Abstract
Impaired nocturnal blood pressure (BP) dipping (i.e., <10% decline in nocturnal BP) is associated with an increased risk of cerebrovascular and cardiovascular diseases. Excess sodium has been shown to impair BP regulation and increase cardiovascular disease risk, yet few studies have assessed the influence of dietary sodium on nocturnal dipping in normotensive adults. The purpose of this study was to determine the effects of dietary sodium on BP dipping in normotensive men and women. Eighty healthy normotensive adults participated in a controlled feeding study (men: n=39, 34±2 yrs; women: n=41, 41±2 yrs). Participants consumed a standardized run-in 100 mmol sodium·day−1 diet for 7 days, followed by 7 days of low sodium (LS; 20 mmol·day−1) and high sodium (HS; 300 mmol·day−1) diets in random order. On the final day of each diet, subjects wore a 24h ambulatory BP monitor, collected a 24h urine sample, and provided a blood sample. During the run-in diet, 24h urinary sodium excretion was 79.4±5.1 mmol·24h−1 in men and 85.3±5.5 mmol·24h−1 in women (p>0.05). Systolic BP dipping was not different between men (11.4±1.0%) and women (11.2±0.9%) (p>0.05). During the HS diet, 24h urinary sodium excretion increased compared to the LS diet in men (LS=31.7±4.6 mmol·24h−1 vs. HS=235.0±13.9 mmol·24h−1, p<0.01) and women (LS=25.8±2.2 mmol·24h−1 vs. HS=234.7±13.8 mmol·24h−1, p<0.01). Despite this large increase in sodium intake and excretion, systolic BP dipping was not blunted in men (LS=8.9±1.0% vs. HS=9.4±1.2%, p>0.05) or women (LS=10.3±0.8% vs. HS=10.5±0.8%, p>0.05). Among normotensive men and women, HS does not blunt nocturnal BP dipping.
Keywords: Nocturnal dipping, dietary sodium, sex differences, salt
INTRODUCTION
Normal blood pressure (BP) follows a circadian pattern, rising to the highest point in the morning and declining during sleeping periods. Nocturnal dipping is the measurement of BP decline during sleep periods compared to wake periods (1). Typically, BP declines ≥10% during sleep periods in most individuals, leading to the classification as “dippers.” Individuals with <10% BP decline are classified as “non-dippers” (2). Non-dippers have been shown to have increased cardiovascular mortality risks (3) and target organ damage (4,5), including silent cerebrovascular damage (6) and kidney damage (7). Most studies examining nocturnal dipping have been performed in clinical populations such as those with hypertension, chronic kidney disease, and primary aldosteronism (3,5,8–10). However, Ohkubo et al (3) provided strong evidence that normotensives with diminished nocturnal dipping have increased cardiovascular risk compared to normotensives with normal nocturnal dipping.
Over a 24h period, BP is influenced by several external factors, including habitual activity, stress, and posture (11). Endogenous factors such as the autonomic nervous system & endocrine factors also influence BP (12,13). Likewise dietary sodium has been shown to affect short- and long-term BP, as well as nocturnal BP dipping in several clinical populations (9,14,15). For example, sodium restriction improves BP dipping in patients with essential hypertension, chronic kidney disease, and hyperaldosteronism (8–10,16). However, there are few studies that have examined the effect of dietary sodium on BP dipping in normotensive men and women (14,17).
Therefore, the purpose of this study was to determine the effects of dietary sodium on BP dipping in normotensive adults. Because habitual sodium intake varies widely within the population (18), it was necessary to conduct a feeding study where sodium content was strictly controlled. We tested the hypothesis that a high sodium (HS) diet would blunt nocturnal dipping in normotensive adults compared to a low sodium (LS) diet. Participants first completed a standardized run-in (100 mmol sodium·day−1) diet before being randomized to a LS (20 mmol·day−1) or HS (300 mmol·day−1) diet. Participants consumed each diet for one week with nocturnal BP dipping assessed on the last day of each diet. Because there are known sex differences in BP regulation (19), our secondary hypothesis was that HS would blunt BP dipping more in men compared to women.
