progression of many liver diseases including NAFLD [22]. In addition, hepatocyte apoptosis may contribute to liver fibrogenesis and the development of cirrhosis, both of which are advanced forms of liver disease [23]. Although hepatocyte apoptosis may occur through a variety of mechanisms, activation of the mitogen-activated protein kinases (MAPKs) is considered a common critical mediator of various apoptotic inducers as in initiating oxidative damage-induced apoptotic cellular events [24]. Indeed, the c-Jun NH2-terminal kinases/stress-activated protein kinases (JNK) and the p38 mitogen-activated protein kinases (p38K) have been widely accepted as the major cell death-related MAPKs and have generally been associated with proapoptotic actions in many cell types [25].
Several models of NAFLD by high fat diet (HFD) have been developed to evaluate the mechanisms of the development and/or progression of NAFLD. HFD-mediated NAFLD has been reported to result from at least partially by the development of fatty liver along with the increased levels of oxidative stress and hepatocyte apoptosis [26, 27]. The healthy benefits of walnuts including reduction of oxidative stress, inflammation, improvement of blood circulation, suppression of the risk of heart disease, cancer, anti-aging properties, eczema prevention, and stabilization of body hormones due to the presence of multiple phytonutrients [28, 29]. Walnuts represent one of the edible nuts that show a very high anti-oxidant property [28]. In addition, Fink et al have shown that walnut oil also ameliorated HFD-induced triglyceride (TG) accumulation in the livers of obese Zucker rats [30]. Consistently, we have recently reported that dietary walnut can improve the levels of hepatic TG, that were markedly elevated by HFD, although walnut supplementation did not significantly alter HFD-related insulin resistance after 20 weeks of feeding [31]. At 20 weeks, The beneficial effects of walnuts appear to result from prevention of hepatic steatosis, adipose tissue inflammation, infiltration of macrophages and apoptosis of adipose tissues [31]. However, the potential protective effects of walnuts on HFD-mediated hepatic fat accumulation, increased nitroxidative stress and the early cell-death related signaling pathways have not been investigated especially after short term exposure to HFD. Thus, the current study is specifically aimed to evaluate whether dietary walnuts can decrease hepatic apoptosis by potentially attenuating some of the early manifestations of NAFLD namely hepatic fat accumulation, oxidative stress, and the cell death signaling pathways in mice fed HFD for only 6 weeks.
2. Materials and methods
2.1. Animals and diets
Age-matched (5 weeks) male C57BL/6 mice were obtained from Jackson Laboratories. After one week of acclimation, mice were randomly assigned to three groups (n≥6/group) fed either a regular chow diet (14% fat by calories; 7017 NIH-31 open formula mouse/rat sterilizable diet) (http://www.envigo.com/products-services/teklad/laboratory-animal-diets/natural-ingredient/traditional/rodent/7017-nih-31-open-formula-mouse-rat-sterilizable-diet.aspx) or HFD (45% fat-derived calories; D12451) (Research Diets, New Brunswick NJ, USA) with or without physiologically relevant amounts of walnuts (21.5% total energy with 18.9% fat-derived calories) for 6 weeks [31]. The HFD and HFD+walnut diets were designed to be isocaloric. The diet composition is shown in Supplementary Table 1. The mice were housed in groups of three per cage at 22°C with a 12 h light/dark cycle and given free access to diet and water. The weight of each mouse was determined once a week and their food intake was recorded daily during the feeding period. The food efficiency ratio (FER) was calculated as gram of body weight gain per gram of food consumed. After 6 weeks, the livers tissues were excised from the mice fasted overnight (12 h) and immediately snap frozen while trunk blood samples following decapitation were collected from the sedated animals into heparinized blood collection tubes. All samples were stored at −80°C until analysis. Animal experiments were performed in accordance with the National Institutes of Health guidelines and approved by the Institutional Animal Care and Use Committee.
2.2. Histopathology analysis
Liver tissue sections from the largest lobe were stained with hematoxylin and eosin (H&E). Following staining, hepatic histological examination was performed with the histological scoring system for NAFLD, as described by Kleiner et al. [32]. All liver specimens were assessed blindly by an independent pathologist who did not know the identities of the study groups.
2.3. Measurements of the hepatic contents of triglyceride (TG) and adenosine triphosphate (ATP) as well as serum levels of alanine aminotransferase (ALT) and leptin
For hepatic TG measurement, liver tissues (50 mg wet weight) were homogenized in 5% Triton X-100 solution and heated in 80–100°C water bath for 2–5 min to solubilize the TG. The samples were then centrifuged at 10,000 × g for 10 min, and the resulting supernatant was used to determine the TG level. Ten µL of either the samples or the standards were used for the assay and 100 µL of the working reagent (100 µL assay buffer, 2 µL enzyme mix, 5 µL lipase, 1 µL ATP, and 1 µL dye reagent) provided by the manufacturer, was transferred into the respective standards and sample wells. Samples were then incubated for 30 min at room temperature and optical density at 570 nm was then read. Values were then extrapolated from the standard curve according to the manufacturer’s instruction provided with the EnzyChrom™ Triglyceride Assay Kit (BioAssay Systems, Hayward, CA). ATP levels in snap frozen liver tissues were evaluated by using the ATP Assay Kit (Abcam Inc., Cambridge, MA) following the manufacturer’s protocol. The level of serum ALT was measured for each animal using a clinical IDEXX Vet Test Chemistry Analyzer system from IDEXX Laboratories (West brook, ME). Serum leptin concentration was measured using the Mouse Leptin ELISA kit (Life Technologies, Grand Island, NY).
2.4. Insulin resistance (IR) and glucose tolerance (GT) tests
After feeding for 4 and 5 weeks with the different diets without or with walnuts, GT and IR tests were conducted in mice fasted for 6 h and overnight, respectively, after subsequent intraperitoneal (i.p.) injection of glucose (2 g/kg) (4 weeks) or insulin (0.75 U/kg; Eli Lilly) (5 weeks). This one week period between the first tests was given to allow sufficient time for the mice to recover from the test and also to examine the mice over an extended period of time. Glucose levels were then measured from the tail blood of each mouse right before the injection (0 time point) as well as at 30, 60, 90, and 120 minutes following the intraperitoneal injection of glucose or insulin. Blood glucose levels were determined using the Elite glucometer (Bayer, Leverkusen, Germany) and the kinetic curve as well as area under the curve (AUC) were generated to compare the levels among the different treatment groups.
2.5. Immunoblot and immunoprecipitation analyses
Total hepatic homogenate proteins were prepared as previously published [33]. Cytosolic or mitochondrial proteins (50 µg/sample), prepared by differential centrifugation as previously described [34, 35], were separated by 10 or 12 % SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and electrophoretically transferred to nitrocellulose membranes. Upon completion of electrophoretic transfer of the proteins, membranes were blocked for 1 h in 4% milk powder in Tris-HCl buffered saline containing 0.01% Tween 20 (TBS-T). Membranes were then probed with specific primary antibodies. The primary antibodies for sirtuin-1 (SIRT-1), fatty acid synthase (FAS), phosphorylated- AMP-activated protein kinase (P-AMPK), AMPK, P-JNK, JNK, P-p38K, p38K, BCL2, P-BCL2, heat shock protein 90 (HSP90) and GAPDH were purchased from Cell Signaling Inc. (Danvers, MA) while specific antibodies to poly-ADP-ribosyl polymerase-1 (PARP-1) or ATP synthase β-subunit (ATP5B) were obtained from Santa Cruz Biotechnology Inc. (Dallas, TX, USA). Specific antibodies for CYP2E1, iNOS, or 3-NT were from Abcam Inc. (Cambridge, MA, USA). After removing the respective primary antibody followed by three washing steps the nitrocellulose membranes were either incubated with the goat anti-rabbit or anti-mouse horseradish peroxidase-conjugated secondary antibody (Santa Cruz Inc, Dallas, TX, USA) (1:5,000 dilution in 5% milk powder in TBS-T to complete the immunoblot analyses).
For immunoprecipitation (IP) analysis, equal amounts of hepatic lysate proteins from each mouse within the same group (n≥6/group) were pooled to prepare a total of 1 mg proteins/sample. Pooled proteins were incubated with the specific primary anti-HSP90 antibody overnight at 4°C with head-to-tail rotations and then pulled down with protein-A/G conjugated magnetic beads (Promega Inc., CA, USA). Following three separate washing steps and elution of the immunoprecipitated proteins by the manufacturer’s protocol, equal amounts of protein/sample were loaded onto 10% SDS-polyacrylamide gels followed by immunoblot analyses using anti-HSP90 or anti-3-NT antibodies, as described previously [36]. Protein bands were detected by enhanced chemiluminescence and their densities quantified using UN-SCAN-IT gel version 6.1 from Silk Scientific, as previously described [26, 33].
2.6. Immunohistochemistry
Formalin-fixed liver samples were processed and 5-µm thick paraffin sections were used for immunohistochemistry (IHC). Briefly, de-paraffinized liver sections were treated with 3% hydrogen peroxide followed by antigen retrieval. The sections were blocked with 2% non-fat skim milk solution, and incubated with the primary antibody against 3-NT and 4-hydroxynonenal (HNE). After incubation and subsequent washing steps, the attached primary antibody was then linked to the dextran polymer by following the manufacturer’s protocol (Envision kit, Dako, Carpinteria, CA, USA). The final reaction was performed by immersing the sections in a solution of 3,3’-diaminobenzidine (DAB). The sections were then counterstained with hematoxylin.
2.7. Measurements of MDA + HAE concentration
The concentration of malondialdehyde (MDA) + 4-hydroxyalkenals (HAE) (µM) in cytosolic or mitochondrial fractions was measured using the commercially available kits (Oxford Biomedical Research, Oxford, MI, USA) following the manufacturer’s protocol.
2.8. Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay
The ApopTag peroxidase in situ apoptosis detection kit from Millipore Corporation (Billerica, MA, USA) was used to identify apoptotic hepatocytes by labeling and detecting DNA strand breaks by the TUNEL method following the manufacturer’s protocol. Numbers of TUNEL-positive hepatocytes were counted in 10 high-power (200×) microscope fields (HPF).
2.9. Statistical analysis
Data represent results from at least two separate measurements, unless otherwise stated. Each point represents the mean ± SEM (n≥6/group). The significance of differences between groups was determined by One-way ANOVA with Tukey’s post hoc test. Statistical analysis was conducted using Graphpad Prism software (GraphPad Software Inc.) and values with p < 0.05 were considered significant.
