Abstract
BACKGROUND
The objective of this analysis was to assess the cost-effectiveness of TB diagnosis using Microscopic Observation Drug Susceptibility (MODS), Xpert MTB/RIF (Xpert), and empiric treatment for all patients, in addition to current clinical diagnostic practices in children less than 5 years of age in a national tuberculosis (TB) referral hospital in Uganda.
METHODS
A decision analysis was conducted from the healthcare perspective, with a primary outcome of incremental cost effectiveness expressed as cost per year of life gained (YLG).
RESULTS
Cost-effectiveness of the algorithms depended strongly on three variables: the prevalence of TB, probability of death if TB was untreated, and accuracy of existing diagnostic algorithms. Xpert and MODS had similar cost-effectiveness profiles and were preferred in settings where the prevalence of TB and probability of death from untreated TB were low. As the underlying probability of TB disease and death increased, treating all children with clinically suspected disease became more cost-effective. In settings where the probability that an untreated child will die of TB – whether a result of high prevalence of TB or high mortality from untreated TB , treating all children for TB is likely to be the most cost-effective approach until better diagnostic tests can be developed.
CONCLUSION
The cost-effectiveness of diagnostic tools for TB in children depends on the population, natural history of untreated TB, and existing diagnostic practices. In settings where the risk of TB death is high, empiric treatment of all children for TB should be considered until a more sensitive, low-cost diagnostic test is available.
Keywords: pediatric TB, tuberculosis, diagnostics
INTRODUCTION
Nearly 10% of the estimated 9.6 million annual incident cases of active tuberculosis (TB) (1, 2) and 8% of the 1.5 million estimated deaths occur in children, and these projected case and mortality numbers likely underestimate the true disease burden in this population due to difficulties in pediatric TB diagnosis (2). TB in children under 5 is more difficult to diagnose because of the low bacillary burden and difficulty in obtaining appropriate samples; as a result, less than 15% of children with active TB are positive using sputum smear microscopy, the current cornerstone of TB diagnosis worldwide (3). Whereas newly developed diagnostic tests for pulmonary TB have high sensitivity and specificity when performed on sputum samples from adults, their performance in children remains suboptimal (4, 5). As a result, diagnosis of active TB in children is rarely confirmed microbiologically. Most current algorithms for diagnosis of pediatric TB rely on approaches which perform poorly in the pediatric population (6). As none of these tests are specific for active TB, diagnosis of TB in children younger than five is often delayed or missed, resulting in increased morbidity and mortality in a population that is particularly susceptible to rapid clinical progression of TB disease (7).
Economic considerations are important for diagnosis of TB in young children, and are likely to be different than for adult pulmonary TB (5). Most young children presenting with possible TB undergo an initial battery of tests that may include physical examination, non-specific laboratory testing (e.g., complete blood count), and chest radiograph. This clinical evaluation may also include TB-specific tests such as smear microscopy, mycobacterial culture, and nucleic acid amplification testing (e.g., Xpert MTB/RIF, now recommended for use in children (8)). Given the poor sensitivity of these tests in children, particularly those less than five years, a reasonable alternative in settings of high pre-test probability (e.g., national TB reference hospitals) may be to treat all children who present with suspected TB. We performed a cost-effectiveness analysis from an economic perspective to understand the settings in which current clinical diagnosis alone, current clinical diagnosis augmented with MODS or Xpert, or empiric treatment for all children (the decision to treat all children who present for suspected TB) might be preferred.
METHODS
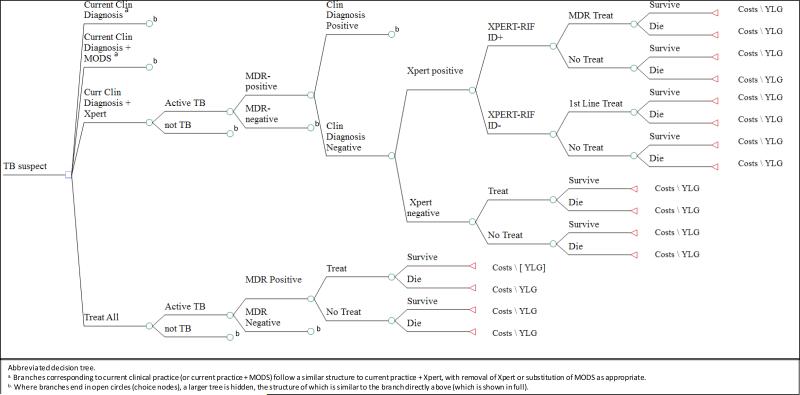
We developed a decision analysis model based on previous analyses of TB diagnostics (9-11) (Figure 1). We considered a hypothetical cohort of 1,000 children under age 5 presenting to a national TB referral hospital in Uganda, a high TB-burden country, for consideration of pediatric TB diagnosis (“children with suspected TB”). We did not model a specific algorithm as each child will have his/her own diagnostic work up before presenting to the referral center. Therefore, we did not hold strictly to the NIH consensus criteria for pediatric TB (12). We understand that clinical practice will vary based on the setting; as such, we modeled the baseline variables applying rates from an analysis of symptom-based approaches (13). There are instances in which some children will receive additional workup after presentation to the referral center, whereas others (for example, those being treated empirically) will not. In those cases, as our reference scenario for the current standard of clinical care (“current clinical diagnosis”), we assumed that the incremental cost of the additional workup would be comparable to the cost of clinical assessment, chest X-ray, smear, and the collection of gastric aspirate specimens. We compare this reference to a scenario in which the standard of care is augmented by 1) MODS, 2) Xpert, and/or 3) a broad policy to treat all children empirically for TB, without confirming that diagnosis using any TB-specific test.