METHODS
Subjects
All experimental protocols were approved by the Institutional Review Board at the University of Delaware and were in compliance with guidelines set forth by the Declaration of Helsinki. Therefore, a total of 101 participants completed the controlled feeding study. Using the criteria of O’Brien et al. (1), we excluded participants if they did not have at least 15 daytime and 8 nocturnal BP measurements. Thus, the data presented herein are the 80 participants that had an adequate number of daytime and nocturnal BP measurements. Utilizing 80 participants allowed for the reliable detection (β>0.80) of a small effect of dietary sodium on nocturnal BP dipping (d=0.133, α=0.05).
Prior to a screening visit, all participants provided both a verbal and written consent before entering the study. During the initial screening visit, participants completed a medical history questionnaire. A 12-lead electrocardiogram, resting brachial BP (Dinamap, Dash 2000; GE Medical Systems, Milwaukee, WI), height, weight, and a fasted blood sample were obtained. All 80 participants were healthy normotensive adults (22–59 years) with a resting systolic BP of <140mmHg and a diastolic BP <90mmHg. Study participants were free of any known cardiovascular disease, and had no evidence of renal, metabolic, pulmonary, or neurological diseases. Participants were non-obese (body mass index, BMI<30 kg·m−2) and did not use nicotine products.
21-Day Controlled Feeding Study
All food was prepared by a registered dietitian. Participants first completed a standardized 7 day run-in (100.2±0.3 mmol sodium·day−1) diet. Immediately following the final day of the run-in diet, participants completed a 7 day LS (22.6±0.2 mmol·day−1) and a 7 day HS (309.5±3.6 mmol·day−1) diet in random order. Dietary potassium was controlled across all three diets (Run-in=69.4±1.2 mmol·day−1; LS=71.1±1.3 mmol·day−1, HS=69.8±1.3 mmol·day−1). In order to assure participants maintained a constant body weight throughout the controlled feeding study, energy content was appropriately adjusted using the Mifflin-St Jeor equation (20); therefore all diets were designed to be eucaloric. The macronutrient content was comprised of ~50% carbohydrate, ~30% fats, and ~20% protein. Participants were instructed to consume all of the provided food. Due to the robust differences in dietary sodium content, it was not possible to blind the participants.
Ambulatory Blood Pressure Monitoring
On the final day of each diet, participants wore a 24h ambulatory BP monitor (Model 90207; Spacelabs Medical, Issaquah, WA, USA) properly fitted for each individuals’ non-dominant arm size, as recommended by the manufacturer. Participants were instructed to maintain their daily activities and refrain from exercise and caffeine. Participants were instructed to maintain their normal waking and sleeping patterns on the final day of each diet and monitors were set accordingly to capture wake and sleep periods. BP was taken every 20 minutes during waking periods and every 30 minutes during sleeping periods. The relatively high frequency BP measurements allows for reliable week to week ambulatory BP measures, as previously demonstrated (21).
In order to reliably measure nocturnal dipping, ≥15 BP measurements were needed during wake periods, and ≥8 BP measurements were needed during sleep periods under each diet, as previously established (1). Nocturnal dipping was calculated as the percent decline in nocturnal BP: Nocturnal Dipping % = [(average daytime BP − average nocturnal BP)/average daytime BP]*100.
Blood and Urine Analysis
A fasted blood sample and a 24h urine collection were obtained on the final day of each diet. Blood and urine was analyzed for electrolyte concentration on each sodium diet (EasyElectrolyte Analyzer; Medica, Bedford, MA, USA). Urinary electrolyte content was calculated and normalized to 24h. Hemoglobin (Hb 201+ model, Hemocue, Lake Forest, CA, USA) and hematocrit (Pre-Calibrated Clay Adams, Readacrit Centrifuge, Becton Dickinson, Sparks, MD, USA) were analyzed from collected whole blood samples.
Statistical Analysis
Baseline screening anthropometrics, screening biochemical parameters, and the run-in diet were compared between men and women using unpaired t-Tests (GraphPad Prism 5, GraphPad Software, Inc., La Jolla, CA, USA). Participants were divided into subgroups based on sex and 2×2 (diet x sex) ANOVAs were used to compare biochemical parameters, ambulatory 24h BP (systolic, diastolic, mean arterial pressure), and nocturnal dipping. Post hoc analyses were performed using the Bonferroni method when appropriate. A two-way ANOVA was used to test for any sequencing effects, the first factor was diet and the second was the order in which participants received LS first (n=40) and HS first (n=40). The main outcome variables met the assumptions of the test (e.g. normal distribution and variance, SPSS Statistics 23, IBM, Armonk, NY). Significance was set at p<0.05 and values are reported as means ± SE.
