Abstract
Aerobic cycling has been repeatedly shown to induce hypertrophy in skeletal muscle across a variety of populations, while there has been a lack of investigation into the impact of running upon hypertrophy. An increasingly popular model of aerobic exercise is high-intensity interval training (HIIT); in addition to its positive impact upon cardiovascular health, HIIT may be sufficient for inducing significant muscular hypertrophy. Therefore, the purpose of this investigation was to examine the influence of a high-intensity interval running protocol upon hypertrophy of the vastus lateralis in an untrained, young population. Twelve recreationally active university students (Male: 2; Female: 10; 19.9±0.5 yr.; 169.8±1.9 cm; 63.8±2.3 kg; VO2max: 42.1±1.6 ml•kg−1min−1) completed 24.5±0.6 sessions of high-intensity interval run training over 10 weeks. The protocol consisted of four sets of 4 minutes running at 90–95% HRmax followed by 3 minutes active rest at 70% HRmax. Relative and absolute aerobic capacity increased 5.2±2.2% and 6.0±2.3% respectively as a result of the intervention (p< 0.05). Cross-sectional area (CSA) of the vastus lateralis was measured via panoramic ultrasound imaging pre- and post-intervention. Following the protocol, CSA of the intervention group was 10.6±2.7% greater (p< 0.05), while that of the control group did not change. This is the first data to demonstrate hypertrophy of the vastus lateralis in a young population following a running protocol. These data support the existing body of evidence suggesting aerobic exercise to be an effective mode of improving cardiorespiratory fitness as well as increasing whole muscle size of the quadriceps.
Keywords: Physical activity, muscle growth, ambulation, treadmill, sprinting
INTRODUCTION
Traditionally, aerobic exercise (AE) has been considered as the optimal mode for improving factors of cardiorespiratory fitness such as: increases in stroke volume, enzyme activity, mitochondrial protein synthesis, and capillarization (8, 11, 14). In contrast, anecdotally, few people would consider aerobic exercise as a means to induce muscle hypertrophy, an adaptation more commonly associated with resistance exercise (17). However, aerobic exercise training has been demonstrated to precipitate skeletal muscular hypertrophy in a variety of populations comparable to the hypertrophy induced by resistance training (7, 12). It is important to note that the majority of AE investigations on muscle hypertrophy have been conducted using cycling as the mode of exercise. There is a noticeable gap in research examining the effects of walking and running upon muscle hypertrophy.
A recent review examined the existing body of evidence demonstrating that AE has a far greater influence on hypertrophy than previously thought (17). A progressive 12-week cycling protocol effectively induced hypertrophy of the quadriceps in untrained older women (7, 16) and in untrained young and older men (9), with growth ranging between 5% and 12%. Similar adaptations were demonstrated in middle-aged and older men following 16 weeks of either moderate (18) or high-intensity cycling (4%–10%) (13). Even as little as 6 weeks of high-intensity cycling has resulted in significant hypertrophy in young women (7%) (19). In addition, a 12-week comparative study demonstrated analogous hypertrophy of the knee extensor in middle-aged women following either classic resistance training or cycling (3.1%–3.7%)(12). In contrast, only one study has examined the impact of a walk/jog protocol on muscle hypertrophy in young and older men (23). The older cohort demonstrated a 9% increase in mid-thigh CSA, while the young men did not exhibit growth. However, it should be noted that the young men, though untrained, had a relatively high fitness level for their age group, which may have limited their adaptive capabilities.
Given hypertrophy has been observed in untrained young, middle aged, and older populations utilizing an aerobic cycling protocol, as well as in an older male population through an aerobic walk/jog protocol, it is conceivable that comparable results could be observed in an untrained, young population through an aerobic running program.