Figure 1. Simplified Diagram of the Model detailing two arms.
This is an abbreviated version of the study decision tree depicting the analytic famework for the decision analysis. The branches for the base case (Current Clinical Practice) is compared to the 3 algorithms, as described in the text. Values for each node, including probabilities, costs and YLG are presented in Table 1.
Model parameters were extrapolated from ongoing studies at the National TB Referral Hospital in Uganda and existing literature from similar settings, where available (Table 1(14-17)). There is substantial uncertainty regarding many parameters, reflecting the heterogeneity of diagnostic practices and the paucity of literature in the absence of a true “gold standard” for pediatric TB diagnosis. Thus, we explored which model parameters were most important to the consideration of cost-effectiveness and then described the settings in which different diagnostic strategies would be preferred. We assumed that 14% of children under five presenting to the TB referral hospital had underlying TB, a low estimate based on culture confirmation (18). We conservatively assumed (i.e., favoring the standard of care) that, in the reference case, TB could be diagnosed on clinical grounds with a sensitivity of 68% and specificity of 81% (13). We did not presume any particular algorithm used, but rather assumed that children diagnosed by any such algorithm (e.g., those with persistent non-remitting cough and failure to thrive) would be treated in all scenarios. Thus, if clinically diagnosed positive, a negative Xpert or MODS test would never be taken as an indication not to treat, and all remaining parameters (for example, test sensitivity/specificity) refer not to all children, but to the 32% of children with underlying TB (and 81% of those without underlying TB) who would not be treated on the basis of these reference tests.
Table 1.
Parameter Estimates
| Parameter | Base Case | Sensitivity range | References |
|---|---|---|---|
| TB Dynamics | |||
| Prevalence of TB Disease | 0.14a | 0.1-0.5 | (18) |
| MDR Prevalence among all TB in Uganda | 0.01 | 0.006-0.022 | (2) |
| Sensitivity in Children Under Five | |||
| Sensitivity of Current Clinical Practice for TB | 0.68 | 0.3-0.9 | (13) |
| Sensitivity of MODS for TB | 0.47 | 0.3-0.9 | (20) |
| Sensitivity of Xpert MTB/RIF (Xpert) for TB | 0.44 | 0.09-0.9 | (20) |
| Sensitivity of MODS for resistance to rifampin | 0.83 | 0.749-1.0 | (31) |
| Sensitivity of Xpert for resistance to rifampin | 0.83 | 0.66666-1.0 | (31) |
| Specificity for TB | |||
| Specificity of Current Clinical Diagnosis for TB | 0.80 | 0.22-0.93 | (13) |
| Specificity of MODS for TB | 0.97b | 0.86 - 0.997 | (20) |
| Specificity of Xpert for TB | 0.97b | 0.86 - 0.997 | (20) |
| Specificity of MODS for resistance to rifampin | 0.99 | 0.819-1.0 | (31) |
| Specificity of Xpert for resistance to rifampin | 0.99 | 0.819-1.0 | (31) |
| Treatment | |||
| Probability of not completing treatment | 0.14 | 0.112-0.168 | (14) |
| Probability of death due to MODS treatment delayc | 0.06 | 0.01-0.10 | (19) |
| Treatment Outcome | |||
| Probability of Death in Patients with Untreated TBd | 0.35a | 0.15-1.0 | (19) |
| Probability of Death in Patients Without TBe | 0.10 | 0.01-0.34 | (15) |
| Probability of Death in patients appropriately treated for MDR TB | 0.18 | 0.144-0.216 | (16) |
| Probability of Death in patients appropriately treated for non-MDR TB | 0.13 | 0.113-0.138 | (17) |
| Probability of Death in patients appropriately treated for DS TB after delay in diagnosing | 0.18 | 0.160-0.195 | (17, 19) |
| Probability of Death in patients appropriately treated for MDR TB after delay in diagnosing | 0.23 | 0.206 - 0.252 | (17, 19) |
| Outcome measure | |||
| Life Expectancy after TB cure (years) | 50 | 37-54 | (22) |
| Unit Cost Estimates | |||
|---|---|---|---|
| Variable costs | Costs ($) | Sensitivity Range | Reference |
| Specimen collection - Gastric Aspirate | 8.99 | 8.09-9.90 | (26) |
| Current Clinical Practice - cost including X-ray and potential microscopy f | 14.00 | 12.60-15.40 | Current costs USD - Mulago Hospital, Uganda |
| MODS f | 20.00 | 8.80-22.0 | Current costs USD - Mulago Hospital, Uganda |
| Xpert f | 30.00 | 30.00-82.00 | Current costs USD - Mulago Hospital, Uganda |
| Total Cost of treating a single case of drug-sensitive TB | 193.66 | 152.83-234.49 | (5) |
| Total Cost of treating a single case of RTF resistant TB | 1841.36 | 1345.17-2337.56 | (5) |
Given wide uncertainty about this parameter, multiple values were tested in the primary analysis as per figures 3 and 4. Values shown here were used to generate results in table 2.
Given no evidence of differential specificity between MODS and Xpert, we used the same value in this analysis.