3. Results
3.1. Effects of walnuts on HFD-induced hepatic steatosis and metabolic parameters
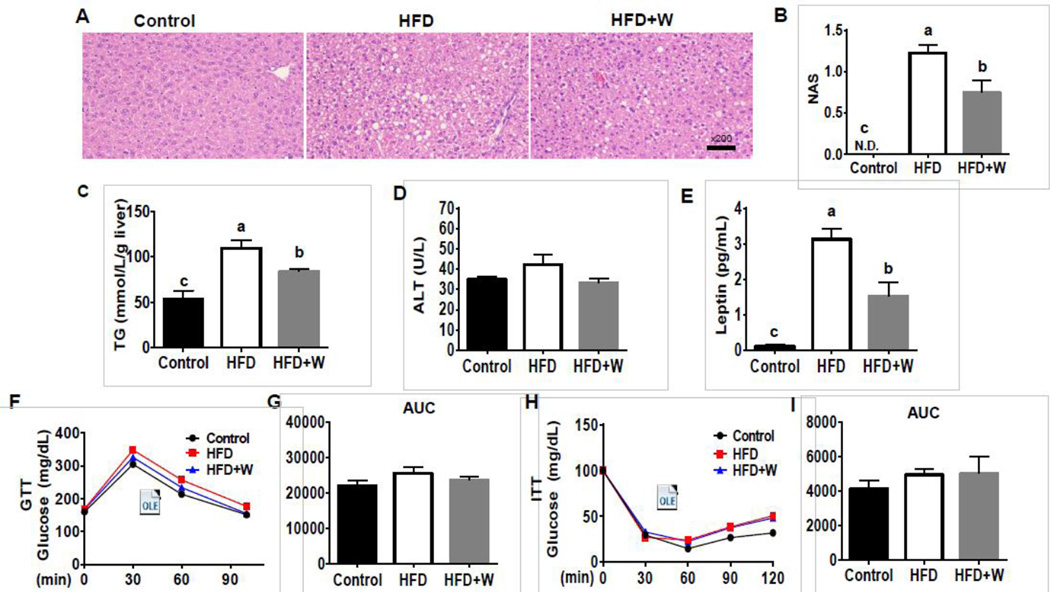
After 6 weeks of feeding, histological analysis revealed the accumulation of lipid droplets in the livers of HFD-fed mice, whereas hepatic fat contents were markedly decreased in mice fed HFD+walnuts (HFD+W) than those of mice fed HFD alone (Fig. 1A). In addition, walnut-fed mice had a significantly lower NAFLD activity score [32] than that of HFD-fed mice (Fig. 1B). Consistent with histological assessment, HFD-fed mice exhibited the highest levels of hepatic TG and these levels were significantly decreased in HFD+W group (Fig. 1C). No significant changes in the hepatic ATP contents were observed among the different groups (data not shown). Serum ALT levels were not significantly changed in either HFD-fed mice or HFD+walnut compared to control group (Fig. 1D). HFD-induced marked elevation in the serum leptin level, compared to the control group, which was significantly decreased by walnut supplementation (Fig. 1E). The body weight gain as well as visceral fat mass of mice fed the HFD or HFD+W were greater than those of control mice, but walnut supplementation did not significantly change these parameters compared with HFD group (Table 1). In addition, walnut supplementation did not change the food intake, whereas HFD- and HFD+W-fed mice had greater food efficiency ratios (FER) compared with control mice (Table 1). Furthermore, walnut supplementation did not affect glucose intolerence or insulin resistance, although HFD for 6 weeks did not alter the rates of both parameters (Fig. 1F–I), possibly due to a relative short exposure of HFD.
Figure 1.
Effects of dietary walnuts on hepatic steatosis and metabolic parameters in mice fed HFD for 6 weeks. (A) Representative liver histology with H&E staining, (B) NASH score, (C) concentration of hepatic TG, (D) serum ALT, and (E) serum leptin of experimental mice. The results of (F) glucose tolerance test (GTT), (G) glucose area under the curve (AUC) during GTT, (H) insulin tolerance test (ITT), and (I) glucose AUC during ITT are shown. All results are presented as mean ± SEM (n=6/group). Significance was determined by one-way ANOVA with the Tukey’s post hoc test (P<0.05) and is denoted by different letters.
Table 1.
Body weight gain, liver weight, and food intake from mice fed experimental diets
| Groups | Control | HFD | HFD+W |
|---|---|---|---|
| Final body weight (g) | 26.99 ± 0.64b | 30.78 ± 0.36a | 30.75 ± 1.16a |
| Body weight gain (g/6 weeks) | 3.68 ± 0.17b | 8.22 ± 0.49a | 7.28 ± 1.21a |
| Visceral fat-pad weight (mg/g body weight) |
12.0 ± 1.47b | 69.2 ± 1.53a | 61.7 ± 2.79a |
| Liver index (mg/g body weight)# | 3.32 ± 0.262 | 3.68 ± 0.058 | 3.34 ± 0.115 |
| Food intake (g/day) | 3.55 ± 0.10a | 2.60 ± 0.05b | 2.89 ± 0.13b |
| Caloric intake (kcal/day) | 10.65 ± 0.3a | 12.22 ± 0.24b | 13.29 ± 0.6b |
| Food efficiency ratio* | 0.024 ± 0.002b | 0.075 ± 0.005a | 0.059 ± 0.004a |
Each value represents the mean ± SEM; Means in the same row not sharing a common superscript are significantly different at P<.05.
liver index = liver weight/body weight
food efficiency ratio = Body weight gain for experimental period (g)/Food intake for experimental period (g)
3.2. Effects of walnuts on the levels of hepatic proteins involved in lipid metabolism
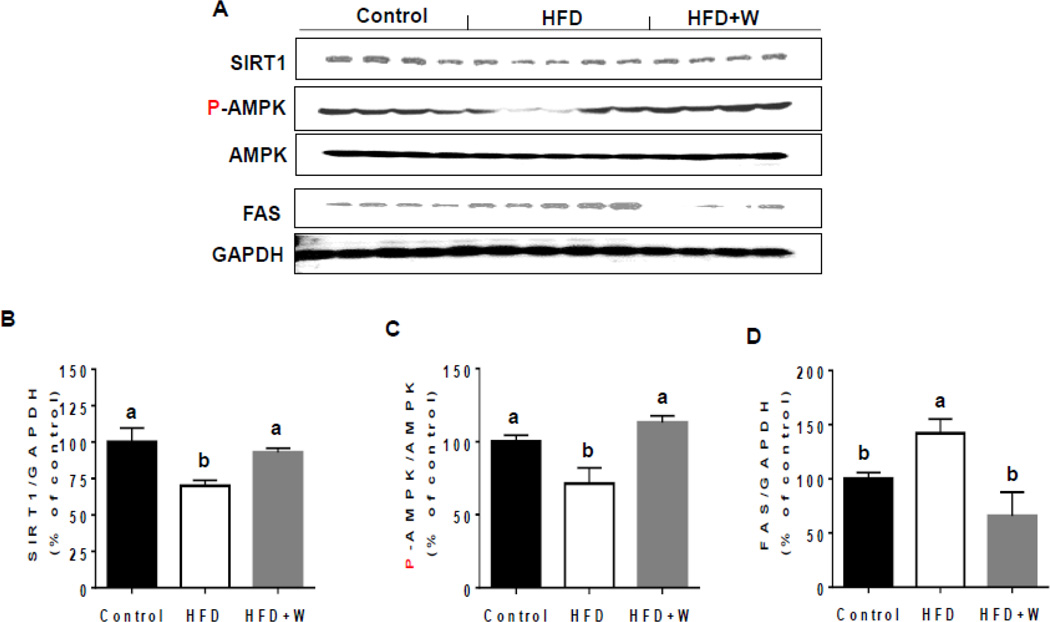
To elucidate potential protective mechanism(s) of walnuts against the HFD-induced hepatic TG increment in this model, we examined the effects of walnuts on the levels of key hepatic proteins involved in lipogenesis and fatty acid oxidation as SIRT1, AMPK, and FAS. SIRT1 and phosphorylated (active) AMPK (p-AMPK) are key regulators of both lipogenesis and fatty acid oxidation in the liver [37, 38]. Expression of SIRT1 and phosphorylation of AMPK were significantly decreased in the livers of HFD-fed mice compared with control mice and walnuts supplementation completely prevented the alteration of the levels of these two proteins (Figs. 2A–C). In addition, the hepatic expression of FAS was significantly elevated in HFD-fed mice compared with control mice and this increase was significantly reduced by walnut supplementation (Figs. 2A and D).
Figure 2.
Effect of dietary walnuts on the levels of key proteins involved in lipid metabolism following exposure to HFD for 6 weeks. (A) Representative images of the immunoblot analysis for SIRT1, P-AMPK, AMPK, FAS or GAPDH in the livers of experimental mice. The densitometric levels of (B) SIRT1, (C) P-AMPK, and (D) FAS measured by immunoblot analysis were normalized to GAPDH are shown. The densities of all samples are presented as mean ± SEM. Significance was determined by one-way ANOVA with the Tukey’s post hoc test (P<0.05) and is denoted by different letters.