An increasingly prevalent model of aerobic exercise is high-intensity interval training (HIIT). In recent years, evidence has demonstrated greater adaptation following HIIT than following traditional continuous run training (CT) (24, 26) in a variety of fitness factors including: VO2max (21); SV; ejection fraction (2); protein synthesis rates; and work economy (27). Comparable results have been shown across a variety of populations from young, healthy males to CAD patients and post-infarction heart failure patients, where both HIIT and CT were matched for caloric expenditure (14,16). Additionally, the increased stimulus of HIIT may be adequate for producing muscle hypertrophy, though this has not been sufficiently examined. As HIIT has been effective in inducing substantial physiological improvements in a variety of populations, we chose to use HIIT as our model for a high-intensity running protocol. Therefore, it was the purpose of this investigation to examine the influence of a HIIT running protocol upon hypertrophy of the vastus lateralis in an untrained, young population.
METHODS
Participants
Twelve (n=12) apparently healthy university students (19.9±0.5 yr; 169.8±1.9 cm; 63.8±2.3 kg) were recruited for participation in the High-Intensity Interval Training (HIIT) program. All subjects were recreationally active, participating in intramurals and other similar physical activities, but had not engaged in any formal endurance or strength training in the previous six months. For muscle size comparison, five additional subjects (21±0.3 yr) were recruited to serve as the Control group (CTL). The Institutional Review Board at Taylor University approved all procedures, and all subjects provided written informed consent prior to participation.
Protocol
To measure maximal aerobic capacity, HIIT subjects performed an incremental treadmill test pre- and post-training intervention (JAS Trackmaster, Full Vision Inc, Newton, KS; TrueOne 2400, Parvo Medics, Sandy, UT). During the test, subjects completed a 4 minute warm-up at 5 kph at 0% grade. Subsequently, there were three 2-minute stages of increasing speed based on the subject’s comfort level, with the highest speed corresponding to a Rate of Perceived Exertion (RPE) of 13–15. The remainder of each test was conducted at the subject’s highest speed, while grade increased by 0.5% every 30 seconds until the subject reached volitional fatigue. Heart rate was monitored throughout