This probability was calculated to account for patients who get diagnosed too late due to diagnostic delay
The probability of death in untreated TB is assumed to be the same for patients with MDR TB if not treated appropriately.
This probability was conservatively assumed to be equal whether TB treatment was initiated or not.
The unit costs are estimated from lab charges in the National TB referral lab in Kampala, Uganda. They are assumed to be representative of market costs, including labor. Therefore, they are all-inclusive from the healthcare perspective.
The probability of death if TB goes undiagnosed and untreated was assumed as 35% based on extrapolation of age-stratified mortality risks observed in the pre-chemotherapy era (19). We assumed that, upon presentation to the referral hospital, children would have one diagnostic attempt, consisting of multiple tests completed over the course of multiple visits in a confined timeframe. Where MODS was included in the attempt, we incorporated an attendant delay (and increased mortality risk) while waiting for results. Similarly, a delay was included for treatment based on current clinical diagnosis given the probability of seeking care but not completing treatment. If TB was not diagnosed at the referral hospital, we assumed that children with underlying TB would experience the natural history of untreated TB (i.e., that no other diagnostic options would be pursued after evaluation at the referral center). The sensitivity of Xpert (and MODS) in children under five differs markedly from the sensitivity in adults and adolescents. We applied the sensitivity reported comparing Xpert and MODS to positive clinical diagnosis, similar to the setting in this analysis (20). We accounted for the possibility of pediatric multidrug-resistant (MDR) TB, including the fact that MDR-TB could only be diagnosed with Xpert or MODS (incorporating imperfect sensitivity).
We performed our economic evaluation from the healthcare perspective. Our primary outcome was the incremental cost-effectiveness ratio (ICER), expressed as the incremental cost per incremental year of life gained (YLG). We measured YLG rather than health utility (e.g., disability-adjusted life years, DALYs) because morbidity in young children is difficult to quantify and strongly outweighed by mortality in any health utility calculation related to pediatric TB (21). Our analytic time horizon was the episode of illness (up to one year, allowing for complete treatment based on diagnosis). We adopted a lifetime time horizon for estimation of YLG, based on an estimated life expectancy in Uganda of 54 years and an average age at presentation of 2.5 years (22). Costs were inflated to the year 2014 using inflation factors from the International Monetary Fund and converted to US dollars using the 2014 exchange rate (23). Future costs and YLG are discounted at 3% per year (24).
Given the uncertainty in many model parameters, we subjected all variables to wide sensitivity analysis, first in one-way fashion and then as multi-way sensitivity analyses including the parameters on which the primary outcome depended most strongly. In multi-way analyses, incremental cost effectiveness of clinical diagnosis, Xpert, and MODS were evaluated across a range of values for TB disease prevalence among children (conservatively estimated at 14% in baseline calculations) with suspected TB. Likewise, the probability of death from untreated TB disease (estimated at 35%) was varied greatly, as the true value for this parameter is unknown. Rather than conduct probabilistic uncertainty analysis to provide uncertainty ranges around specific outcome values, we attempt to describe situations in which different diagnostic algorithms are more likely to be economically preferred, given specified thresholds of willingness to pay (WTP) for one YLG. Analyses were performed using TreeAge Pro 2014 (25).
RESULTS
In our initial calculations evaluating 1000 children presenting with symptoms suggestive of TB to a National TB Referral Hospital, we projected that 140 would have true underlying active TB. Under the current clinical diagnosis, we estimated that 96 of these children would be diagnosed on clinical grounds, of whom 90 would start treatment (Table 2). We estimated that an additional 171 children without TB would be diagnosed as false-positives, of whom 147 would start treatment (Table 2). Of the remaining 44 children with TB whose diagnosis would be missed using the current clinical diagnosis scenario, we estimated that Xpert or MODS would result in treatment of an additional 18 to 20, while also resulting in up to 16 additional false-positive treatments (Table 2). The incremental cost of Xpert (or MODS), relative to standard care, was estimated at $39 ($29) per child evaluated, or $126 ($106) per YLG. The incremental cost of treating all children empirically relative to the incremental cost of clinical diagnosis plus Xpert was estimated to be $232 per YLG (data not shown).
Table 2.
Cost -Effectiveness of TB diagnostic algorithms in 1000 hypothetical children presenting to an urban Referral Hospital, Uganda
| No. Patients with Active TB | No. Diagnosed with True TB | TB Deaths | No. with true TB started on Treatment | No. without TB started on TB treatment | Total costa | Incremental costb | Total Effectiveness (YLG)c | Incremental Effectivness | Incremental Cost-effectiveness (ICER) - USD/YLG | |
|---|---|---|---|---|---|---|---|---|---|---|
| Clinical Diagnosis | 140 | 96 | 29 | 90 | 147 | 66769 | 0 | 22480 | 0 | 0 |
| Clinical Diagnosis plus MODS | 140 | 117 | 26 | 108 | 163 | 95365 | 28596 | 22750 | 270 | 106 |
| Clinical Practice plus Xpert | 140 | 115 | 24 | 110 | 163 | 105691 | 38922 | 22790 | 310 | 126 |
| Treat All children presenting with suspected TB | 140 | 140 | 18 | 140 | 860 | 193662 | 126893 | 23170 | 690 | 184 |
Total cost: includes cost of diagnosis, treatment of true and false positives.