3.3. Effects of walnuts on expressions of CYP2E1, iNOS and NADPH oxidase
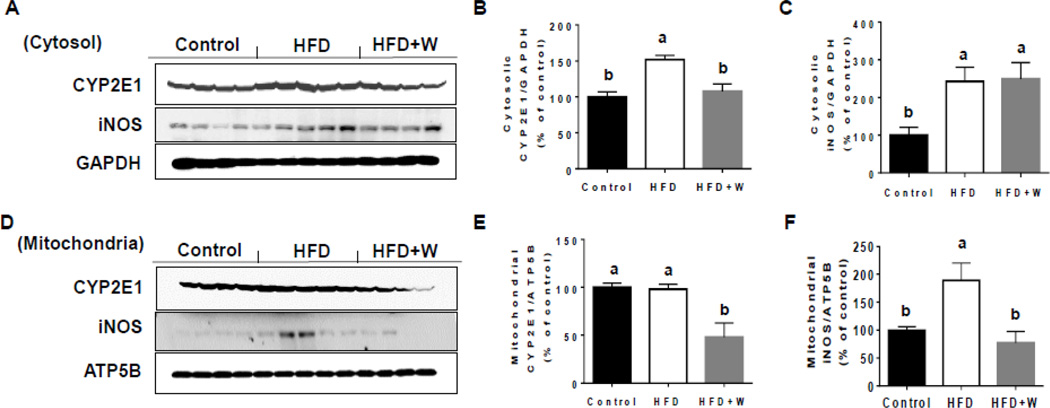
To determine the effect of walnuts on oxidative stress in HFD-fed mice, we evaluated the expressed levels of cytosolic and mitochondrial CYP2E1 proteins, because CYP2E1, an important source of oxidative stress [39], has been reported to increase in response to HFD and play a critical role in the development of NAFLD [33]. Cytosolic CYP2E1 protein levels were significantly upregulated in liver tissues of HFD-fed mice compared to control group and this increase was significantly reversed by the walnut supplementation (Figs. 3A and B). In addition, walnut supplementation to HFD significantly decreased the levels of mitochondrial CYP2E1 compared to the two other groups (Figs. 3D and E). Further, HFD-fed mice exhibited significantly elevated levels of cytosolic and mitochondrial iNOS compared to those of the control group (Figs. 3A, C, D, and F). However, walnut supplementation significantly decreased the mitochondrial iNOS levels (Figs. 3D and F) without affecting the cytosolic contents (Figs. 3A and C), although we do not know the reason for the different outcomes. Finally, the levels of hepatic NAPDH oxidase protein were not statistically different among all the groups (data not shown).
Figure 3.
Effects of dietary walnuts on the expressions of CYP2E1 and iNOS in the liver cytosol or mitochondria from mice fed HFD for 6 weeks. Representative images of the immunoblot analysis for CYP2E1 and iNOS in (A) cytoplasm or (D) mitochondria in livers of experimental mice. The densitometric levels of CYP2E1 and iNOS in cytoplasm (B and C) and mitochondria (E and F) measured by immunoblot analysis and normalized to GAPDH or ATP5B, respectively are shown. All results are presented as mean ± SEM. Significance was determined by one-way ANOVA with the Tukey’s post hoc test (P<0.05) and is denoted by different letters.
3.4. Effects of walnuts on HFD-induced protein nitration and lipid peroxidation
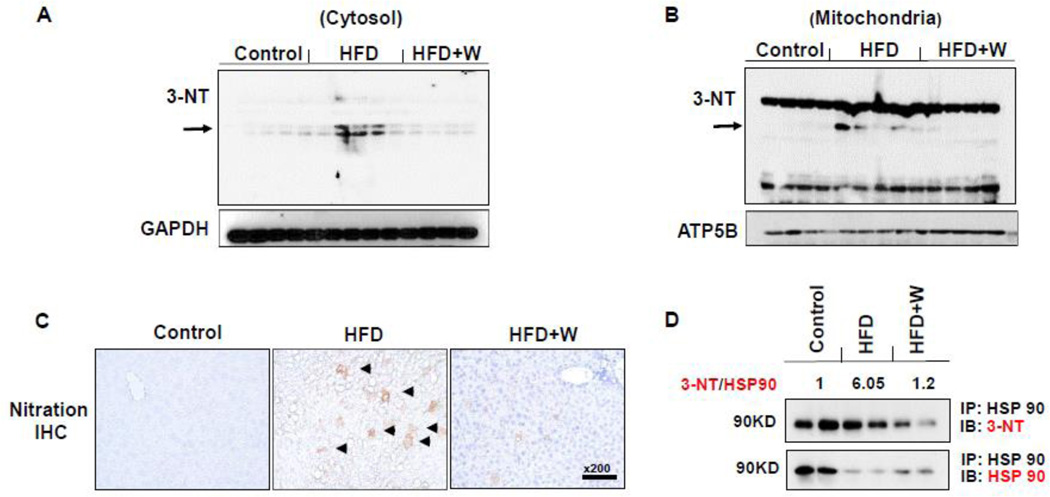
We further evaluated the levels of nitrated proteins by both immunoblot and immunohistochemistry analyses. Western blot analysis of hepatic proteins showed that the markedly increased levels of nitrated proteins in cytosolic and mitochondrial fractions in the HFD-fed mice were considerably prevented in the HFD+W mice group (Figs. 4A and B). Immunohistochemical analysis confirmed the marked decrease of the increased levels of nitrated proteins in HFD-fed mice by the walnut supplementation (Fig. 4C). To further support the result of increased nitrated proteins (Fig. 4), we have also analyzed the nitrated levels of Hsp90 as an examples of protein nitration, since this protein is known to be nitrated and involved in promoting cellular apoptosis [40]. We performed immunoprecipitation of HSP90 followed by immunoblot analysis with the respective antibody against HSP90 or 3-NT. The densitometric levels of nitrated HSP90 were normalized to those of native HSP90 in each group. Our results showed that increased protein nitration, as reflected by the elevated ratio (~6 folds) of nitrated HSP90/native HSP90, was observed in HFD-fed mice compared to the control group and that walnut supplementation prevented the increased levels of the nitration of Hsp90 (Fig. 4D) and would possibly be the case with many other proteins.
Figure 4.
Effects of dietary walnuts on protein nitration in mice exposed to HFD for 6 weeks. Equal amounts of liver lysates from the indicated groups were used to determine the levels of protein nitration using the anti-3-NT antibody in (A) cytoplasm or (B) mitochondria. Immunoblot analysis were normalized to GAPDH (cytosol) or ATP5B (mitochondria). (C) Representative immunohistochemical analysis results of hepatic protein nitration determined with the anti-3-NT antibody in all indicated groups are presented. (D) Results of immunoprecipitation of HSP90 followed by immunoblot analysis are presented. Specific anti-HSP90 and 3-NT antibodies were used for immunoblot analyses, as indicated. The numerical values, shown above the blots, represent the normalized values of nitrated HSP90 to the native HSP90 for the different groups, compared to the control group which was set at 1. All results are presented as mean ± SEM. Significance was determined by one-way ANOVA with the Tukey’s post hoc test (P<0.05) and is denoted by different letters.
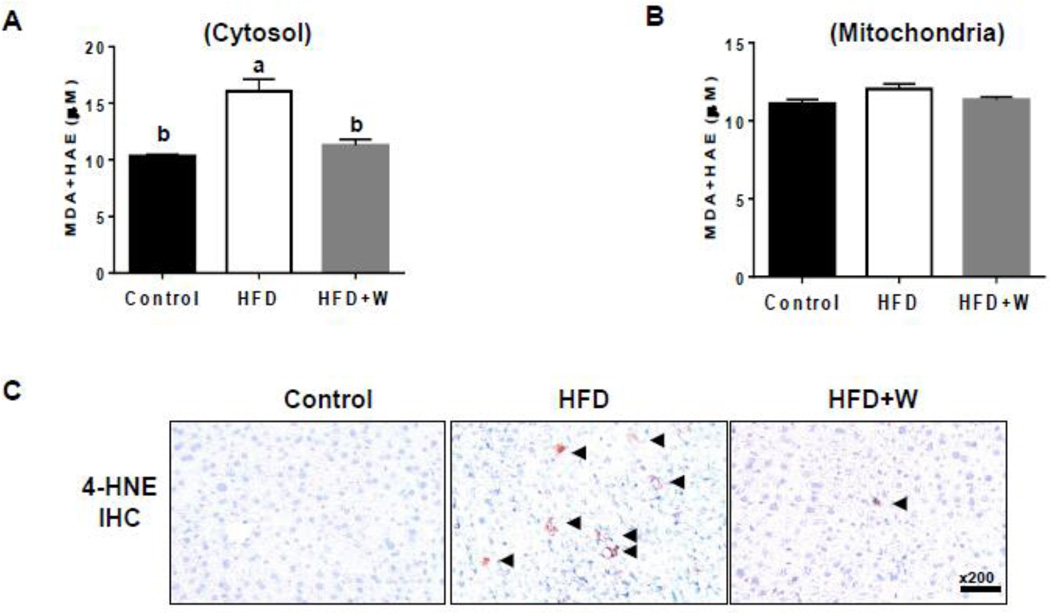
Increased oxidative stress can also elevate the levels of lipid peroxidation, as shown in conditions associated with a HFD and fatty liver [26, 33]. Thus, we evaluated a lipid peroxidation marker, MDA+HNE, in both cytosol and mitochondria. Similar to the results in protein nitration levels, walnut supplementation significantly decreased the levels of cytosolic MDA+HAE that were substantively increased in HFD-fed group (Fig. 5A). However, there was no significant change in the mitochondrial MDA+HNE levels among all the groups (Fig. 5B). Immunohistochemistry analysis supported the increased 4-HNE contents in livers of HFD+W group than those of control mice and that walnut supplementation markedly prevented the increment (Fig. 5C).
Figure 5.
Effects of dietary walnuts on lipid peroxidation in mice fed HFD for 6 weeks. Equal amounts of whole tissue lysates were used to determine the levels of MDA+HAE in (A) cytoplasm or (B) mitochondria from the livers of the indicated groups. (C) Representative immunohistochemical analysis results of hepatic lipid peroxidation determined with the anti-4-HNE antibody in all indicated mouse groups are presented. All results are presented as mean ± SEM (n=6/group). Significance was determined by one-way ANOVA with the Tukey’s post hoc test (P<0.05) and is denoted by different letters.