the test (Polar FT7F, Lake Success, NY). Maximal heart rate achieved during the test was used to determine intensity throughout the exercise intervention. Achievement of VO2max was accepted when two of three criteria were met: 1.) HR within 10 bpm age-predicted HRmax; 2.) Respiratory exchange ratio was higher than 1.10; 3.) Less than 2.0 mL/kg/min change in VO2 observed with each increase in workload. Data was collected every 30 seconds (TrueOne 2400, Parvo Medics, Sandy, UT), and the mean of the highest consecutive values was used for analysis. The maximal aerobic capacity of the CTL group was not assessed, as they were examined for muscle size comparison only.
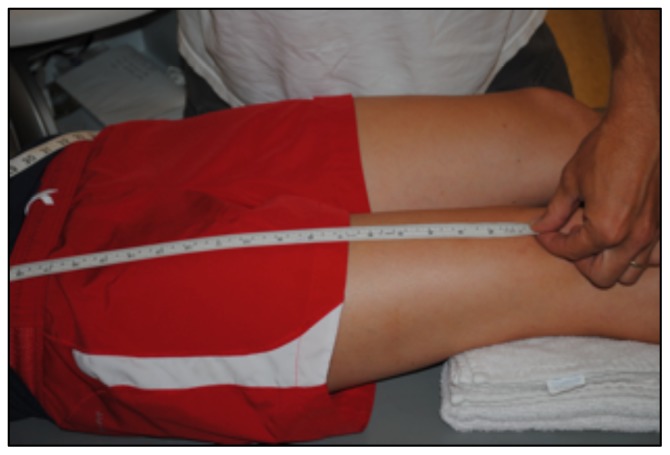
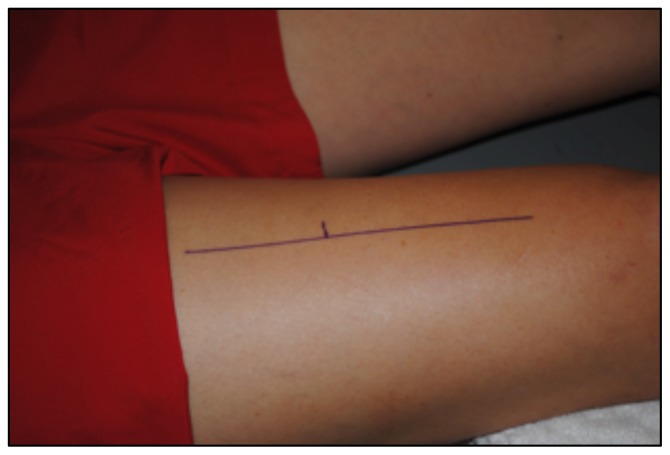
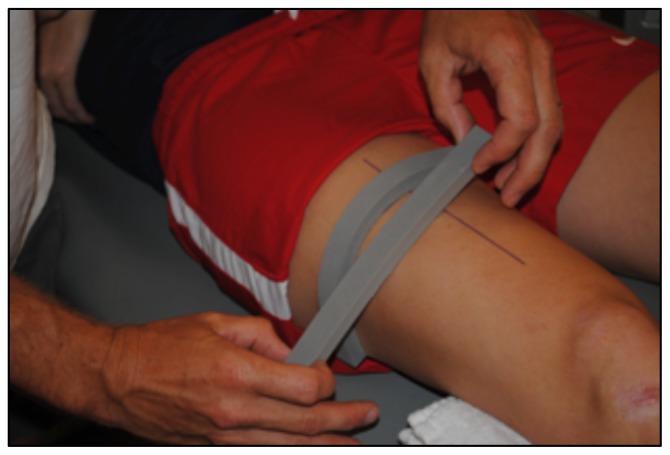
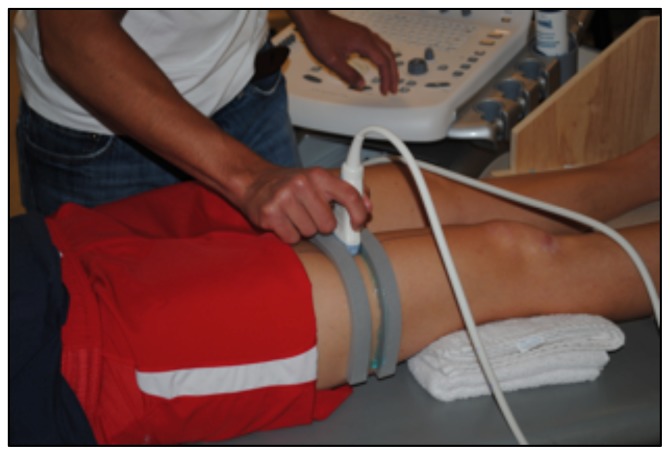
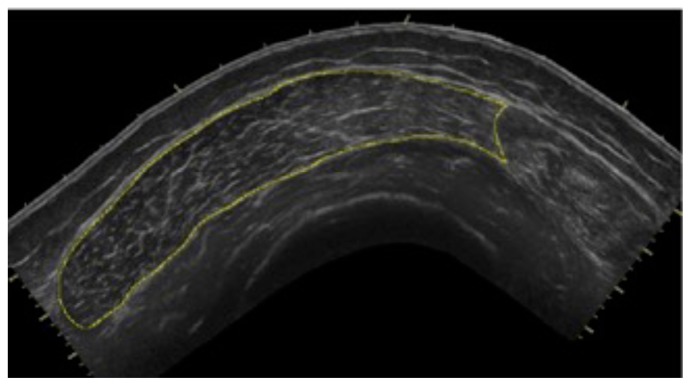
To determine CSA of the vastus lateralis (VL) before the 10-week exercise program, all subjects reported to the lab following a minimum of 36 hours of rest from physical activity extending beyond daily ambulation (i.e. walking to class) (20). CTL subjects followed the same protocol for the post-test, while HIIT subjects reported to the lab 36 hours after their final HIIT session. Upon arrival, subjects completed 30 minutes of supine rest to account for fluid shifts (3) before panoramic ultrasound images were captured using a GE Logic P3 Ultrasound (Fairfield, CT). The length of the femur for each subject was recorded as the distance from the superior border of the patella to the ASIS (Figure 1), and a line was drawn perpendicular to the femur 1/3 of the distance up from patella (Figure 2). High-density foam was adhered to the thigh at and above this marker to act as a guide for the sound head (Figures 3–4), ensuring consistent planes of the muscle were measured pre- and post-intervention. A set of three images was captured at both pre- and post- tests, for all subjects. All images were assessed via manual planimetry using Image J software (Figure 5). The same examiner analyzed both pre- and post-intervention images and was blinded to both group and time. For both time points, each image was measured five times, with a coefficient of variation less than 1.0% between measurements. To prevent any potential outlier images from influencing our results, we chose to use the median, rather than the mean, of the three image values in the statistical analysis.