Incremental cost (and effectiveness): cost-effectiveness is relative to current clinical practice alone scenario (for example: $95,364.59-$66,769.04=an incremental cost of $28,595.55).
Total effectiveness reflects the underlying life expectancy of 54 years for 1000 kids applying a discount of 3%.
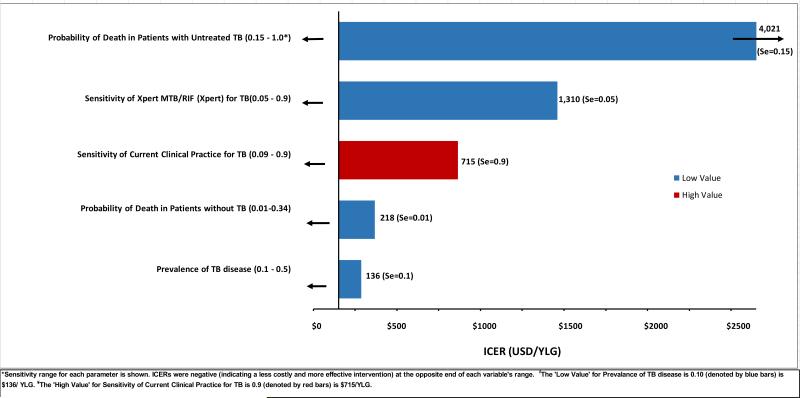
Incremental cost-effectiveness varied widely, the strongest determinants of cost-effectiveness included: accuracy of the existing diagnostic algorithm, probability of underlying TB in the population, accuracy of the TB-specific diagnostic test (Xpert or MODS), and probability of death in children with untreated TB (Figure 2). We, therefore, conducted a series of multi-way sensitivity analyses in which these parameters were varied across reasonable ranges, to identify those settings in which the standard diagnostic algorithm, standard algorithm plus Xpert or MODS, or decision to treat all children with suspected TB would be the most cost-effective option at a WTP of $1716 per YLG (three times the per-capita gross domestic product of Uganda (26, 27)). In these analyses (Figures 3 and 4), we found that addition of Xpert or MODS was preferred to the standard diagnostic algorithm in almost all settings.
Figure 2. One-way sensitivity analyses of incremental cost-effectiveness, comparing Xpert to current clinical practice alone for TB diagnosis in children.
Bars denote the ICER of Xpert relative to the standard of care at the high or low value of each parameter shown. Xpert was highly cost-effective relative to the standard of care except in settings of low probability of death from untreated TB, low Xpert sensitivity, or high sensitivity of current clinical practice. Results are similar for the diagnostic scenario of Clinical Diagnosis plus MODS (data not shown).
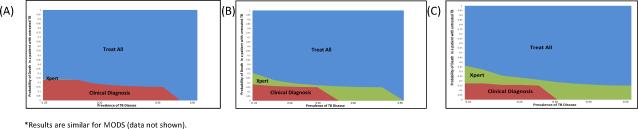
Figure 3. Multi-way sensitivity analysis of the incremental cost-effectiveness of diagnostic algorithms for pediatric TB in a high-burden referral hospital, accounting for varying Sensitivities of the Gene Xpert system*.
The color of the graph at any point denotes the diagnostic algorithm that is most cost-effective (at WTP $1716) under conditions of underlying TB prevalence in the tested population (x-axis) and probability of death if TB goes untreated (y-axis). Panel A assumes a 22.6% sensitivity of Xpert (35), Panel B: 58% (36), and Panel C: 68%(18). Where the probability of death due to untreated TB exceeds, 30-35%, empiric treatment of all children is generally preferred.
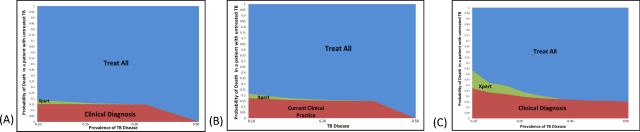
Figure 4. Multi-way sensitivity analysis of the incremental cost-effectiveness of diagnostic algorithms for pediatric TB in a high TB-Burden country referral hospital, accounting for varying Sensitivity of Current Clinical Diagnosis.
This figure evaluates three different scenarios of sensitivity under the current standard of care: Panel A assumes a 30% sensitivity of the current clinical algorithm, Panel B a sensitivity of 50%, and Panel C a sensitivity of 86%. Improving the Current Clinical Diagnosis makes Xpert (or MODS) more likely to be cost-effective relative to empirically “Treat All” children, because fewer children with true underlying TB are left without a diagnosis.
In comparing the standard algorithm plus Xpert or MODS to a decision to empirically treat all TB suspect children, the latter was preferred in most settings considered, including the parameters for the referral setting presented in Table 1. In settings where the sensitivity of Xpert or MODS for pediatric TB was assumed to be higher, Xpert- or MODS-based diagnosis became more cost-effective relative to treatment for all (Figure 3b-c). If current clinical diagnosis is assumed to have a high sensitivity of 86%, clinical diagnosis alone (without Xpert, MODS, or empiric treatment) had a more favorable cost-effectiveness profile, but empiric treatment of all children was still preferred when the probability of death from untreated TB was high (Figure 4c). After accounting for the possibility of treating all children empirically, preference for the standard algorithm plus Xpert or MODS was limited to a small subset of possible settings (green areas in Figures 3 and 4). The decision to treat all empirically was generally preferred in settings where the probability that a given child would die if not treated for TB (i.e., prevalence of underlying TB * probability of death from untreated TB) was greater than 10%.