3.5. Effects of walnuts on activation of JNK, p38K, and hepatic apoptosis
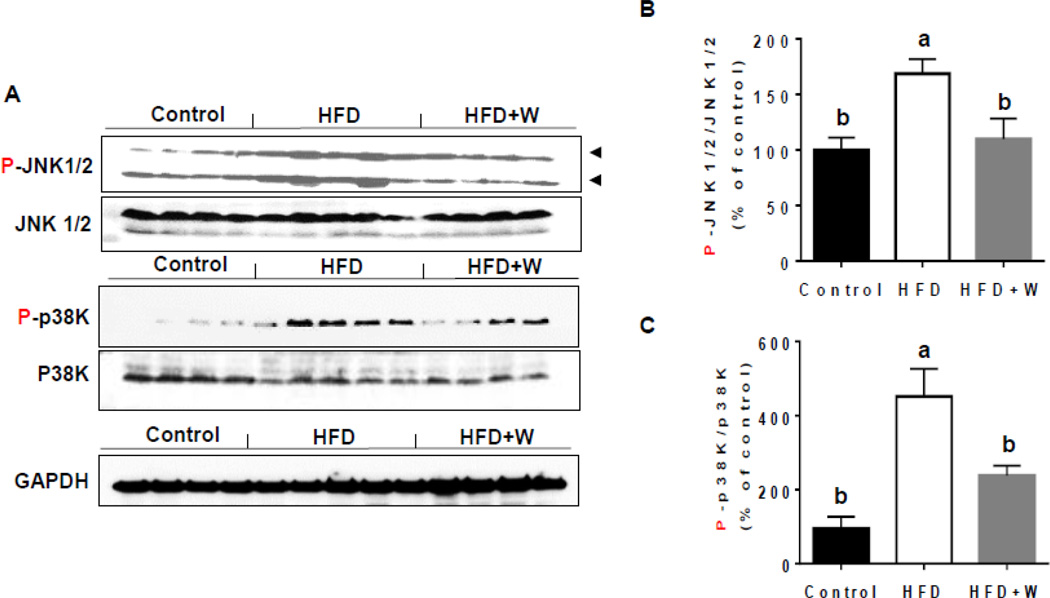
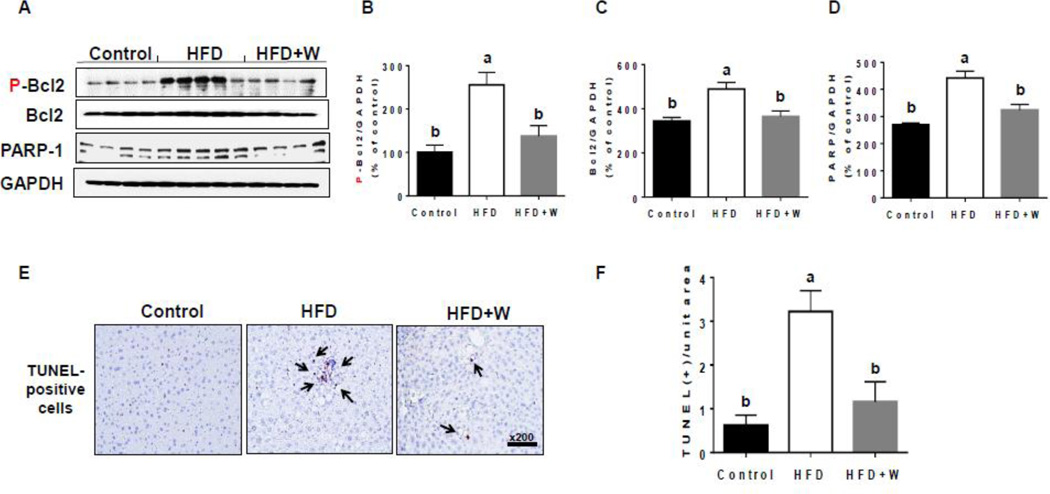
Elevated ROS/RNS can promote the cell death signaling pathways by activating JNK and p38K [41, 42]. The levels of phosphorylated (activated) JNK and p38K were significantly increased in the livers of HFD-fed mice compared to control group (Figs. 6A, B, and C) and walnut supplementation significantly decreased the levels of phosphorylated enzymes. In addition, in order to further demonstrate the protective effects of walnuts on HFD-induced hepatocyte apoptosis, we determined the levels of p-BCL2 and PARP-1 in hepatic lysates of the different groups. Our results showed that the addition of walnut to HFD significantly prevented the increased levels of P-BCL2 (Figs. 7A and B), which has been reported to play a causal role in apoptosis [43], as well as the apoptotic protein PARP-1 [44] (Fig. 7A and D). BCL2 was also slightly but significantly increased in HFD group, probably as a compensatory mechanism to the increased BCL2 phosphorylation (Figs. 7A and C). Consistently, TUNEL analysis in mouse liver tissues of different experimental groups [44] revealed that the number of apoptotic hepatocytes was significantly greater in HFD-fed mice than the other two groups (Figs. 7E and F). Walnut supplementation significantly prevented the hepatocellular apoptosis induced by HFD alone (Figs. 7E and F).
Figure 6.
Effects of dietary walnuts on activation of JNK and p38 kinase in mice fed HFD for 6 weeks. (A) Equal amounts of whole liver lysates were used to determine the levels of P-JNK, JNK, P-p38K, or p38K by immunoblot analysis. The densitometric levels of (B) P-JNK and (C) P-p38K, determined by immunoblot analysis and normalized to JNK and p38K, respectively, are shown. All results are presented as mean ± SEM. Significance was determined by one-way ANOVA with the Tukey’s post hoc test (P<0.05) and is denoted by different letters.
Figure 7.
Effects of dietary walnuts on the hepatic apoptosis. (A) Representative images of the immunoblot analysis for P-BCL-2, BCL2, PARP-1 or GAPDH in the livers of experimental mice exposed to HFD or HFD+walnut for 6 weeks. The densitometric levels of (B) P-BCL2, (C) BCL2, and (D) PARP-1, determined by immunoblot analysis and normalized to GAPDH, are shown. All results are presented as mean ± SEM. Significance was determined by one-way ANOVA with the Tukey’s post hoc test (P<0.05) and is denoted by different letters. (E) Representative images of TUNEL-positive apoptotic hepatocytes (identified by black arrows) in livers of indicated groups are presented. (F) Number of TUNEL-positive hepatocyte in 10 high-power fields (× 200) was calculated. All results are presented as mean ± SEM (n=6/group). Significance was determined by one-way ANOVA with the Tukey’s post hoc test (P<0.05) and is denoted by different letters.
Taken together, these data suggest that the HFD-induced oxidative stress stimulated the JNK- and/or p38K-mediated cell death signaling pathways with elevated hepatocyte apoptosis and that walnut supplementation inhibited the effect of HFD-induced hepatocellular apoptosis partially through inhibiting the HFD-activated cell death signaling pathways.
4. Discussion
As expected, the HFD feeding led to increased hepatic fat accumulation, body weight and visceral fat-pad weight in HFD-fed mice compared to those of the control group. Although food intake was significantly decreased in the mice fed HFD and HFD+W, compared to control, the energy intake was still significantly higher in the latter two groups due to the nature of the high caloric intakes (Table 1). The addition of walnut significantly prevented hepatic TG accumulation despite the fact that it neither improved the body weight nor the relative liver weight. The lack of the effect of walnut on the body weight could be due to the high caloric intake when combining both HFD (45% fat-derived calories) and walnut (21.5% total energy with 18.9% fat-derived calories). This might explain the similar weight gains in both HFD and HFD+W groups likely resulting from both HFD and energy intake in our model. The HFD-induced TG accumulation was moderate in our model partly due to the relatively short duration of HFD exposure. This 6-week feeding time was intentionally chosen to evaluate and monitor the preventive role of walnuts in the early signaling events (e.g., hepatic fat accumulation, increased oxidative stress, and activation of the cell death signaling pathways, leading to hepatocellar apoptosis) in the development and progression of NAFLD. Since the body weight gains were comparable in both HFD and HFD+W groups and the fat accumulation in the liver was not massive, it is conceivable that the relative liver weight was also comparable between the two groups, despite the lower tendency in the HFD+W compared to the HFD alone.
Hepatic steatosis, which is considered the first hit in “the two-hit” hypothesis for the development and/or progression of NAFLD in response to HFD, might be mediated via several pathways [4], including oxidative stress. “The two-hit” model represents a well-established and widely-accepted model for the pathophysiology of NAFLD, based on numerous reports from many laboratories, although additional studies are still needed to fully characterize and understand this disease. In addition, hyperleptinemia is one of the best known and early markers for fatty liver and obesity [45]. Recent reports described that the positive correlation between leptin and hepatic steatosis can be reversed by anti-leptin therapies [46–48]. More importantly, leptin has been reported to be a key to peroxynitrite-mediated oxidative stress in experimental non-alcoholic steatohepatitis [48]. In agreement with these reports [45–48], our results showed that HFD increased leptin levels while walnut supplementation significantly decreased the leptin level (Fig. 1). SIRT1, a mammalian ortholog of Sir2 (silent information regulator 2) and a NAD+-dependent deacetylase, has been implicated in the molecular control of aging and cell proliferation, and more recently has also been involved in protection from metabolic syndrome [49, 50]. Manipulation of SIRT1 levels in the liver has been reported to affect the expression of a number of genes involved in glucose and lipid metabolism [51]. Additionally, recent studies demonstrated that modest overexpression of SIRT1 resulted in a protective effect against high-fat induced hepatic steatosis and glucose intolerance [50, 52]. An earlier report demonstrated that hepatic SIRT1 mediates a fine balance between energy influx and energy expenditure in the liver [53]. Furthermore, SIRT1 overexpression reduces the level of oxygen consumption linked to the generation of ROS, whose levels correlate with the degree of NAFLD [54, 55]. Another player in the SIRT1 activation pathway is AMPK. In human hepatoma cells, AMPK activation through SIRT1 decreased glucose-induced FAS and triglyceride accumulation [56]. Overexpression of SIRT1 increased, while shRNA silencing of SIRT1 dramatically decreased AMPK and glucose-stimulated triglyceride accumulation [37]. These results suggest an important role of AMPK-SIRT1 axis in hepatic lipid metabolism. Furthermore, liver-specific SIRT1-knockout mice developed hepatic steatosis and inflammation [53]. Taken together, these reports [37, 49–56] support the role of SIRT1 and AMPK in suppressing lipogenic pathways in the acute regulation of energy balance in the liver. Because FAS catalyzes the last step in the fatty acid biosynthetic pathway, it is believed to be a determinant of the maximal capacity of a tissue, and liver in particular, to synthesize fatty acids by de novo lipogenesis. Chakravarthy et al. [57] showed that liver-specific deletion of mouse FAS gene results in mutant mice that possess a similar phenotype to control animals when fed normal chow. Similar results of a positive relationship between FAS and hepatic fat accumulation have been reported by Ito et al. [58] in different rodent NASH models. Consistently, Mitsuyoshi et al. [59] also reported increased levels of FAS mRNA expression in human steatotic liver tissues. Thus, it is reasonable to assume that the walnut-mediated inhibition of hepatic steatosis in this study may result from, at least partially, preventing hyperleptinemia (Fig. 1) and normalizing the levels of SIRT-1, p-AMPK, and FAS, as comparable to those of the control group (Fig. 2). In addition, walnut supplementation prevented the HFD-mediated alteration of the cell-death-related signaling pathway proteins such as p-JNK, p-38K, p-BCL2 and PARP-1 as well as hepatocellular apoptosis (Figs. 6 and 7).
