Abstract
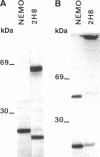
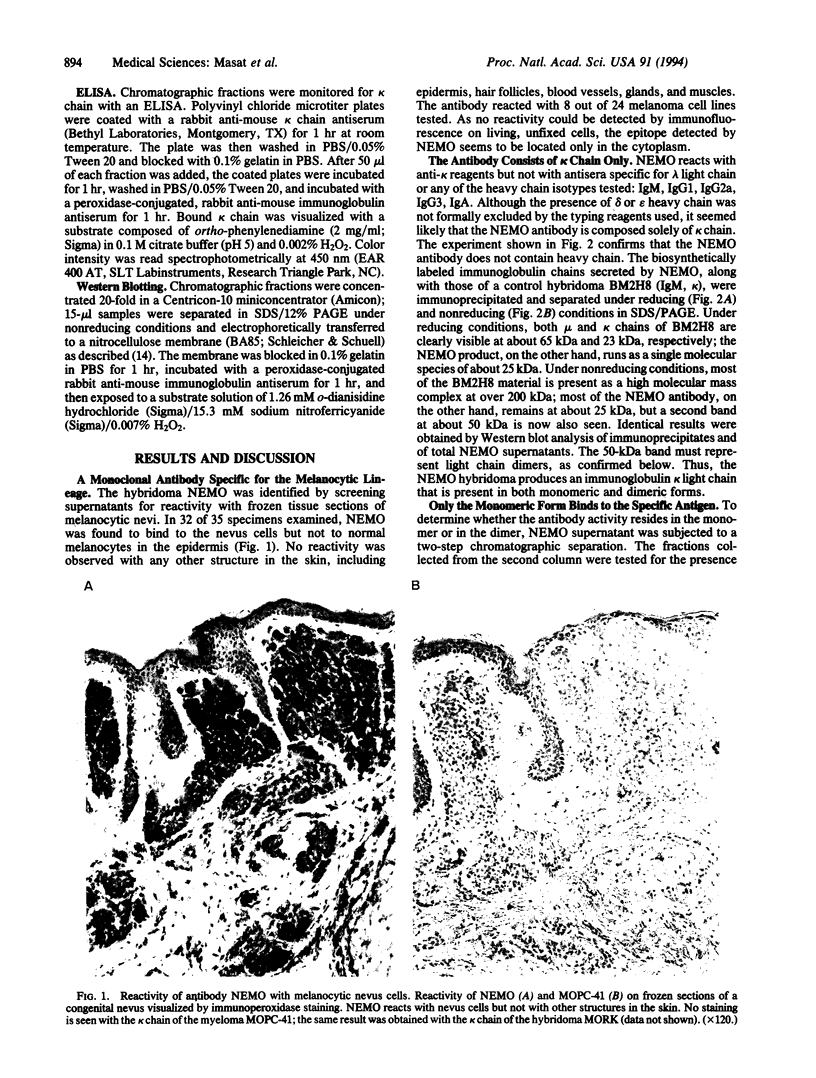
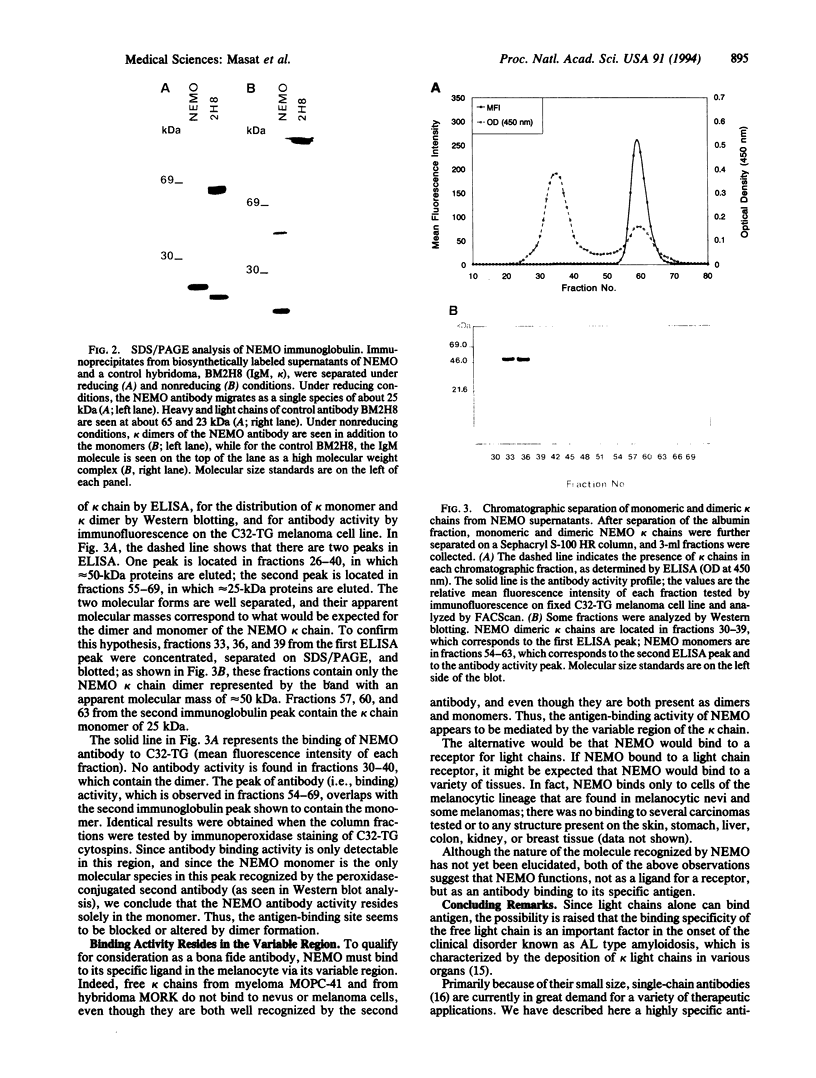
The monoclonal antibody NEMO is directed against a molecule expressed by human cells of the melanocytic lineage. Although obtained by conventional immunization and fusion procedures, NEMO consists solely of kappa light chain. SDS/PAGE analysis indicates that the kappa chains are present as both monomers and dimers. When these two forms were separated by gel filtration, only the monomeric form bound antigen. As kappa light chains from the myeloma MOPC-41 and the hybridoma MORK do not bind to the melanocytic cells, we conclude that the binding specificity of NEMO resides in the variable region.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Edmundson A. B., Ely K. R., Girling R. L., Abola E. E., Schiffer M., Westholm F. A., Fausch M. D., Deutsch H. F. Binding of 2,4-dinitrophenyl compounds and other small molecules to a crystalline lambda-type Bence-Jones dimer. Biochemistry. 1974 Aug 27;13(18):3816–3827. doi: 10.1021/bi00715a031. [DOI] [PubMed] [Google Scholar]
- Edmundson A. B., Ely K. R., Herron J. N. A search for site-filling ligands in the Mcg Bence-Jones dimer: crystal binding studies of fluorescent compounds. Mol Immunol. 1984 Jul;21(7):561–576. doi: 10.1016/0161-5890(84)90041-5. [DOI] [PubMed] [Google Scholar]
- FLEISCHMAN J. B., PORTER R. R., PRESS E. M. THE ARRANGEMENT OF THE PEPTIDE CHAINS IN GAMMA-GLOBULIN. Biochem J. 1963 Aug;88:220–228. doi: 10.1042/bj0880220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göttlinger H. G., Lobo F. M., Grimm T. W., Riethmüller G., Johnson J. P. Biochemical characterization and tissue distribution of the Cora antigen, a cell surface glycoprotein differentially expressed on malignant and benign gastrointestinal epithelia. Cancer Res. 1988 Apr 15;48(8):2198–2203. [PubMed] [Google Scholar]
- Jaton J. C., Klinman N. R., Givol D., Sela M. Recovery of antibody activity upon reoxidation of completely reduced polyalanyl heavy chain and its Fd fragment derived from anti-2,4-dinitrophenyl antibody. Biochemistry. 1968 Dec;7(12):4185–4195. doi: 10.1021/bi00852a008. [DOI] [PubMed] [Google Scholar]