DISCUSSION
This analysis highlights settings in which different algorithms for diagnosis of pediatric TB in a high-burden national TB referral hospital are likely to be cost-effective. Specifically, we found that the addition of TB-specific tests (whether Xpert or MODS) was almost always cost-effective relative to the existing diagnostic algorithm alone. However, in settings where the probability that a given child would die, if not treated for TB, approaches 10% (whether due to high prevalence of TB or high mortality from untreated TB), a broad decision to treat all children under five with suspected TB may be more appropriate.
Our findings support the recent WHO recommendation to use Xpert MTB/RIF as a diagnostic test for pediatric TB, especially in settings where the prevalence of underlying TB is low (or the specificity of existing diagnostic algorithms for TB is high). Where MODS is equally or more accessible than Xpert, MODS is also a reasonable alternative. MODS is used as an example of liquid culture that is inexpensive and equally sensitive and specific to other types of liquid culture. These tests are likely to be highly cost-effective relative to no additional TB-specific testing unless their sensitivity for TB (accounting also for the fact that not every child with TB can produce an adequate sputum sample) is <10%. Since these tests are unlikely to be available at the periphery in most high-burden settings, it is likely that they would only be used on patients with a higher underlying TB prevalence than these thresholds. Thus, while this analysis of TB testing at a referral hospital cannot be generalized to lower-level clinical settings, it is likely that Xpert/MODS would make a cost-effective addition to TB testing algorithms in most referral-hospital settings where the pre-test probability of TB is reasonably high.
Perhaps more controversial is the finding that a decision to empirically treat all children for TB might be cost-effective, relative even to Xpert or MODS (plus all other clinical diagnostic tools), in a reasonable range of settings characterized by high TB prevalence and/or high TB mortality. Treating even ten children with TB drugs to prevent one TB death is likely to be cost-effective from the healthcare perspective. Importantly, such treatment may be beyond the capacity of the existing healthcare system or pose an unacceptable burden to parents – in other words, a cost-effective algorithm might still not be implementable due to other important considerations. Nevertheless, our findings suggest that, until better diagnostic tests for pediatric TB can be developed, universal empiric treatment may be the most cost-effective approach to diagnosing TB among children under five in settings where the risk of death from untreated TB is reasonably high.
Our results have important limitations. TB is very difficult to diagnose in young children, such that the true sensitivity and specificity of any diagnostic algorithm for pediatric TB is uncertain. Given this limitation, we have used reasonable values from the literature, but we acknowledge that these estimates may differ in many clinical settings. As such, we attempted to use conservative estimates (i.e., biased against empiric treatment of all children), including low rates of TB disease, high accuracy of the standard diagnostic algorithm, low rate of death if TB left untreated. In this analysis, we used a conservative rate of disease for children presenting to the referral TB clinic (14%), per a recent publication from Uganda (18). This estimate is based on culture-confirmed TB in a study with a small sample size, and therefore, likely to be an underestimate. Similar studies defining children under 16 with varying specimen collection methods report culture-confirmed TB in children from 5% to 41% (28-32). Two studies including clinical diagnosis as the reference standard, reported higher rates of TB disease in the study populations. Given the difficulties in culture-based methodologies in children under five, TB disease rates based on culture confirmation are likely to underestimate true burden of TB disease in young children (28, 32). There is little information on the sensitivity of a clinical, non-microbiological pediatric TB diagnosis. We chose conservative estimates based on a study reviewing a symptom-based diagnostic approach in childhood pulmonary TB in an endemic area. The study combined different symptoms upon presentation to provide the highest degree of accuracy of diagnosis. The combination of different symptoms increased sensitivity in different risk-groups according to age in HIV-uninfected children. A 2-symptom approach including non-remitting cough and failure to thrive provided the best diagnostic accuracy when considering the high risk group of children <3 as well as the lower-risk group of children >3, providing a sensitivity of 68.3% and a specificity of 80.1% with sensitivity being increased in each group with the addition of symptoms appropriate for specific risk groups (13). Similarly, information regarding the sensitivity of MODS or Xpert in a pediatric population is limited, even more so in children under five. In addition, there are few studies comparing the sensitivity of these tests to clinical diagnosis as opposed to culture confirmed samples, which are unlikely in children under 5.
The sensitivity and specificity estimates used in our analysis are based on a study comparing MODS and Xpert, among other diagnostics, to clinical diagnosis in children under 16 (20). The categorization of children 0 - 16, rather than children under 5, likely results in an overestimation of the sensitivity of these diagnostics. The estimated probability of death in TB patients not treated for TB (35%) was extrapolated from a study in the 1950s reporting that 90% of deaths in children occurred within the first year of discovery of primary TB. In infants first diagnosed at ≤6 months of age, 55% died; 28% of children ages 1-2 years and up to 15% of children ages 3-9 years old died of TB (19). These data were not stratified at age 5; therefore, we considered a mortality probability of 35% with a broad sensitivity range to cover estimates in the literature.
The prevalence of MDR-TB in this analysis was low, reflecting the rates of MDR-TB among children in Uganda; however, these rates may not be generalizable to other settings where MDR-TB transmission is more widespread. While this analysis was not primarily intended as an analysis of MDR-TB outcomes, we included costs of MDR-TB given that they often comprise a large proportion of overall TB control costs. The cost of treatment for both drug-sensitive and MDR-TB were adapted from the Vassal et al study in 2011 in which they estimated costs for Uganda, specifically. We converted these values to 2014 prices. While these estimates were validated with previous costing studies, the costs were estimated for treatment of adult TB, and the treatment costs may be different on a per patient basis to treat pediatric TB.