Figure 1.
The distance from the superior border of the patella to the ASIS was measured to determine the length of the femur for each subject.
Figure 2.
A line was drawn perpendicular to the femur 1/3 of the distance up from patella, to establish the plane of muscle to be measured.
Figure 3.
High-density foam was adhered to the thigh perpendicular to the femur, at and above this marker (Figure 2).
Figure 4.
The high-density foam served as a guide for the sound head, thus minimizing tilt and rotation.
Figure 5.
All images were assessed using Image J software.
Training sessions were conducted on average 3 d•wk−1 on non-consecutive days, for 10 weeks; sessions included a 10 minute warm-up period before HIIT, and concluded with a 3 minute cool-down at 70% HRmax, as determined by the maximal aerobic capacity test. During each trial, HIIT subjects completed four sets of 4 minutes running at 90–95% HRmax followed by 3 minutes active rest at a predetermined speed that would elicit ~70% HRmax, as previously described (10). In addition to the HIIT protocol, a subset of seven of the original twelve subjects completed an additional 2 d•wk−1 of continuous running for 30 min at 70–75% HRmax, as part of a larger study. However, as there were no significant differences in response between groups, all data were combined for analysis. All sessions were performed on a treadmill (Integrity Series, LifeFitness, Rosemont, IL) at 0% grade. All exercise sessions were supervised by a member of the research team, and heart rate was monitored (Polar FT7F, Lake Success, NY) to ensure desired intensity was attained. CTL subjects were advised to maintain their current activity level, while abstaining from any formal training for the 10-week intervention period.
Statistical Analysis
The mean pre- and post-intervention measurements of aerobic capacity were analyzed through SPSS software using a dependent t-test with a significance level of p<0.05. Mean muscle CSA values were analyzed using a one-way ANOVA with significance level set at p<0.05.
RESULTS
Subjects completed 24.5±0.6 training sessions each, averaging 92% HRmax during intervals and 72.4% during recovery. Aerobic capacity, assessed as maximal oxygen uptake (VO2max), increased significantly following the high-intensity interval protocol. Subjects exhibited an increase in both relative (5.2±2.2%) and absolute (6.0±2.3%) VO2max. Absolute changes in VO2max were 2.4±1.1 ml/kg/min and 0.2±0.1 L/min, respectively.
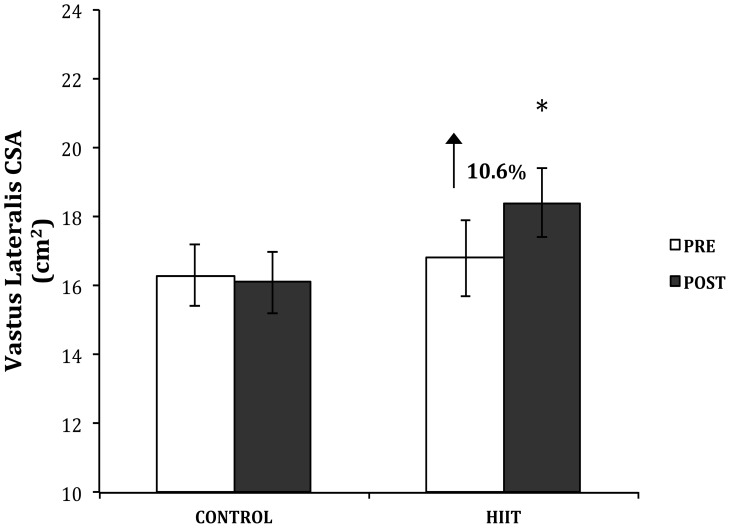
VL CSA significantly increased by an average of 10.6±2.7% in the HIIT group following the intervention (Figure 6). Absolute muscle CSA increased from 16.1±1.1 cm2 to 17.6±1.0 cm2 (p<0.05, η2=0.34). There was no change in CSA of CTL (−1.2±1.3%).