Oxidative stress has been shown to play a causal role in the development and/or progression of NAFLD as one of the second hits of the “two-hit” hypothesis [60] (Fig. 8). In the present study, the both cytosolic fraction and mitochondria of liver tissues of walnut-fed mice showed significantly decreased CYP2E1 protein levels in HFD-fed mice. In agreement, CYP2E1 was reported to play a critical role in the development of HFD-mediated NAFLD, as demonstrated with the WT and Cyp2e1-null mice [33, 61, 62]. Conversely, transgenic mice containing over-expressed CYP2E1 also exhibited elevated levels of insulin resistance, hepatic fat accumulation and histological liver injury than the WT mice, when these mice were chronically fed with a moderate-fat diet (20% fat-derived energy), further supporting the important function of CYP2E1 in promoting fat-mediated NAFLD/NASH [63]. Furthermore, there is accumulating evidence that HFD-mediated upregulation of CYP2E1 may initiate lipid peroxidation by the production of ROS [33]. In view of the cytotoxic effect of ROS and lipid peroxides [64, 65] and the capacity of CYP2E1 to generate such reactive intermediates, it is likely that this enzyme plays a key role in the pathogenesis of liver injury [65–67]. HFD feeding is likely to increase CYP2E1 levels in response to elevated liver concentrations of ketones and fatty acids that serve as inducers and substrates of P450 enzymes [9]. Importantly, hepatic CYP2E1 levels are increased in NASH patients [68]. These results are in agreement with our study since cytosolic lipid peroxidation was also increased in the HFD-fed mice relative to control group, while the oxidative stress in HFD+W fed mice was similar to that observed in control group. Human livers with NASH have increased levels of lipid peroxidation and its by-products, providing further evidence of an increased oxidative stress in this condition [12]. ROS are relatively short-lived molecules that exert local effects [69]. However, they can attack polyunsaturated fatty acids (PUFAs) and initiate lipid peroxidation within the cell, leading to the formation of aldehyde by-products such as 4-HNE and MDA [12]. These molecules have longer half-lives than ROS and can potentially diffuse from their sites of origin to reach distant intracellular and extracellular targets, thereby amplifying the effects of oxidative stress. The 4-HNE and MDA can be produced only through the peroxidation of PUFAs, which are preferentially oxidized owing to elevated electron density and decreased carbon-hydrogen bond length in double bonds between unsaturated carbon pairs [70]. Further, ROS may oxidize fat deposits, releasing lipid peroxidation products that damage mitochondrial DNA and proteins to partially block the normal flow of electrons along the respiratory chain, which might also ultimately increase ROS levels via elevating mitochondrial ROS production.
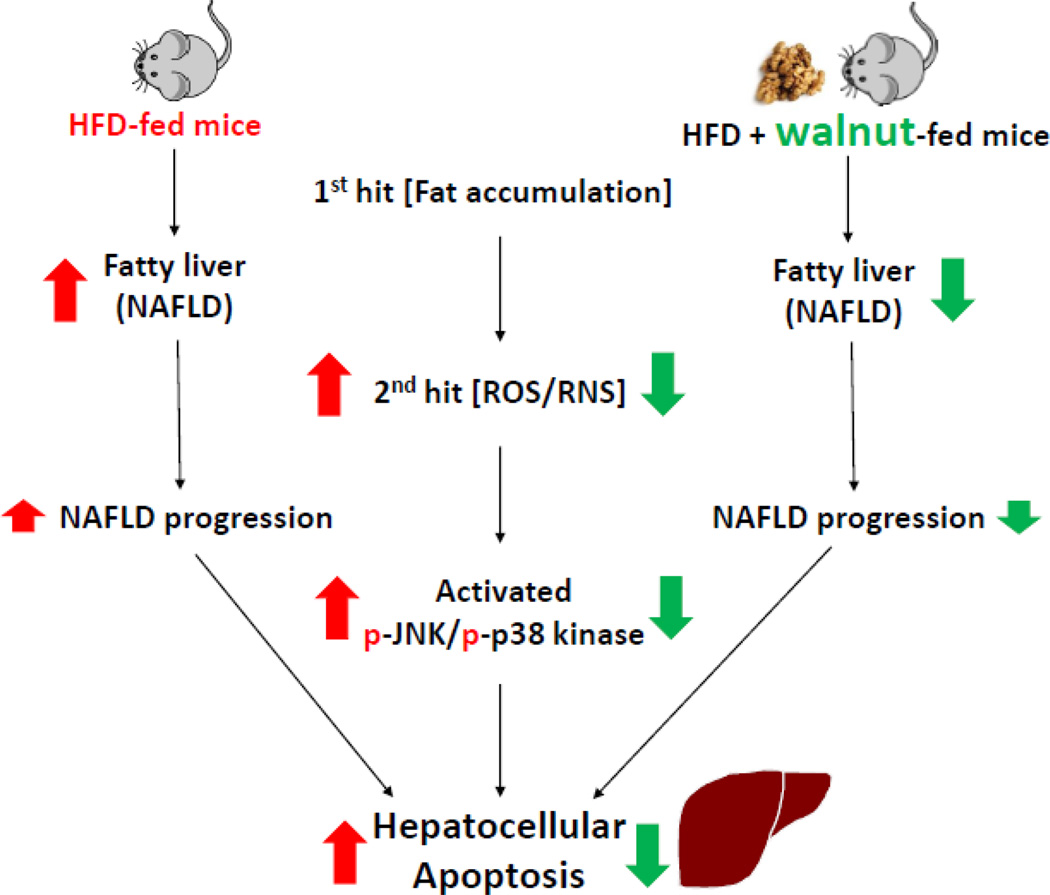
Figure 8.
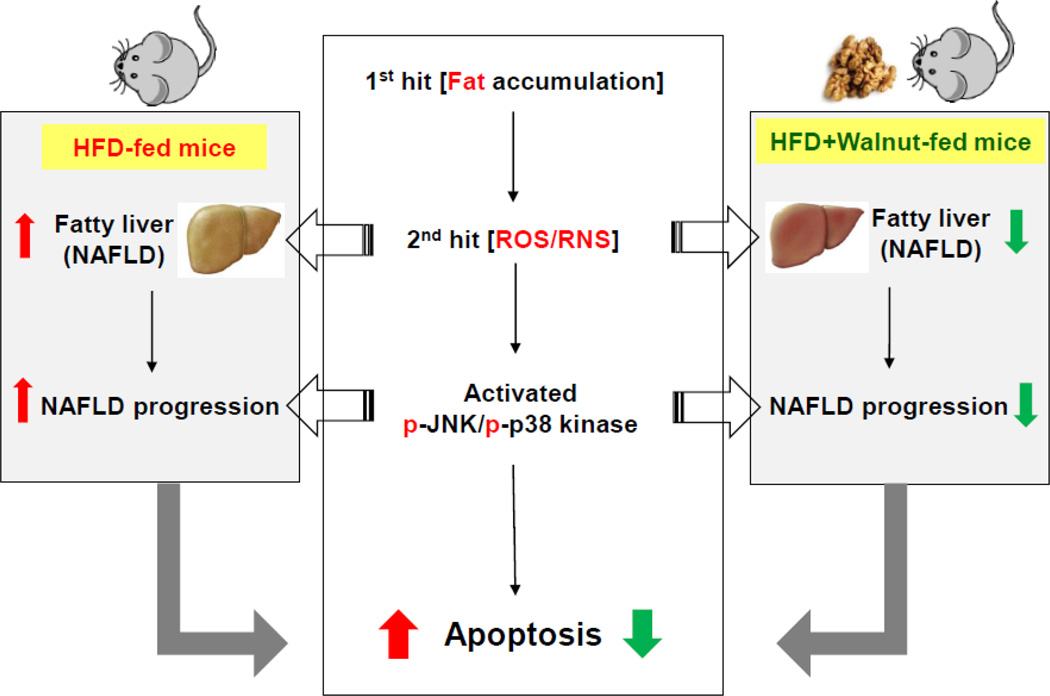
Schematic representation for the protective effects of dietary walnuts against hepatocellular apoptosis in HFD-fed mice.
Nitric oxide (NO) is now known to control mitochondrial respiration [71–73], insulin signaling [74], oxidative stress [75]. NO and iNOS have been reported to play a pivotal role in the development of various types of liver disease such as alcoholic cirrhosis [76], primary biliary cirrhosis, autoimmune hepatitis [77], and especially NASH [78] in humans. Furthermore, oxidative stress seems to be the most important candidate proposed so far where NO and nitrotyrosine (3-NT), which are strongly associated with oxidative stress, may be indicated to progress NASH as the second hitter. Peroxynitrite, derived from the combination of superoxide (may be originated by CYP2E1) and NO, immediately attacks tyrosine residues of many cellular proteins to form nitrotyrosine, which in turn causes dysfunction and degradation of many functional proteins in the body [79]. Thus, nitrotyrosine formation is considered a consequence of oxidative stress. There are numerous reports in the literature showing that increased levels of iNOS, CYP2E1, and protein nitration play an important role, at least partially, in the development and/or progression of NAFLD, as shown in various experimental models and people with NASH [33, 68, 80]. Peroxynitrite and protein nitration also play a central role in the suppression of the mitochondrial respiratory chain activity, leading to mitochondrial dysfunction in a NASH model using ob/ob mice while these effects were ameliorated by melatonin administration [81]. In addition, elevated levels of peroxynitrite were observed in the liver of individuals with NASH and ob/ob mice. For instance, Sanyal et al. [7] found that there was considerable staining for 3-NT in individuals with fatty liver or NASH. Laurent et al. [82] showed that the concentrations of nitrites and nitrates were significantly increased in liver homogenates of ob/ob mice, and it has been demonstrated that iNOS protein expression is upregulated in the liver of ob/ob mice [83]. These results are in accordance with our data where the upregulated mitochondrial, but not cytosolic, iNOS in response to HFD was reversed by the walnut supplementation. In addition, both upregulated cytosolic and mitochondrial nitrated proteins in HFD-fed mice were also prevented by the walnut addition. However, it seems that the major protective role of walnuts against the development of HFD-induced nitroxidative stress seems to stem from its inhibitory effect of both cytosolic and mitochondrial CYP2E1, which can be also involved in protein nitration [84] and the development of NAFLD [32, 58].