We did not include HIV-infected children in the analysis. With increased implementation of highly effective regimens for the prevention of mother to child transmission of HIV, the number of newly infected children is dramatically decreasing. For the relatively small number of newly infected children, it is recommended that HIV treatment start immediately upon diagnosis. In addition to lessening HIV progression, it also decreases likelihood of other infections such as TB. However, it is important to note that HIV is still a factor of importance in high prevalence settings as the rates of TB will remain higher than those of the non HIV-infected population.
We did not include the risk and associated costs of hepatotoxicity as it would not change any of the conclusions of the analysis. While adverse events associated with empiric treatment are important considerations, the risk of hepatotoxicity in the adult population is small, ranging from 0.1-0.6% (33). This risk is even smaller in children. The data on children less than 5 years is extremely limited, with a recent publication reporting zero cases of hepatotoxicity among more than 1000 children with latent TB (34). Our analysis does not ultimately claim that Xpert, MODS, or universal treatment are cost-effective in a generic sense, but rather provides guidance as to likely cost-effectiveness under different local conditions of TB prevalence, Xpert/MODS test sensitivity, sensitivity of the existing diagnostic algorithm, and probability of death if TB is untreated in an under-five population.
Given the lack of data on societal costs of pediatric TB treatment in kids, we performed this from a healthcare perspective. Future analyses could refine our estimates by collecting local-level data from different settings and incorporate these estimates into the analysis. Other important directions for future research include patient-level costing, evaluation of more peripherally located clinical centers, and evaluation of the impact of empiric TB treatment among young children with TB symptoms across a range of clinical settings. While waiting for these data to emerge, greater consideration should be given to the possibility of universal empiric treatment in referral settings where the risk of death from untreated TB remains unacceptably high. The findings of this analysis underscore the urgent need for rapid and accurate diagnostic tests for TB in children, so that more targeted treatment algorithms can be effectively implemented.
Acknowledgments
Funding Source: DWD was supported by the B. Frank and Kathleen Polk Assistant Professorship in Epidemiology at the Johns Hopkins Bloomberg School of Public Health. Other sources of support include: NIH/NICHHD award # R01HD059005 (AR, RHG) and Johns Hopkins University Center for AIDS Research P30AI094189 (AR)
References
- 1.Jenkins HE, Tolman AW, Yuen CM, Parr JB, Keshavjee S, Pérez-Vélez CM, et al. Incidence of multidrug-resistant tuberculosis disease in children: systematic review and global estimates. The Lancet. 2014;383(9928):1572–9. doi: 10.1016/S0140-6736(14)60195-1. doi: 10.1016/s0140-6736(14)60195-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO. Global Tuberculosis Report 2014. World Health Organization; Geneva, Switzerland: 2014. [Google Scholar]
- 3.Swaminathan S, Rekha B. Pediatric tuberculosis: global overview and challenges. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2010;50(Suppl 3):S184–94. doi: 10.1086/651490. Epub 2010/04/20. doi: 10.1086/651490. PubMed PMID: 20397947. [DOI] [PubMed] [Google Scholar]
- 4.Oberhelman RA, Soto-Castellares G, Caviedes L, Castillo ME, Kissinger P, Moore DA, et al. Improved recovery of Mycobacterium tuberculosis from children using the microscopic observation drug susceptibility method. Pediatrics. 2006;118(1):e100–6. doi: 10.1542/peds.2005-2623. doi: 10.1542/peds.2005-2623. PubMed PMID: 16751616. [DOI] [PubMed] [Google Scholar]
- 5.Vassall A, van Kampen S, Sohn H, Michael JS, John KR, den Boon S, et al. Rapid diagnosis of tuberculosis with the Xpert MTB/RIF assay in high burden countries: a cost-effectiveness analysis. PLoS Med. 2011;8(11):e1001120. doi: 10.1371/journal.pmed.1001120. Epub 2011/11/17. doi: 10.1371/journal.pmed.1001120. PubMed PMID: 22087078; PubMed Central PMCID: PMCPmc3210757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hesseling AC, Schaaf HS, Gie RP, Starke JR, Beyers N. A critical review of diagnostic approaches used in the diagnosis of childhood tuberculosis. The international journal of tuberculosis and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease. 2002;6(12):1038–45. Epub 2003/01/28. PubMed PMID: 12546110. [PubMed] [Google Scholar]
- 7.Marais BJ. Childhood tuberculosis: epidemiology and natural history of disease. Indian J Pediatr. 2011;78(3):321–7. doi: 10.1007/s12098-010-0353-1. doi: 10.1007/s12098-010-0353-1. PubMed PMID: 21213073. [DOI] [PubMed] [Google Scholar]