Figure 6.
The muscle cross-sectional area (cm2) of the vastus lateralis was measured for each group pre- and post-intervention. Data presented as mean ± standard error. * p< 0.05.
DISCUSSION
While evidence continues to accrue regarding the positive impacts of aerobic exercise upon muscle hypertrophy, the majority of studies have utilized cycling as their mode of intervention. There is a significant gap in the research examining the affects of a running protocol upon muscular hypertrophy. To address this, we examined hypertrophy of the vastus lateralis following a 10-week HIIT running protocol in untrained college students. Post-intervention assessment demonstrated a 10.6% increase in CSA of the vastus lateralis. Additionally, subjects exhibited a 6% increase in VO2max, a value in accordance with those of comparable studies (6, 10, 15, 22).
Adaptation in CSA of the vastus lateralis was congruent with that demonstrated through cycling protocols of comparable duration and intensity (9, 18, 19). To our knowledge, this is the first study to examine run training induced hypertrophy in an untrained, young population including both males and females, though similar adaptation has been exhibited by an older, male population following a high-intensity walk/jog protocol (23). Schwartz et al. conducted a 27-week study wherein they examined the impact of a high-intensity walk/jog intervention upon hypertrophy of the quadriceps in both young and older men. The protocol consisted of 45 min. of exercise at 85% HRR, 5 d•wk−1. Following the intervention, the older cohort exhibited a 9% increase in mid-thigh CSA, a value comparable to that induced through cycling protocols and the current study; however, the young men did not experience any adaptation. Upon closer examination, we discovered that these young men, though untrained, would have been categorized in the 85th percentile of fitness for their age group, according to current ACSM standards in relation to VO2max (1). In contrast, the older cohort in the Schwartz study fell into the 25th percentile, or “poor” fitness category. Further investigation revealed that subjects examined in the existing body of cycling interventions would have fallen into or below the “below average” fitness category (5th – 40th percentiles), according to their initial VO2max values (7, 9, 16, 18, 19). Additionally, our young subjects fell into the 50th percentile, or “average” fitness category. As the young cohort of the Schwartz study were the only subjects assessed to surpass the 50th percentile of fitness before intervention, as well as the only subjects that did not experience hypertrophy, we suspect the high initial fitness of the young men inhibited their adaptive capabilities, thereby preventing muscle growth, despite the intensity of the intervention.
The results of this study are consistent with the existing body of evidence in regards to muscle hypertrophy. Additionally, although we did not assess cellular changes, mechanisms associated with muscle growth and aerobic exercise have been reported. Specifically, aerobic exercise has been demonstrated to inhibit myostatin within the cell (16), thereby allowing for heightened activity of the AKT-mTOR pathway, which results in muscle protein synthesis. Simultaneously, the repeated muscle contraction of aerobic exercise augments the functioning of PGC-1σ (4), leading to a decrease in muscle protein breakdown through the inhibition of FOXO3a, a catalyst for muscular catabolism. Both of these aerobically induced adaptations lead to a positive muscle protein balance within the cell, and therefore to hypertrophy of the fiber.
Aerobic exercise has been characterized by inducing significant cardiorespiratory adaptation across a wide variety of populations. Furthermore, it has been demonstrated to be effective in producing significant muscular hypertrophy following various aerobic cycling and now running protocols in both young and older populations (23). These findings suggest that beginners could utilize high-intensity running as an effective method of achieving concurrent increases in VO2max and muscle CSA. Given the often-cited reason of lack of time for exercise, our findings may offer motivation for novices looking to increase aerobic fitness as well as quadriceps muscle size. In addition, as skeletal muscle is a primary site of glucose disposal within the body, we speculate that the increase in muscle size may contribute to the improved whole body glucose disposal rates associated with AE, though further investigations are necessary (5, 25).
