Abstract
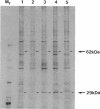
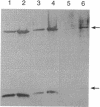
Mutants of CHO-K1 cells with varied levels of A system activity, probably the result of increases in absolute amount of the A system transporter, have corresponding increases in levels of peptides banding at 62-66 and 29 kDa. Mutant alar4-H3.9, showing the highest increase of A system activity and of 62- to 66- and 29-kDa peptides, was selected for this study. The N terminus 16-amino acid sequence of the 62- to 66-kDa peptide(s) of this mutant showed between 80% and 100% identity with the mammalian mitochondrial 60-kDa heat shock protein P1 (hsp60). Two-dimensional gel electrophoresis of the 62- to 66-kDa band showed two major, a minor, and several smaller spots (of same mass but different pI values) for both wild type (WT) and mutant, with the two major spots being of greater density in the mutant. Immunoblots with antibody to P1 identified the two major and minor peptides as P1 related. Two-dimensional gels of whole cell extracts of the WT and alar4-H3.9 confirmed these findings and indicated that the two major bands of the mutant were 2.4 times as abundant as that found for the WT. A plasma membrane fraction of the mutant, exhibiting 4.8 times more A system activity than the WT, contained 3.6 times as much P1 as the WT. Immunoblots with antibodies to P1, mitochondrial malate dehydrogenase, and to the mitochondrial F1/F0-ATPase demonstrated that the increased amount of P1 observed in the mutant was not the result of increases in amount of mitochondrial protein. Northern blot analysis demonstrated that the mutant had 2.5 times as much mRNA for P1 as the WT. The close analogy with the relationship between A system and Na+,K(+)-ATPase suggests that there is a coordinate regulation of the A system of amino acid transport, Na+,K(+)-ATPase, and P1 protein, probably as a result of mutation in a shared regulatory element. The possible role of P1 in A system function is discussed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdel-Ghany M., el-Gendy K., Zhang S., Racker E. Control of src kinase activity by activators, inhibitors, and substrate chaperones. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7061–7065. doi: 10.1073/pnas.87.18.7061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass R., Hedegaard H. B., Dillehay L., Moffett J., Englesberg E. The A, ASC, and L systems for the transport of amino acids in Chinese hamster ovary cells (CHO-K1). J Biol Chem. 1981 Oct 25;256(20):10259–10266. [PubMed] [Google Scholar]
- Brudzynski K., Martinez V., Gupta R. S. Immunocytochemical localization of heat-shock protein 60-related protein in beta-cell secretory granules and its altered distribution in non-obese diabetic mice. Diabetologia. 1992 Apr;35(4):316–324. doi: 10.1007/BF00401198. [DOI] [PubMed] [Google Scholar]
- Cheng M. Y., Hartl F. U., Horwich A. L. The mitochondrial chaperonin hsp60 is required for its own assembly. Nature. 1990 Nov 29;348(6300):455–458. doi: 10.1038/348455a0. [DOI] [PubMed] [Google Scholar]
- Cheng M. Y., Hartl F. U., Martin J., Pollock R. A., Kalousek F., Neupert W., Hallberg E. M., Hallberg R. L., Horwich A. L. Mitochondrial heat-shock protein hsp60 is essential for assembly of proteins imported into yeast mitochondria. Nature. 1989 Feb 16;337(6208):620–625. doi: 10.1038/337620a0. [DOI] [PubMed] [Google Scholar]
- Curriden S., Englesberg E. Inhibition of growth of proline-requiring Chinese hamster ovary cells (CHO-k1) resulting from antagonism by a system amino acids. J Cell Physiol. 1981 Feb;106(2):245–252. doi: 10.1002/jcp.1041060210. [DOI] [PubMed] [Google Scholar]