Oxidant-induced apoptosis is not the simple result of the biochemical interactions of ROS and cellular macromolecules but rather is actively regulated through the cell signaling cascades [14]. For instance, oxidative stress partly activates mitochondrial dysfunction and cell-death associated MAPK pathways, in particular the p38K and JNK pathway [42, 85]. This condition occurs when the cellular ROS levels exceed the capacity of antioxidant defense. ROS formation and accumulation above a certain threshold can induce cell damage, which is a common mechanism for liver injury [86]. It has been clearly demonstrated that a high rate of hepatocyte apoptosis was developed in a NASH rat model induced by a HFD. Our observation is in agreement with findings from both human studies of NASH patients [22, 87] and an animal model of NASH [88]. These results suggest that increased hepatocyte apoptosis could contribute to NASH pathogenesis. Moreover, the biological importance of HFD-induced hepatocyte apoptosis in this model is more appropriate, because the rodents consumed the diets ad libitum, similar to human eating patterns, rather than being force-fed. The higher apoptotic hepatocytes in HFD-induced NASH were associated with high oxidative stress, as reflected by significantly elevated MDA and 4-HNE levels, indicating a potential major contribution to hepatocyte apoptosis from increased oxidative stress. In addition, this increased oxidative stress associated with high apoptosis could, at least partially, result from the induction of CYP2E1 by HFD feeding. CYP2E1 has been shown to be invariably elevated and responsible for generation of free radicals in the liver of NASH patients [89, 90]. Many previous studies have demonstrated that JNK and p38K activation contributes to stress-induced apoptosis in several cell types, including hepatocytes [26, 91]. Our results of higher phosphorylated JNK and p38K indicated that JNK and p38K could be involved in HFD-induced NAFLD pathogenesis. All these observations suggest an essential role of JNK and p38K in the development of NASH. Current concepts suggest that a high rate of hepatocyte apoptosis in steatohepatitis (NASH) patients, with the magnitude of apoptosis correlating with hepatic inflammation instead of simple steatosis. This implies that apoptosis could be involved in NASH progression [22, 87], possibly through stimulating other liver cells such as macrophages and stellate cells along with increased infiltration of immune cells for inflammatory liver disease. Moreover, activation (phosphorylation) of JNK and p38K (Fig. 6), that can be stimulated by ROS and blocked by antioxidants [92], could also contribute to cell death [26]. The hepatocellular apoptosis in HFD-fed mice and its prevention by walnut supplementation were further supported by TUNEL assay results with increased levels of apoptotic proteins PARP-1 and p-BCL2 (Fig. 7).
In summary, these results implicate that the protective effects of walnut against the development and/or the progression of NAFLD are mediated, at least partially, by attenuating the widely-accepted mechanisms of NAFLD namely hepatic steatosis (1st hit) and oxidative stress (2nd hit), leading to inhibition of the cell death signal pathways in HFD-fed obese mice. Potential hepatoprotective mechanisms of walnut against NAFLD are summarized in Fig. 8. The inhibition of oxidative stress may either directly result from the antioxidant ingredients of walnuts or the prevention of steatosis which primes the liver for increased oxidative liver damage usually observed in later times. However, more work is still warranted to fully characterize the protective mechanism(s) of walnut.
Supplementary Material
Acknowledgments
This work was supported by the Intramural Research Program of National Institute on Alcohol Abuse and Alcoholism and a grant to Youngshim Choi from the KRIBB Research Initiative Program (Korean Biomedical Scientist Fellowship Program), Korea Research Institute of Bioscience and Biotechnology, Republic of Korea. We were thankful to Dr. Klaus Gawrisch for supporting this study. We also appreciated Dr. Carol Sloan at California Walnut Commission for providing fresh walnuts used for our experiments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology. 2012;142:1592–1609. doi: 10.1053/j.gastro.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 2.Anania FA. Non-alcoholic fatty liver disease and fructose: bad for us, better for mice. Journal of hepatology. 2011;55:218–220. doi: 10.1016/j.jhep.2011.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lim JS, Mietus-Snyder M, Valente A, Schwarz JM, Lustig RH. The role of fructose in the pathogenesis of NAFLD and the metabolic syndrome. Nature reviews Gastroenterology & hepatology. 2010;7:251–264. doi: 10.1038/nrgastro.2010.41. [DOI] [PubMed] [Google Scholar]
- 4.Day CP, James OF. Steatohepatitis: a tale of two "hits"? Gastroenterology. 1998;114:842–845. doi: 10.1016/s0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- 5.Salt WB., 2nd Nonalcoholic fatty liver disease (NAFLD): a comprehensive review. Journal of insurance medicine. 2004;36:27–41. [PubMed] [Google Scholar]
- 6.Allard JP, Aghdassi E, Mohammed S, Raman M, Avand G, Arendt BM, et al. Nutritional assessment and hepatic fatty acid composition in non-alcoholic fatty liver disease (NAFLD): a cross-sectional study. Journal of hepatology. 2008;48:300–307. doi: 10.1016/j.jhep.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 7.Sanyal AJ, Campbell-Sargent C, Mirshahi F, Rizzo WB, Contos MJ, Sterling RK, et al. Nonalcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities. Gastroenterology. 2001;120:1183–1192. doi: 10.1053/gast.2001.23256. [DOI] [PubMed] [Google Scholar]
- 8.Araya J, Rodrigo R, Videla LA, Thielemann L, Orellana M, Pettinelli P, et al. Increase in long-chain polyunsaturated fatty acid n - 6/n - 3 ratio in relation to hepatic steatosis in patients with non-alcoholic fatty liver disease. Clinical science. 2004;106:635–643. doi: 10.1042/CS20030326. [DOI] [PubMed] [Google Scholar]
- 9.Lieber CS. CYP2E1: from ASH to NASH. Hepatology research : the official journal of the Japan Society of Hepatology. 2004;28:1–11. doi: 10.1016/j.hepres.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 10.Hensley K, Kotake Y, Sang H, Pye QN, Wallis GL, Kolker LM, et al. Dietary choline restriction causes complex I dysfunction and increased H(2)O(2) generation in liver mitochondria. Carcinogenesis. 2000;21:983–989. doi: 10.1093/carcin/21.5.983. [DOI] [PubMed] [Google Scholar]
- 11.Yang S, Zhu H, Li Y, Lin H, Gabrielson K, Trush MA, et al. Mitochondrial adaptations to obesity-related oxidant stress. Archives of biochemistry and biophysics. 2000;378:259–268. doi: 10.1006/abbi.2000.1829. [DOI] [PubMed] [Google Scholar]
- 12.Seki S, Kitada T, Yamada T, Sakaguchi H, Nakatani K, Wakasa K. In situ detection of lipid peroxidation and oxidative DNA damage in non-alcoholic fatty liver diseases. Journal of hepatology. 2002;37:56–62. doi: 10.1016/s0168-8278(02)00073-9. [DOI] [PubMed] [Google Scholar]
- 13.Mantena SK, King AL, Andringa KK, Eccleston HB, Bailey SM. Mitochondrial dysfunction and oxidative stress in the pathogenesis of alcohol- and obesity-induced fatty liver diseases. Free radical biology & medicine. 2008;44:1259–1272. doi: 10.1016/j.freeradbiomed.2007.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singh R, Czaja MJ. Regulation of hepatocyte apoptosis by oxidative stress. Journal of gastroenterology and hepatology. 2007;22(Suppl 1):S45–S48. doi: 10.1111/j.1440-1746.2006.04646.x. [DOI] [PubMed] [Google Scholar]
- 15.Curzio M, Esterbauer H, Dianzani MU. Chemotactic activity of hydroxyalkenals on rat neutrophils. International journal of tissue reactions. 1985;7:137–142. [PubMed] [Google Scholar]
- 16.Lee KS, Buck M, Houglum K, Chojkier M. Activation of hepatic stellate cells by TGF alpha and collagen type I is mediated by oxidative stress through c-myb expression. The Journal of clinical investigation. 1995;96:2461–2468. doi: 10.1172/JCI118304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kalinowski L, Dobrucki IT, Malinski T. Race-specific differences in endothelial function: predisposition of African Americans to vascular diseases. Circulation. 2004;109:2511–2517. doi: 10.1161/01.CIR.0000129087.81352.7A. [DOI] [PubMed] [Google Scholar]
- 18.Wedman J, Balm AJ, Hart AA, Loftus BM, Hilgers FJ, Gregor RT, et al. Value of resection of pulmonary metastases in head and neck cancer patients. Head & neck. 1996;18:311–316. doi: 10.1002/(SICI)1097-0347(199607/08)18:4<311::AID-HED1>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 19.Hara MR, Agrawal N, Kim SF, Cascio MB, Fujimuro M, Ozeki Y, et al. S-nitrosylated GAPDH initiates apoptotic cell death by nuclear translocation following Siah1 binding. Nature cell biology. 2005;7:665–674. doi: 10.1038/ncb1268. [DOI] [PubMed] [Google Scholar]
- 20.Czaja MJ. Induction and regulation of hepatocyte apoptosis by oxidative stress. Antioxidants & redox signaling. 2002;4:759–767. doi: 10.1089/152308602760598909. [DOI] [PubMed] [Google Scholar]
- 21.Rosseland CM, Wierod L, Oksvold MP, Werner H, Ostvold AC, Thoresen GH, et al. Cytoplasmic retention of peroxide-activated ERK provides survival in primary cultures of rat hepatocytes. Hepatology. 2005;42:200–207. doi: 10.1002/hep.20762. [DOI] [PubMed] [Google Scholar]