- 8.WHO. Automated real-time nucleic acid amplification technology for rapid and simultaneous detection of tuberculosis and rifampicin resistance: Xpert MTB/RIF assay for the diagnosis of pulmonary and extrapulmonary TB in adults and children: Policy Update. Geneva, Switzerland: p. 2013. [PubMed] [Google Scholar]
- 9.O'Brien RJ, Talbot EA. The utility of an antibiotic trial for diagnosis of AFB-negative tuberculosis. The international journal of tuberculosis and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease. 2003;7(2):198. Epub 2003/02/18. PubMed PMID: 12588024. [PubMed] [Google Scholar]
- 10.Harries AD, Banda HT, Boeree MJ, Welby S, Wirima JJ, Subramanyam VR, et al. Management of pulmonary tuberculosis suspects with negative sputum smears and normal or minimally abnormal chest radiographs in resource-poor settings. The international journal of tuberculosis and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease. 1998;2(12):999–1004. Epub 1998/12/30. PubMed PMID: 9869116. [PubMed] [Google Scholar]
- 11.Githui WA. Laboratory methods for diagnosis and detection of drug resistant Mycobacterium tuberculosis complex with reference to developing countries: a review. East African medical journal. 2002;79(5):242–8. doi: 10.4314/eamj.v79i5.8861. Epub 2003/03/18. PubMed PMID: 12638807. [DOI] [PubMed] [Google Scholar]
- 12.Graham SM, Ahmed T, Amanullah F, Browning R, Cardenas V, Casenghi M, et al. Evaluation of tuberculosis diagnostics in children: 1. Proposed clinical case definitions for classification of intrathoracic tuberculosis disease. Consensus from an expert panel. The Journal of infectious diseases. 2012;205(Suppl 2):S199–208. doi: 10.1093/infdis/jis008. doi: 10.1093/infdis/jis008. PubMed PMID: 22448023; PubMed Central PMCID: PMC3334506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marais BJ, Gie RP, Hesseling AC, Schaaf HS, Lombard C, Enarson DA, et al. A refined symptom-based approach to diagnose pulmonary tuberculosis in children. Pediatrics. 2006;118(5):e1350–9. doi: 10.1542/peds.2006-0519. Epub 2006/11/03. doi: 10.1542/peds.2006-0519. PubMed PMID: 17079536. [DOI] [PubMed] [Google Scholar]
- 14.MacPherson P, Houben RM, Glynn JR, Corbett EL, Kranzer K. Pre-treatment loss to follow-up in tuberculosis patients in low- and lower-middle-income countries and high-burden countries: a systematic review and meta-analysis. Bull World Health Organ. 2014;92(2):126–38. doi: 10.2471/BLT.13.124800. Epub 2014/03/14. doi: 10.2471/blt.13.124800. PubMed PMID: 24623906; PubMed Central PMCID: PMCPmc3949536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zucker JR, Lackritz EM, Ruebush TK, 2nd, Hightower AW, Adungosi JE, Were JB, et al. Childhood mortality during and after hospitalization in western Kenya: effect of malaria treatment regimens. The American journal of tropical medicine and hygiene. 1996;55(6):655–60. doi: 10.4269/ajtmh.1996.55.655. Epub 1996/12/01. PubMed PMID: 9025694. [DOI] [PubMed] [Google Scholar]
- 16.Seddon JA, Hesseling AC, Willemse M, Donald PR, Schaaf HS. Culture-confirmed multidrug-resistant tuberculosis in children: clinical features, treatment, and outcome. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2012;54(2):157–66. doi: 10.1093/cid/cir772. Epub 2011/11/05. doi: 10.1093/cid/cir772. PubMed PMID: 22052896. [DOI] [PubMed] [Google Scholar]
- 17.Wu XR, Yin QQ, Jiao AX, Xu BP, Sun L, Jiao WW, et al. Pediatric tuberculosis at Beijing Children's Hospital: 2002-2010. Pediatrics. 2012;130(6):e1433–40. doi: 10.1542/peds.2011-3742. doi: 10.1542/peds.2011-3742. PubMed PMID: 23184116. [DOI] [PubMed] [Google Scholar]
- 18.Sekadde MP, Wobudeya E, Joloba ML, Ssengooba W, Kisembo H, Bakeera-Kitaka S, et al. Evaluation of the Xpert MTB/RIF test for the diagnosis of childhood pulmonary tuberculosis in Uganda: a cross-sectional diagnostic study. BMC infectious diseases. 2013;13:133. doi: 10.1186/1471-2334-13-133. Epub 2013/03/19. doi: 10.1186/1471-2334-13-133. PubMed PMID: 23497044; PubMed Central PMCID: PMCPmc3602671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lincoln EM. Course and prognosis of tuberculosis in children. The American journal of medicine. 1950;9(5):623–32. doi: 10.1016/0002-9343(50)90212-9. Epub 1950/11/01. PubMed PMID: 14783132. [DOI] [PubMed] [Google Scholar]
- 20.Nhu NT, Ha DT, Anh ND, Thu DD, Duong TN, Quang ND, et al. Evaluation of Xpert MTB/RIF and MODS assay for the diagnosis of pediatric tuberculosis. BMC Infect Dis. 2013;13:31. doi: 10.1186/1471-2334-13-31. Epub 2013/01/25. doi: 10.1186/1471-2334-13-31. PubMed PMID: 23343418; PubMed Central PMCID: PMCPmc3562258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robberstad B. QALYs vs DALYs vs LYs gained: What are the differences, and what difference do they make for health care priority setting? Norsk Epidemiologie. 2005;15(2):183–91. [Google Scholar]
- 22.IndexMundi [11/21/2014];Uganda Demographics Profile 2014. 2014 [2014]. Available from: http://www.indexmundi.com/uganda/demographics_profile.html.