Our findings support evidence suggesting aerobic exercise to be an effective mode of activity for the promotion of cardiorespiratory fitness and increase in whole muscle size of the quadriceps.
REFERENCES
- 1.ACSM’s Guidelines for Exercise Testing and Prescription. 9th ed. Philadelphia: LWW; 2013. [DOI] [PubMed] [Google Scholar]
- 2.Adachi H, Koike A, Obayashi T. Does appropriate endurance exercise training improve cardiac function in patients with prior myocardial infarction? Eur Heart J. 1996;17(10):1511–1521. doi: 10.1093/oxfordjournals.eurheartj.a014715. [DOI] [PubMed] [Google Scholar]
- 3.Berg HE, Tedner B, Tesch PA. Changes in lower limb muscle cross-sectional area and tissue fluid volume after transition from standing to supine. Acta Physiol Scand. 1993;148(4):379–385. doi: 10.1111/j.1748-1716.1993.tb09573.x. [DOI] [PubMed] [Google Scholar]
- 4.Brault JJ, Jespersen JG, Goldberg AL. Peroxisome proliferator-activated receptor gamma coactivator 1alpha or 1beta overexpression inhibits muscle protein degradation, induction of ubiquitin ligases, and disuse atrophy. J Biol Chem. 2010;285(25):19460–19471. doi: 10.1074/jbc.M110.113092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colberg SR, Albright AL, Blissmer BJ. Exercise and type 2 diabetes: American College of Sports Medicine and the American Diabetes Association: joint position statement. Exercise and type 2 diabetes. Med Sci Sports Exerc. 2010;42(12):2282–2303. doi: 10.1249/MSS.0b013e3181eeb61c. [DOI] [PubMed] [Google Scholar]
- 6.Franch J, Madsen K, Djurhuus MS, Pedersen PK. Improved running economy following intensified training correlates with reduced ventilatory demands. Med Sci Sports Exerc. 1998;30(8):1250–1256. doi: 10.1097/00005768-199808000-00011. [DOI] [PubMed] [Google Scholar]
- 7.Harber MP, Konopka AR, Douglass MD. Aerobic exercise training improves whole muscle and single myofiber size and function in older women. Am J Physiol Regul Integr Comp Physiol. 2009;297(5):R1452–1459. doi: 10.1152/ajpregu.00354.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harber MP, Konopka AR, Jemiolo B, Trappe SW, Trappe TA, Reidy PT. Muscle protein synthesis and gene expression during recovery from aerobic exercise in the fasted and fed states. Am J Physiol Regul Integr Comp Physiol. 2010;299(5):R1254–1262. doi: 10.1152/ajpregu.00348.2010. [DOI] [PubMed] [Google Scholar]
- 9.Harber MP, Konopka AR, Undem MK. Aerobic exercise training induces skeletal muscle hypertrophy and age-dependent adaptations in myofiber function in young and older men. J Appl Physiol. 2012;113(9):1495–1504. doi: 10.1152/japplphysiol.00786.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Helgerud J, Høydal K, Wang E. Aerobic high-intensity intervals improve VO2max more than moderate training. Med Sci Sports Exerc. 2007;39(4):665–671. doi: 10.1249/mss.0b013e3180304570. [DOI] [PubMed] [Google Scholar]
- 11.Holloszy JO, Coyle EF. Adaptations of skeletal muscle to endurance exercise and their metabolic consequences. J Appl Physiol. 1984;56(4):831–838. doi: 10.1152/jappl.1984.56.4.831. [DOI] [PubMed] [Google Scholar]
- 12.Hudelmaier M, Wirth W, Himmer M, Ring-Dimitriou S, Sänger A, Eckstein F. Effect of exercise intervention on thigh muscle volume and anatomical cross-sectional areas--quantitative assessment using MRI. Magn Reson Med. 2010;64(6):1713–1720. doi: 10.1002/mrm.22550. [DOI] [PubMed] [Google Scholar]