RESULTS
Baseline Characteristics
Table 1 displays baseline demographic, anthropometric, and biochemical parameters (n=80) obtained during the screening visit during their habitual sodium intake. Men were younger than women participants (p<0.05), however men and women had similar BMI. Height, weight, systolic BP, and mean arterial pressure were significantly greater in men compared to women (p<0.05). Serum chloride, total cholesterol, and fasting HDL cholesterol were lower in men compared to women (p<0.05), while hemoglobin and hematocrit were greater in men (p<0.01). All participants had liver and kidney function within normal limits. All other biochemical parameters were similar during the initial screening visit between men and women.
Table 1.
Participant Baseline Data
| Men | Women | |
|---|---|---|
| Demographic & Anthropometric Data | ||
| Number of participants | 39 | 41 |
| Race | W29, B6, A4 | W35, B5, A1 |
| Age, yr. | 34 ± 2 | 41 ± 2* |
| Height, cm | 178 ± 1 | 167 ± 1* |
| Weight, kg | 78 ± 2 | 68 ± 1* |
| BMI, kg·m−2 | 25.0 ± 0.5 | 24.2 ± 0.4 |
| SBP, mmHg | 123 ± 2 | 116 ± 2* |
| DBP, mmHg | 77 ± 1 | 73 ± 2 |
| PP, mmHg | 46 ± 1 | 43 ± 1 |
| MAP, mmHg | 92 ± 1 | 87 ± 1* |
| Heart rate, beats·min−1 | 63 ± 1 | 64 ± 2 |
| Biochemical Parameters | ||
| Serum sodium, mmol·L−1 | 138.6 ± 0.9 | 139.0 ± 0.3 |
| Serum potassium, mmol·L−1 | 4.50 ± 0.10 | 4.52 ± 0.06 |
| Serum chloride, mmol·L−1 | 103.8 ± 0.4 | 106.0 ± 0.3* |
| Fasting total cholesterol, mg·dL−1 | 174 ± 5 | 192 ± 5* |
| Fasting LDL, mg·dL−1 | 102 ± 4 | 105 ± 4 |
| Fasting HDL, mg·dL−1 | 54 ± 2 | 72 ± 3* |
| Fasting triglycerides, mg·dL−1 | 92 ± 6 | 80 ± 5 |
| Blood urea nitrogen, mg·dL−1 | 14.2 ± 0.7 | 14.0 ± 0.6 |
| Hemoglobin, g·dL−1 | 15.2 ± 0.1 | 13.0 ± 0.1* |
| Hematocrit, % | 45 ± 0 | 39 ± 0* |
Values are means ± SE. A, Asian; B, black; BMI, body mass index; DBP, diastolic blood pressure; HDL, high-density lipoprotein;; LDL, low-density lipoprotein; MAP, mean arterial blood pressure; PP, pulse pressure; SBP, systolic blood pressure; W, white.
p <0.05 (men vs. women)
Responses to Standardized Run-in Diet
During the standardized run-in diet, 24h urine sodium excretion was not different between men and women (Fig 1, p>0.05). Systolic BP and mean arterial pressure were higher in men compared to women (see Table 2). Blood electrolyte analysis revealed that serum chloride was lower in men compared to women (p<0.05), while men also had an expected higher hematocrit and hemoglobin levels compared to women (p<0.01). Nocturnal dipping was not different between men and women (p>0.05) as presented in Table 2 and in Figure 2.
Figure 1.