- Ellis R. J., van der Vies S. M. Molecular chaperones. Annu Rev Biochem. 1991;60:321–347. doi: 10.1146/annurev.bi.60.070191.001541. [DOI] [PubMed] [Google Scholar]
- Fisch P., Malkovsky M., Kovats S., Sturm E., Braakman E., Klein B. S., Voss S. D., Morrissey L. W., DeMars R., Welch W. J. Recognition by human V gamma 9/V delta 2 T cells of a GroEL homolog on Daudi Burkitt's lymphoma cells. Science. 1990 Nov 30;250(4985):1269–1273. doi: 10.1126/science.1978758. [DOI] [PubMed] [Google Scholar]
- Gething M. J., Sambrook J. Protein folding in the cell. Nature. 1992 Jan 2;355(6355):33–45. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- Gupta R. S., Dudani A. K. Mitochondrial binding of a protein affected in mutants resistant to the microtubule inhibitor podophyllotoxin. Eur J Cell Biol. 1987 Oct;44(2):278–285. [PubMed] [Google Scholar]
- Gupta R. S., Ho T. K., Moffat M. R., Gupta R. Podophyllotoxin-resistant mutants of Chinese hamster ovary cells. Alteration in a microtubule-associated protein. J Biol Chem. 1982 Jan 25;257(2):1071–1078. [PubMed] [Google Scholar]
- Gupta R. S. Mitochondria, molecular chaperone proteins and the in vivo assembly of microtubules. Trends Biochem Sci. 1990 Nov;15(11):415–418. doi: 10.1016/0968-0004(90)90276-h. [DOI] [PubMed] [Google Scholar]
- Gupta R. S., Picketts D. J., Ahmad S. A novel ubiquitous protein 'chaperonin' supports the endosymbiotic origin of mitochondrion and plant chloroplast. Biochem Biophys Res Commun. 1989 Sep 15;163(2):780–787. doi: 10.1016/0006-291x(89)92290-0. [DOI] [PubMed] [Google Scholar]
- Gupta R. S., Venner T. J., Chopra A. Genetic and biochemical studies with mutants of mammalian cells affected in microtubule-related proteins other than tubulin: mitochondrial localization of a microtubule-related protein. Can J Biochem Cell Biol. 1985 Jun;63(6):489–502. doi: 10.1139/o85-068. [DOI] [PubMed] [Google Scholar]
- Gupta R. S., Venner T. J., Chopra A. Genetic and biochemical studies with mutants of mammalian cells affected in microtubule-related proteins other than tubulin: mitochondrial localization of a microtubule-related protein. Can J Biochem Cell Biol. 1985 Jun;63(6):489–502. doi: 10.1139/o85-068. [DOI] [PubMed] [Google Scholar]
- Hartl F. U., Martin J., Neupert W. Protein folding in the cell: the role of molecular chaperones Hsp70 and Hsp60. Annu Rev Biophys Biomol Struct. 1992;21:293–322. doi: 10.1146/annurev.bb.21.060192.001453. [DOI] [PubMed] [Google Scholar]
- Ikawa S., Weinberg R. A. An interaction between p21ras and heat shock protein hsp60, a chaperonin. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2012–2016. doi: 10.1073/pnas.89.6.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs E., Clad A. Electroelution of fixed and stained membrane proteins from preparative sodium dodecyl sulfate-polyacrylamide gels into a membrane trap. Anal Biochem. 1986 May 1;154(2):583–589. doi: 10.1016/0003-2697(86)90033-3. [DOI] [PubMed] [Google Scholar]
- Kaur I., Voss S. D., Gupta R. S., Schell K., Fisch P., Sondel P. M. Human peripheral gamma delta T cells recognize hsp60 molecules on Daudi Burkitt's lymphoma cells. J Immunol. 1993 Mar 1;150(5):2046–2055. [PubMed] [Google Scholar]
- Mamounas M., Ross S., Luong C. L., Brown E., Coulter K., Carroll G., Englesberg E. Analysis of the genes involved in the insulin transmembrane mitogenic signal in Chinese hamster ovary cells, CHO-K1, utilizing insulin-independent mutants. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3530–3534. doi: 10.1073/pnas.88.9.3530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizzen L. A., Chang C., Garrels J. I., Welch W. J. Identification, characterization, and purification of two mammalian stress proteins present in mitochondria, grp 75, a member of the hsp 70 family and hsp 58, a homolog of the bacterial groEL protein. J Biol Chem. 1989 Dec 5;264(34):20664–20675. [PubMed] [Google Scholar]