- 13.Izquierdo M, Häkkinen K, Ibáñez J, Kraemer WJ, Gorostiaga EM. Effects of combined resistance and cardiovascular training on strength, power, muscle cross-sectional area, and endurance markers in middle-aged men. Eur J Appl Physiol. 2005;94(1–2):70–75. doi: 10.1007/s00421-004-1280-5. [DOI] [PubMed] [Google Scholar]
- 14.Klausen K, Andersen LB, Pelle I. Adaptive changes in work capacity, skeletal muscle capillarization and enzyme levels during training and detraining. Acta Physiol Scand. 1981;113(1):9–16. doi: 10.1111/j.1748-1716.1981.tb06854.x. [DOI] [PubMed] [Google Scholar]
- 15.Knuttgen HG, Nordesjö LO, Ollander B, Saltin B. Physical conditioning through interval training with young male adults. Med Sci Sports. 1973;5(4):220–226. [PubMed] [Google Scholar]
- 16.Konopka AR, Douglass MD, Kaminsky LA. Molecular adaptations to aerobic exercise training in skeletal muscle of older women. J Gerontol A Biol Sci Med Sci. 2010;65(11):1201–1207. doi: 10.1093/gerona/glq109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Konopka AR, Harber MP. Skeletal muscle hypertrophy after aerobic exercise training. Exerc Sport Sci Rev. 2014;42(2):53–61. doi: 10.1249/JES.0000000000000007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lovell DI, Cuneo R, Gass GC. Can aerobic training improve muscle strength and power in older men? J Aging Phys Act. 2010;18(1):14–26. doi: 10.1123/japa.18.1.14. [DOI] [PubMed] [Google Scholar]
- 19.McPhee JS, Williams AG, Degens H, Jones DA. Inter-individual variability in adaptation of the leg muscles following a standardised endurance training programme in young women. Eur J Appl Physiol. 2010;109(6):1111–1118. doi: 10.1007/s00421-010-1454-2. [DOI] [PubMed] [Google Scholar]
- 20.Ploutz-Snyder LL, Convertino VA, Dudley GA. Resistance exercise-induced fluid shifts: change in active muscle size and plasma volume. Am J Physiol. 1995;269(3 Pt 2):R536–543. doi: 10.1152/ajpregu.1995.269.3.R536. [DOI] [PubMed] [Google Scholar]
- 21.Rognmo Ø, Hetland E, Helgerud J, Hoff J, Slørdahl SA. High intensity aerobic interval exercise is superior to moderate intensity exercise for increasing aerobic capacity in patients with coronary artery disease. Eur J Cardiovasc Prev Rehabil. 2004;11(3):216–222. doi: 10.1097/01.hjr.0000131677.96762.0c. [DOI] [PubMed] [Google Scholar]
- 22.Saltin B, Nazar K, Terjung RL. International Perspectives in Exercise Physiology. Champaign: Human Kinetics Publishers; 1990. Maximal oxygen uptake: limitations and malleability. [Google Scholar]
- 23.Schwartz RS, Shuman WP, Larson V. The effect of intensive endurance exercise training on body fat distribution in young and older men. Metabolism. 1991;40(5):545–551. doi: 10.1016/0026-0495(91)90239-s. [DOI] [PubMed] [Google Scholar]
- 24.Thomas TR, Adeniran SB, Etheridge GL. Effects of different running programs on VO2 max, percent fat, and plasma lipids. Can J Appl Sport Sci. 1984;9(2):55–62. [PubMed] [Google Scholar]
- 25.Tjønna AE, Lee SJ, Rognmo Ø. Aerobic interval training versus continuous moderate exercise as a treatment for the metabolic syndrome: a pilot study. Circulation. 2008;118(4):346–354. doi: 10.1161/CIRCULATIONAHA.108.772822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wenger HA, Bell GJ. The interactions of intensity, frequency and duration of exercise training in altering cardiorespiratory fitness. Sports Med. 1986;3(5):346–356. doi: 10.2165/00007256-198603050-00004. [DOI] [PubMed] [Google Scholar]
- 27.Wisløff U, Støylen A, Loennechen JP. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: a randomized study. Circulation. 2007;115(24):3086–3094. doi: 10.1161/CIRCULATIONAHA.106.675041. [DOI] [PubMed] [Google Scholar]