- Johnson J. P., Stade B. G., Hupke U., Holzmann B., Riethmüller G. The melanoma progression-associated antigen P3.58 is identical to the intercellular adhesion molecule, ICAM-1. Immunobiology. 1988 Dec;178(3):275–284. doi: 10.1016/S0171-2985(88)80071-8. [DOI] [PubMed] [Google Scholar]
- Jäck H. M., Beck-Engeser G., Sloan B., Wong M. L., Wabl M. A different sort of Mott cell. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):11688–11691. doi: 10.1073/pnas.89.24.11688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Ledbetter J. A., Fell H. P., Grosmaire L. S., Norris N. A., Tsu T. T. An immunoglobulin light chain dimer with CD4 antigen specificity. Mol Immunol. 1987 Dec;24(12):1255–1261. doi: 10.1016/0161-5890(87)90119-2. [DOI] [PubMed] [Google Scholar]
- Lehmann J. M., Holzmann B., Breitbart E. W., Schmiegelow P., Riethmüller G., Johnson J. P. Discrimination between benign and malignant cells of melanocytic lineage by two novel antigens, a glycoprotein with a molecular weight of 113,000 and a protein with a molecular weight of 76,000. Cancer Res. 1987 Feb 1;47(3):841–845. [PubMed] [Google Scholar]
- Painter R. G., Sage H. J., Tanford C. Contributions of heavy and light chains of rabbit immunoglobulin G to antibody activity. I. Binding studies on isolated heavy and light chains. Biochemistry. 1972 Apr 11;11(8):1327–1337. doi: 10.1021/bi00758a001. [DOI] [PubMed] [Google Scholar]
- Potter M. Immunoglobulin-producing tumors and myeloma proteins of mice. Physiol Rev. 1972 Jul;52(3):631–719. doi: 10.1152/physrev.1972.52.3.631. [DOI] [PubMed] [Google Scholar]
- Sipe J. D. Amyloidosis. Annu Rev Biochem. 1992;61:947–975. doi: 10.1146/annurev.bi.61.070192.004503. [DOI] [PubMed] [Google Scholar]
- UTSUMI S., KARUSH F. THE SUBUNITS OF PURIFIED RABBIT ANTIBODY. Biochemistry. 1964 Sep;3:1329–1338. doi: 10.1021/bi00897a024. [DOI] [PubMed] [Google Scholar]
- Ward E. S., Güssow D., Griffiths A. D., Jones P. T., Winter G. Binding activities of a repertoire of single immunoglobulin variable domains secreted from Escherichia coli. Nature. 1989 Oct 12;341(6242):544–546. doi: 10.1038/341544a0. [DOI] [PubMed] [Google Scholar]
- Winter G., Milstein C. Man-made antibodies. Nature. 1991 Jan 24;349(6307):293–299. doi: 10.1038/349293a0. [DOI] [PubMed] [Google Scholar]