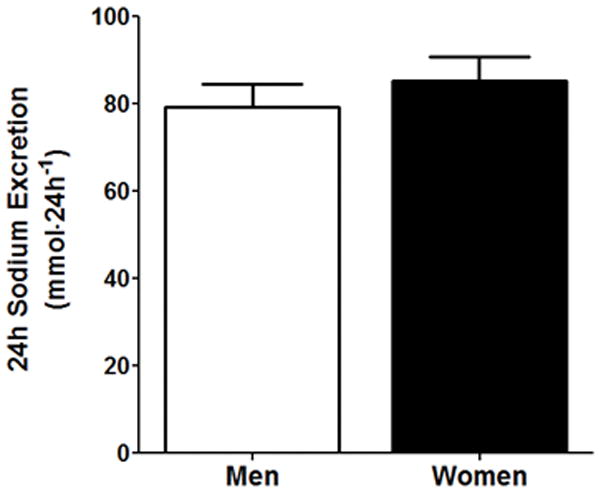
24h urinary sodium excretion between men and women during the standardized run-in diet. p>0.05 vs. men.
Table 2.
Blood Pressure and Biochemical Parameters during the Run-in Diet
| Men | Women | |
|---|---|---|
| 24h SBP, mmHg | 119 ± 1 | 112 ± 1* |
| 24h DBP, mmHg | 71 ± 1 | 70 ± 1 |
| 24h MAP, mmHg | 87 ± 1 | 84 ± 1* |
| Daytime SBP, mmHg | 124 ± 1 | 116 ± 1* |
| Daytime DBP, mmHg | 75 ± 1 | 74 ± 1 |
| Daytime MAP, mmHg | 90 ± 1 | 88 ± 1 |
| Nocturnal SBP, mmHg | 109 ± 2 | 103 ± 1* |
| Nocturnal DBP, mmHg | 63 ± 1 | 61 ± 1 |
| Nocturnal MAP, mmHg | 79 ± 1 | 76 ± 1* |
| 24h Heart Rate, bpm | 67 ± 1 | 68 ± 1 |
| Daytime Heart Rate, bpm | 70 ± 1 | 71 ± 1 |
| Nocturnal Heart Rate, bpm | 58 ± 1 | 61 ± 1 |
| Serum Sodium, mmol·L−1 | 138.2 ± 0.5 | 138.4 ± 0.3 |
| Serum Potassium, mmol·L−1 | 4.16 ± 0.07 | 4.06 ± 0.09 |
| Serum Chloride, mmol·L−1 | 102.1 ± 0.5 | 105.1 ± 0.5* |
| Hemoglobin, g·dL−1 | 16 ± 0.9 | 13 ± 0.2* |
| Hematocrit, % | 43 ± 1 | 38 ± 1* |
| Nocturnal DBP Dipping, % | 14.9 ± 1.3 | 16.8 ± 1.2 |
| Nocturnal MAP Dipping, % | 12.8 ± 1.1 | 13.8 ± 1.0 |
Values are means ± SE. DBP, Diastolic blood pressure; MAP, Mean arterial pressure; SBP, Systolic blood pressure.
p <0.05 (men vs. women)
Figure 2.
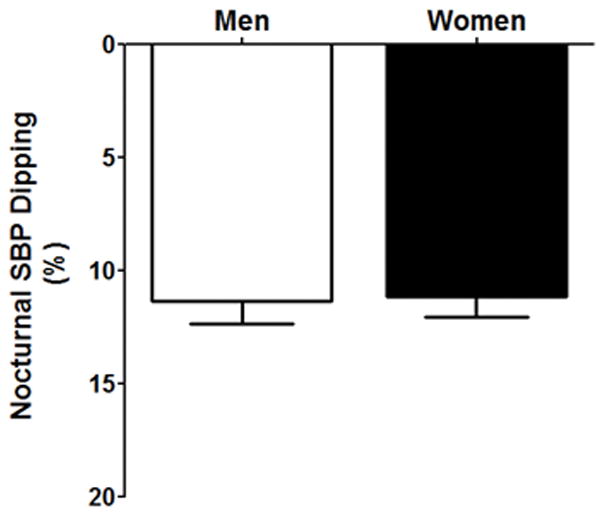
Nocturnal systolic blood pressure dipping (%) during the standardized run-in diet between men (white bar) and women (black bar). p>0.05 vs. men.