- 23.International Monetary Fund [11/21/2014];World Economic Outlook Database, 2013. 2014 [2014]. Available from: http://www.imf.org/external/pubs/ft/weo/2013/02/weodata/weorept.aspx?sy=2010&ey=2018&scsm=1&ssd=1&sort=country&ds=.&br=1&c=111&s=PCPI%2CPCPIE&grp=0&a=&pr1.x=15&pr1.y=10.
- 24.Weinstein MC, Siegel JE, Gold MR, Kamlet MS, Russell LB. Recommendations of the Panel on Cost-effectiveness in Health and Medicine. JAMA : the journal of the American Medical Association. 1996;276(15):1253–8. Epub 1996/10/16. PubMed PMID: 8849754. [PubMed] [Google Scholar]
- 25.TreeAge Software I.; Williamstown, MA: 2014. Available from: https://www.treeage.com/ [Google Scholar]
- 26.WHO. Cost effectiveness and strategic planning (WHO-CHOICE) 2014 Available from: http://www.who.int/choice/costs/CER_thresholds/en/
- 27.WorldBank GDP per capita (current US$) 2014 Available from: http://data.worldbank.org/indicator/NY.GDP.PCAP.CD.
- 28.Iriso R, Mudido PM, Karamagi C, Whalen C. The diagnosis of childhood tuberculosis in an HIV-endemic setting and the use of induced sputum. The international journal of tuberculosis and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease. 2005;9(7):716–26. Epub 2005/07/15. PubMed PMID: 16013765. [PubMed] [Google Scholar]
- 29.Rachow A, Clowes P, Saathoff E, Mtafya B, Michael E, Ntinginya EN, et al. Increased and expedited case detection by Xpert MTB/RIF assay in childhood tuberculosis: a prospective cohort study. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2012;54(10):1388–96. doi: 10.1093/cid/cis190. doi: 10.1093/cid/cis190. PubMed PMID: 22474220. [DOI] [PubMed] [Google Scholar]
- 30.Nicol MP, Workman L, Isaacs W, Munro J, Black F, Eley B, et al. Accuracy of the Xpert MTB/RIF test for the diagnosis of pulmonary tuberculosis in children admitted to hospital in Cape Town, South Africa: a descriptive study. The Lancet Infectious diseases. 2011;11(11):819–24. doi: 10.1016/S1473-3099(11)70167-0. Epub 2011/07/19. doi: 10.1016/s1473-3099(11)70167-0. PubMed PMID: 21764384; PubMed Central PMCID: PMCPmc4202386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zar HJ, Workman L, Isaacs W, Munro J, Black F, Eley B, et al. Rapid molecular diagnosis of pulmonary tuberculosis in children using nasopharyngeal specimens. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2012;55(8):1088–95. doi: 10.1093/cid/cis598. doi: 10.1093/cid/cis598. PubMed PMID: 22752518; PubMed Central PMCID: PMC3529610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Togun TO, Egere U, Sillah AK, Ayorinde A, Mendy F, Tientcheu L, et al. Contribution of Xpert(R) MTB/RIF to the diagnosis of pulmonary tuberculosis among TB-exposed children in The Gambia. The international journal of tuberculosis and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease. 2015;19(9):1091–7. i–ii. doi: 10.5588/ijtld.15.0228. Epub 2015/08/12. doi: 10.5588/ijtld.15.0228. PubMed PMID: 26260831. [DOI] [PubMed] [Google Scholar]
- 33.Pooran A, Booth H, Miller RF, Scott G, Badri M, Huggett JF, et al. Different screening strategies (single or dual) for the diagnosis of suspected latent tuberculosis: a cost effectiveness analysis. BMC pulmonary medicine. 2010;10:7. doi: 10.1186/1471-2466-10-7. Epub 2010/02/23. doi: 10.1186/1471-2466-10-7. PubMed PMID: 20170555; PubMed Central PMCID: PMCPmc2837635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mathew JL, John TJ, Parakh A. Intermittent Short Course Rifapentine-Isoniazid Combination for Preventing Tuberculosis in Children: Evidence based Medicine Viewpoint. Indian pediatrics. 2015;52(5):421–5. doi: 10.1007/s13312-015-0648-4. Epub 2015/06/11. PubMed PMID: 26061929. [DOI] [PubMed] [Google Scholar]
- 35.Bunyasi EW, Tameris M, Geldenhuys H, Schmidt BM, Luabeya AK, Mulenga H, et al. Evaluation of Xpert(R) MTB/RIF Assay in Induced Sputum and Gastric Lavage Samples from Young Children with Suspected Tuberculosis from the MVA85A TB Vaccine Trial. PloS one. 2015;10(11):e0141623. doi: 10.1371/journal.pone.0141623. Epub 2015/11/12. doi: 10.1371/journal.pone.0141623. PubMed PMID: 26554383; PubMed Central PMCID: PMCPmc4640848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zar HJ, Workman L, Isaacs W, Dheda K, Zemanay W, Nicol MP. Rapid diagnosis of pulmonary tuberculosis in African children in a primary care setting by use of Xpert MTB/RIF on respiratory specimens: a prospective study. The Lancet Global health. 2013;1(2):e97–104. doi: 10.1016/S2214-109X(13)70036-6. Epub 2014/08/12. doi: 10.1016/s2214-109x(13)70036-6. PubMed PMID: 25104164. [DOI] [PubMed] [Google Scholar]