- 22.Feldstein AE, Canbay A, Angulo P, Taniai M, Burgart LJ, Lindor KD, et al. Hepatocyte apoptosis and fas expression are prominent features of human nonalcoholic steatohepatitis. Gastroenterology. 2003;125:437–443. doi: 10.1016/s0016-5085(03)00907-7. [DOI] [PubMed] [Google Scholar]
- 23.Wang Y, Ausman LM, Russell RM, Greenberg AS, Wang XD. Increased apoptosis in high-fat diet-induced nonalcoholic steatohepatitis in rats is associated with c-Jun NH2-terminal kinase activation and elevated proapoptotic Bax. The Journal of nutrition. 2008;138:1866–1871. doi: 10.1093/jn/138.10.1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martindale JL, Holbrook NJ. Cellular response to oxidative stress: signaling for suicide and survival. Journal of cellular physiology. 2002;192:1–15. doi: 10.1002/jcp.10119. [DOI] [PubMed] [Google Scholar]
- 25.Raman M, Chen W, Cobb MH. Differential regulation and properties of MAPKs. Oncogene. 2007;26:3100–3112. doi: 10.1038/sj.onc.1210392. [DOI] [PubMed] [Google Scholar]
- 26.Abdelmegeed MA, Yoo SH, Henderson LE, Gonzalez FJ, Woodcroft KJ, Song BJ. PPARalpha expression protects male mice from high fat-induced nonalcoholic fatty liver. The Journal of nutrition. 2011;141:603–610. doi: 10.3945/jn.110.135210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakamura A, Terauchi Y. Lessons from mouse models of high-fat diet-induced NAFLD. International journal of molecular sciences. 2013;14:21240–21257. doi: 10.3390/ijms141121240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berryman CE, Grieger JA, West SG, Chen CY, Blumberg JB, Rothblat GH, et al. Acute consumption of walnuts and walnut components differentially affect postprandial lipemia, endothelial function, oxidative stress, and cholesterol efflux in humans with mild hypercholesterolemia. The Journal of nutrition. 2013;143:788–794. doi: 10.3945/jn.112.170993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mullner E, Brath H, Pleifer S, Schiermayr C, Baierl A, Wallner M, et al. Vegetables and PUFA-rich plant oil reduce DNA strand breaks in individuals with type 2 diabetes. Molecular nutrition & food research. 2013;57:328–338. doi: 10.1002/mnfr.201200343. [DOI] [PubMed] [Google Scholar]
- 30.Fink A, Rufer CE, Le Grandois J, Roth A, Aoude-Werner D, Marchioni E, et al. Dietary walnut oil modulates liver steatosis in the obese Zucker rat. European journal of nutrition. 2014;53:645–660. doi: 10.1007/s00394-013-0573-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Youngshim Choi MAA, Akbar Mohammed, Song Byoung-Joon. Dietary walnut reduces hepatic triglyceride content in high-fat-fed mice via modulation of hepatic fatty acid metabolism and adipose tissue inflammation. The Journal of nutritional biochemistry. 2016;30:116–125. doi: 10.1016/j.jnutbio.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 33.Abdelmegeed MA, Banerjee A, Yoo SH, Jang S, Gonzalez FJ, Song BJ. Critical role of cytochrome P450 2E1 (CYP2E1) in the development of high fat-induced non-alcoholic steatohepatitis. Journal of hepatology. 2012;57:860–866. doi: 10.1016/j.jhep.2012.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moon KH, Hood BL, Mukhopadhyay P, Rajesh M, Abdelmegeed MA, Kwon YI, et al. Oxidative inactivation of key mitochondrial proteins leads to dysfunction and injury in hepatic ischemia reperfusion. Gastroenterology. 2008;135:1344–1357. doi: 10.1053/j.gastro.2008.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moon KH, Hood BL, Kim BJ, Hardwick JP, Conrads TP, Veenstra TD, et al. Inactivation of oxidized and S-nitrosylated mitochondrial proteins in alcoholic fatty liver of rats. Hepatology. 2006;44:1218–1230. doi: 10.1002/hep.21372. [DOI] [PubMed] [Google Scholar]
- 36.Abdelmegeed MA, Choi Y, Ha SK, Song BJ. Cytochrome P450-2E1 promotes aging-related hepatic steatosis, apoptosis and fibrosis through increased nitroxidative stress. Free radical biology & medicine. 2015;91:188–202. doi: 10.1016/j.freeradbiomed.2015.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hou X, Xu S, Maitland-Toolan KA, Sato K, Jiang B, Ido Y, et al. SIRT1 regulates hepatocyte lipid metabolism through activating AMP-activated protein kinase. The Journal of biological chemistry. 2008;283:20015–20026. doi: 10.1074/jbc.M802187200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Choi Y, Yanagawa Y, Kim S, Park T. Involvement of SIRT1-AMPK signaling in the protective action of indole-3-carbinol against hepatic steatosis in mice fed a high-fat diet. The Journal of nutritional biochemistry. 2013;24:1393–1400. doi: 10.1016/j.jnutbio.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 39.Caro AA, Cederbaum AI. Oxidative stress, toxicology, and pharmacology of CYP2E1. Annual review of pharmacology and toxicology. 2004;44:27–42. doi: 10.1146/annurev.pharmtox.44.101802.121704. [DOI] [PubMed] [Google Scholar]
- 40.Franco MC, Ye Y, Refakis CA, Feldman JL, Stokes AL, Basso M, et al. Nitration of Hsp90 induces cell death. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:E1102–E1111. doi: 10.1073/pnas.1215177110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim BJ, Ryu SW, Song BJ. JNK- and p38 kinase-mediated phosphorylation of Bax leads to its activation and mitochondrial translocation and to apoptosis of human hepatoma HepG2 cells. The Journal of biological chemistry. 2006;281:21256–21265. doi: 10.1074/jbc.M510644200. [DOI] [PubMed] [Google Scholar]
- 42.Song BJ, Akbar M, Abdelmegeed MA, Byun K, Lee B, Yoon SK, et al. Mitochondrial dysfunction and tissue injury by alcohol, high fat nonalcoholic substances and pathological conditions through post-translational protein modifications. Redox biology. 2014;3:109–123. doi: 10.1016/j.redox.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Upreti VV, Moon KH, Yu LR, Lee IJ, Eddington ND, Ye X, et al. Increased oxidative-modifications of cytosolic proteins in 3,4-methylenedioxymethamphetamine (MDMA, ecstasy)-exposed rat liver. Proteomics. 2011;11:202–211. doi: 10.1002/pmic.201000203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nikoletopoulou V, Markaki M, Palikaras K, Tavernarakis N. Crosstalk between apoptosis, necrosis and autophagy. Biochimica et biophysica acta. 2013;1833:3448–3459. doi: 10.1016/j.bbamcr.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 45.Unger RH, Orci L. Diseases of liporegulation: new perspective on obesity and related disorders. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2001;15:312–321. doi: 10.1096/fj.00-0590. [DOI] [PubMed] [Google Scholar]
- 46.Oral EA, Simha V, Ruiz E, Andewelt A, Premkumar A, Snell P, et al. Leptin-replacement therapy for lipodystrophy. The New England journal of medicine. 2002;346:570–578. doi: 10.1056/NEJMoa012437. [DOI] [PubMed] [Google Scholar]
- 47.Das S, Kumar A, Seth RK, Tokar EJ, Kadiiska MB, Waalkes MP, et al. Proinflammatory adipokine leptin mediates disinfection byproduct bromodichloromethane-induced early steatohepatitic injury in obesity. Toxicology and applied pharmacology. 2013;269:297–306. doi: 10.1016/j.taap.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chatterjee S, Ganini D, Tokar EJ, Kumar A, Das S, Corbett J, et al. Leptin is key to peroxynitrite-mediated oxidative stress and Kupffer cell activation in experimental non-alcoholic steatohepatitis. Journal of hepatology. 2013;58:778–784. doi: 10.1016/j.jhep.2012.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guarente L. Sirtuins as potential targets for metabolic syndrome. Nature. 2006;444:868–874. doi: 10.1038/nature05486. [DOI] [PubMed] [Google Scholar]
- 50.Pfluger PT, Herranz D, Velasco-Miguel S, Serrano M, Tschop MH. Sirt1 protects against high-fat diet-induced metabolic damage. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:9793–9798. doi: 10.1073/pnas.0802917105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rodgers JT, Puigserver P. Fasting-dependent glucose and lipid metabolic response through hepatic sirtuin 1. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:12861–12866. doi: 10.1073/pnas.0702509104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Banks AS, Kon N, Knight C, Matsumoto M, Gutierrez-Juarez R, Rossetti L, et al. SirT1 gain of function increases energy efficiency and prevents diabetes in mice. Cell metabolism. 2008;8:333–341. doi: 10.1016/j.cmet.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Purushotham A, Schug TT, Xu Q, Surapureddi S, Guo X, Li X. Hepatocyte-specific deletion of SIRT1 alters fatty acid metabolism and results in hepatic steatosis and inflammation. Cell metabolism. 2009;9:327–338. doi: 10.1016/j.cmet.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nemoto S, Fergusson MM, Finkel T. SIRT1 functionally interacts with the metabolic regulator and transcriptional coactivator PGC-1{alpha} The Journal of biological chemistry. 2005;280:16456–16460. doi: 10.1074/jbc.M501485200. [DOI] [PubMed] [Google Scholar]
- 55.Oliveira CP, Coelho AM, Barbeiro HV, Lima VM, Soriano F, Ribeiro C, et al. Liver mitochondrial dysfunction and oxidative stress in the pathogenesis of experimental nonalcoholic fatty liver disease. Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas / Sociedade Brasileira de Biofisica [et al] 2006;39:189–194. doi: 10.1590/s0100-879x2006000200004. [DOI] [PubMed] [Google Scholar]
- 56.Imai K, Inukai K, Ikegami Y, Awata T, Katayama S. LKB1, an upstream AMPK kinase, regulates glucose and lipid metabolism in cultured liver and muscle cells. Biochemical and biophysical research communications. 2006;351:595–601. doi: 10.1016/j.bbrc.2006.10.056. [DOI] [PubMed] [Google Scholar]