- Moffett J., Curriden S., Ertsey R., Mendiaz E., Englesberg E. Alanine-resistant mutants of Chinese hamster ovary cells, CHO-K1, producing increases in velocity of proline transport through the A, ASC, and P systems. Somatic Cell Genet. 1983 Mar;9(2):189–213. doi: 10.1007/BF01543177. [DOI] [PubMed] [Google Scholar]
- Moffett J., Englesberg E. Recessive constitutive mutant Chinese hamster ovary cells (CHO-K1) with an altered A system for amino acid transport and the mechanism of gene regulation of the A system. Mol Cell Biol. 1984 Apr;4(4):799–808. doi: 10.1128/mcb.4.4.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffett J., Englesberg E. Regulation of the A system of amino acid transport in Chinese hamster ovary cells, CHO-K1: the difference in specificity between the apo-repressor inactivator (apo-ri) and the transporter and the characterization of the proposed apo-ri. J Cell Physiol. 1986 Mar;126(3):421–429. doi: 10.1002/jcp.1041260313. [DOI] [PubMed] [Google Scholar]
- Moffett J., Jones M., Englesberg E. Amino acid transport in membrane vesicles from CHO-K1 and alanine-resistant transport mutants. Biochemistry. 1987 May 5;26(9):2487–2494. doi: 10.1021/bi00383a013. [DOI] [PubMed] [Google Scholar]
- Moffett J., Mendiaz E., Jones M., Englesberg E. Two membrane-bound proteins associated with alanine resistance and increased A-system amino acid transport in mutants of CHO-K1. Somat Cell Mol Genet. 1988 Jan;14(1):1–12. doi: 10.1007/BF01535044. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Picketts D. J., Mayanil C. S., Gupta R. S. Molecular cloning of a Chinese hamster mitochondrial protein related to the "chaperonin" family of bacterial and plant proteins. J Biol Chem. 1989 Jul 15;264(20):12001–12008. [PubMed] [Google Scholar]
- Qian N. X., Jones M., McDonough A., Englesberg E. alar4, a constitutive mutant of the A system for amino acid transport, has increased abundance of the Na+,K+-ATPase and mRNA for alpha 1 subunit of this enzyme. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7984–7988. doi: 10.1073/pnas.86.20.7984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian N. X., Pastor-Anglada M., Englesberg E. Evidence for coordinate regulation of the A system for amino acid transport and the mRNA for the alpha 1 subunit of the Na+,K(+)-ATPase gene in Chinese hamster ovary cells. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3416–3420. doi: 10.1073/pnas.88.8.3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shotwell M. A., Jayme D. W., Kilberg M. S., Oxender D. L. Neutral amino acid transport systems in Chinese hamster ovary cells. J Biol Chem. 1981 Jun 10;256(11):5422–5427. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viitanen P. V., Lorimer G. H., Seetharam R., Gupta R. S., Oppenheim J., Thomas J. O., Cowan N. J. Mammalian mitochondrial chaperonin 60 functions as a single toroidal ring. J Biol Chem. 1992 Jan 15;267(2):695–698. [PubMed] [Google Scholar]
- Waldinger D., Eckerskorn C., Lottspeich F., Cleve H. Amino-acid sequence homology of a polymorphic cellular protein from human lymphocytes and the chaperonins from Escherichia coli (groEL) and chloroplasts (Rubisco-binding protein). Biol Chem Hoppe Seyler. 1988 Oct;369(10):1185–1189. doi: 10.1515/bchm3.1988.369.2.1185. [DOI] [PubMed] [Google Scholar]
- Waldinger D., Subramanian A. R., Cleve H. The polymorphic human chaperonin protein HuCha60 is a mitochondrial protein sensitive to heat shock and cell transformation. Eur J Cell Biol. 1989 Dec;50(2):435–441. [PubMed] [Google Scholar]