- 57.Chakravarthy MV, Pan Z, Zhu Y, Tordjman K, Schneider JG, Coleman T, et al. "New" hepatic fat activates PPARalpha to maintain glucose, lipid, and cholesterol homeostasis. Cell metabolism. 2005;1:309–322. doi: 10.1016/j.cmet.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 58.Ito M, Suzuki J, Tsujioka S, Sasaki M, Gomori A, Shirakura T, et al. Longitudinal analysis of murine steatohepatitis model induced by chronic exposure to high-fat diet. Hepatology research : the official journal of the Japan Society of Hepatology. 2007;37:50–57. doi: 10.1111/j.1872-034X.2007.00008.x. [DOI] [PubMed] [Google Scholar]
- 59.Mitsuyoshi H, Yasui K, Harano Y, Endo M, Tsuji K, Minami M, et al. Analysis of hepatic genes involved in the metabolism of fatty acids and iron in nonalcoholic fatty liver disease. Hepatology research : the official journal of the Japan Society of Hepatology. 2009;39:366–373. doi: 10.1111/j.1872-034X.2008.00464.x. [DOI] [PubMed] [Google Scholar]
- 60.Matsuzawa N, Takamura T, Kurita S, Misu H, Ota T, Ando H, et al. Lipid-induced oxidative stress causes steatohepatitis in mice fed an atherogenic diet. Hepatology. 2007;46:1392–1403. doi: 10.1002/hep.21874. [DOI] [PubMed] [Google Scholar]
- 61.Aubert J, Begriche K, Knockaert L, Robin MA, Fromenty B. Increased expression of cytochrome P450 2E1 in nonalcoholic fatty liver disease: mechanisms and pathophysiological role. Clinics and research in hepatology and gastroenterology. 2011;35:630–637. doi: 10.1016/j.clinre.2011.04.015. [DOI] [PubMed] [Google Scholar]
- 62.Zong H, Armoni M, Harel C, Karnieli E, Pessin JE. Cytochrome P-450 CYP2E1 knockout mice are protected against high-fat diet-induced obesity and insulin resistance. American journal of physiology Endocrinology and metabolism. 2012;302:E532–E539. doi: 10.1152/ajpendo.00258.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kathirvel E, Morgan K, French SW, Morgan TR. Overexpression of liver-specific cytochrome P4502E1 impairs hepatic insulin signaling in a transgenic mouse model of nonalcoholic fatty liver disease. European journal of gastroenterology & hepatology. 2009;21:973–983. doi: 10.1097/MEG.0b013e328328f461. [DOI] [PubMed] [Google Scholar]
- 64.Curzio M, Esterbauer H, Poli G, Biasi F, Cecchini G, Di Mauro C, et al. Possible role of aldehydic lipid peroxidation products as chemoattractants. International journal of tissue reactions. 1987;9:295–306. [PubMed] [Google Scholar]
- 65.Gorsky LD, Koop DR, Coon MJ. On the stoichiometry of the oxidase and monooxygenase reactions catalyzed by liver microsomal cytochrome P-450. Products of oxygen reduction. The Journal of biological chemistry. 1984;259:6812–6817. [PubMed] [Google Scholar]
- 66.Ekstrom G, Ingelman-Sundberg M. Rat liver microsomal NADPH-supported oxidase activity and lipid peroxidation dependent on ethanol-inducible cytochrome P-450 (P-450IIE1) Biochemical pharmacology. 1989;38:1313–1319. doi: 10.1016/0006-2952(89)90338-9. [DOI] [PubMed] [Google Scholar]
- 67.Dai Y, Rashba-Step J, Cederbaum AI. Stable expression of human cytochrome P4502E1 in HepG2 cells: characterization of catalytic activities and production of reactive oxygen intermediates. Biochemistry. 1993;32:6928–6937. doi: 10.1021/bi00078a017. [DOI] [PubMed] [Google Scholar]
- 68.Chalasani N, Gorski JC, Asghar MS, Asghar A, Foresman B, Hall SD, et al. Hepatic cytochrome P450 2E1 activity in nondiabetic patients with nonalcoholic steatohepatitis. Hepatology. 2003;37:544–550. doi: 10.1053/jhep.2003.50095. [DOI] [PubMed] [Google Scholar]
- 69.Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free radical biology & medicine. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 70.Gardner HW. Oxygen radical chemistry of polyunsaturated fatty acids. Free radical biology & medicine. 1989;7:65–86. doi: 10.1016/0891-5849(89)90102-0. [DOI] [PubMed] [Google Scholar]
- 71.Begriche K, Igoudjil A, Pessayre D, Fromenty B. Mitochondrial dysfunction in NASH: causes, consequences and possible means to prevent it. Mitochondrion. 2006;6:1–28. doi: 10.1016/j.mito.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 72.Fromenty B, Robin MA, Igoudjil A, Mansouri A, Pessayre D. The ins and outs of mitochondrial dysfunction in NASH. Diabetes & metabolism. 2004;30:121–138. doi: 10.1016/s1262-3636(07)70098-8. [DOI] [PubMed] [Google Scholar]
- 73.Perez-Carreras M, Del Hoyo P, Martin MA, Rubio JC, Martin A, Castellano G, et al. Defective hepatic mitochondrial respiratory chain in patients with nonalcoholic steatohepatitis. Hepatology. 2003;38:999–1007. doi: 10.1053/jhep.2003.50398. [DOI] [PubMed] [Google Scholar]
- 74.Choudhury J, Sanyal AJ. Insulin resistance in NASH. Frontiers in bioscience : a journal and virtual library. 2005;10:1520–1533. doi: 10.2741/1636. [DOI] [PubMed] [Google Scholar]
- 75.Sakaida I, Okita K. The role of oxidative stress in NASH and fatty liver model. Hepatology research : the official journal of the Japan Society of Hepatology. 2005;33:128–131. doi: 10.1016/j.hepres.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 76.McNaughton L, Puttagunta L, Martinez-Cuesta MA, Kneteman N, Mayers I, Moqbel R, et al. Distribution of nitric oxide synthase in normal and cirrhotic human liver. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:17161–17166. doi: 10.1073/pnas.0134112100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sanz-Cameno P, Medina J, Garcia-Buey L, Garcia-Sanchez A, Borque MJ, Martin-Vilchez S, et al. Enhanced intrahepatic inducible nitric oxide synthase expression and nitrotyrosine accumulation in primary biliary cirrhosis and autoimmune hepatitis. Journal of hepatology. 2002;37:723–729. doi: 10.1016/s0168-8278(02)00266-0. [DOI] [PubMed] [Google Scholar]
- 78.Wei CL, Hon WM, Lee KH, Khoo HE. Temporal expression of hepatic inducible nitric oxide synthase in liver cirrhosis. World journal of gastroenterology. 2005;11:362–367. doi: 10.3748/wjg.v11.i3.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Abdelmegeed MA, Song BJ. Functional roles of protein nitration in acute and chronic liver diseases. Oxidative medicine and cellular longevity. 2014;2014:149627. doi: 10.1155/2014/149627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Leung TM, Nieto N. CYP2E1 and oxidant stress in alcoholic and non-alcoholic fatty liver disease. Journal of hepatology. 2013;58:395–398. doi: 10.1016/j.jhep.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 81.Solis-Munoz P, Solis-Herruzo JA, Fernandez-Moreira D, Gomez-Izquierdo E, Garcia-Consuegra I, Munoz-Yague T, et al. Melatonin improves mitochondrial respiratory chain activity and liver morphology in ob/ob mice. Journal of pineal research. 2011;51:113–123. doi: 10.1111/j.1600-079X.2011.00868.x. [DOI] [PubMed] [Google Scholar]
- 82.Laurent A, Nicco C, Tran Van Nhieu J, Borderie D, Chereau C, Conti F, et al. Pivotal role of superoxide anion and beneficial effect of antioxidant molecules in murine steatohepatitis. Hepatology. 2004;39:1277–1285. doi: 10.1002/hep.20177. [DOI] [PubMed] [Google Scholar]
- 83.Garcia-Ruiz I, Rodriguez-Juan C, Diaz-Sanjuan T, del Hoyo P, Colina F, Munoz-Yague T, et al. Uric acid and anti-TNF antibody improve mitochondrial dysfunction in ob/ob mice. Hepatology. 2006;44:581–591. doi: 10.1002/hep.21313. [DOI] [PubMed] [Google Scholar]
- 84.Abdelmegeed MA, Moon KH, Chen C, Gonzalez FJ, Song BJ. Role of cytochrome P450 2E1 in protein nitration and ubiquitin-mediated degradation during acetaminophen toxicity. Biochemical pharmacology. 2010;79:57–66. doi: 10.1016/j.bcp.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- 86.Papa S, Bubici C, Zazzeroni F, Franzoso G. Mechanisms of liver disease: cross-talk between the NF-kappaB and JNK pathways. Biological chemistry. 2009;390:965–976. doi: 10.1515/BC.2009.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ribeiro PS, Cortez-Pinto H, Sola S, Castro RE, Ramalho RM, Baptista A, et al. Hepatocyte apoptosis, expression of death receptors, and activation of NF-kappaB in the liver of nonalcoholic and alcoholic steatohepatitis patients. The American journal of gastroenterology. 2004;99:1708–1717. doi: 10.1111/j.1572-0241.2004.40009.x. [DOI] [PubMed] [Google Scholar]
- 88.Sellmann C, Priebs J, Landmann M, Degen C, Engstler AJ, Jin CJ, et al. Diets rich in fructose, fat or fructose and fat alter intestinal barrier function and lead to the development of nonalcoholic fatty liver disease over time. The Journal of nutritional biochemistry. 2015;26:1183–1192. doi: 10.1016/j.jnutbio.2015.05.011. [DOI] [PubMed] [Google Scholar]
- 89.Gonzalez FJ. Role of cytochromes P450 in chemical toxicity and oxidative stress: studies with CYP2E1. Mutation research. 2005;569:101–110. doi: 10.1016/j.mrfmmm.2004.04.021. [DOI] [PubMed] [Google Scholar]
- 90.Weltman MD, Farrell GC, Hall P, Ingelman-Sundberg M, Liddle C. Hepatic cytochrome P450 2E1 is increased in patients with nonalcoholic steatohepatitis. Hepatology. 1998;27:128–133. doi: 10.1002/hep.510270121. [DOI] [PubMed] [Google Scholar]
- 91.Marderstein EL, Bucher B, Guo Z, Feng X, Reid K, Geller DA. Protection of rat hepatocytes from apoptosis by inhibition of c-Jun N-terminal kinase. Surgery. 2003;134:280–284. doi: 10.1067/msy.2003.237. [DOI] [PubMed] [Google Scholar]
- 92.Seki E, Brenner DA, Karin M. A liver full of JNK: signaling in regulation of cell function and disease pathogenesis, and clinical approaches. Gastroenterology. 2012;143:307–320. doi: 10.1053/j.gastro.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.