Responses to Controlled LS & HS Diets
In response to randomized HS and LS, 24h urine sodium excretion was significantly greater during HS compared to LS (main effect of diet, p>0.05, see Figure 3), with no differences between men and women. Men had no change in 24h BP (systolic, diastolic, and mean arterial pressure, p>0.05), while women had a significant increase in systolic BP under HS (p<0.05) and no difference in diastolic BP and mean arterial pressure (see Table 3). Hematocrit and hemoglobin were lower during HS compared to LS (main effect of diet p<0.05) and this response occurred in both men and women (p<0.05 for both). Serum sodium and chloride were significantly increased under HS compared to LS (p<0.05) with no difference between men and women. Nocturnal BP dipping was not different between LS and HS or between sexes (p>0.05), as presented in Table 3 and Figure 4. A two-way ANOVA tested for a potential confounding by sequencing effects, comparing diets and orders. The results of this analysis found no effect of order and no diet by order interaction (p=0.72, and p=0.38, respectively). A significant interaction or order effect would have suggested a potential confounding effect.
Figure 3.
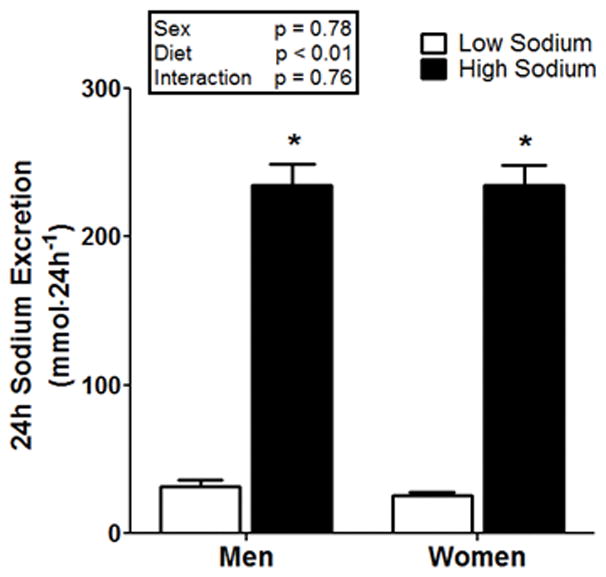
24h urinary sodium excretion in 39 normotensive men and 41 normotensive women after LS (white bars) and HS (black bars), each for 7 days in randomized order. *p<0.05 compared to LS.
Table 3.
24h Ambulatory Blood Pressure and Biochemical Parameters Response to Dietary Sodium Manipulation in Men and Women
| Men | Women | |||
|---|---|---|---|---|
| Low Sodium | High Sodium | Low Sodium | High Sodium | |
| 24h SBP, mmHg | 119 ± 1 | 119 ± 1 | 112 ± 1* | 114 ± 1*† |
| 24h DBP, mmHg | 72 ± 1 | 71 ± 1 | 70 ± 1 | 70 ± 1 |
| 24h MAP, mmHg | 87 ± 1 | 87 ± 1 | 84 ± 1* | 85 ± 1* |
| Daytime SBP, mmHg | 122 ± 1 | 124 ± 1† | 116 ± 1* | 118 ± 1*† |
| Daytime DBP, mmHg | 76 ± 1 | 75 ± 1 | 74 ± 1 | 74 ± 1 |
| Daytime MAP, mmHg | 91 ± 1 | 90 ± 1 | 88 ± 1* | 89 ± 1 |
| Nocturnal SBP, mmHg | 111 ± 1 | 111 ± 2 | 103 ± 1* | 106 ± 1* |
| Nocturnal DBP, mmHg | 64 ± 1 | 64 ± 1 | 62 ± 1 | 63 ± 1 |
| Nocturnal MAP, mmHg | 79 ± 1 | 80 ± 1 | 76 ± 1* | 78 ± 1 |
| 24h Heart Rate, bpm | 68 ± 1 | 65 ± 1 | 69 ± 2 | 65 ± 1† |
| Daytime Heart Rate, bpm | 71 ± 1 | 68 ± 1 | 73 ± 2 | 66 ± 2† |
| Nocturnal Heart Rate, bpm | 60 ± 1 | 58 ± 1 | 61 ± 1 | 60 ± 1† |
| Serum Sodium, mmol·L−1 | 137.9 ± 0.4 | 140.0 ± 0.3† | 137.4 ± 0.3 | 139.5 ± 0.4† |
| Serum Potassium, mmol·L−1 | 4.13 ± 0.08 | 4.08 ± 0.05 | 4.02 ± 0.06 | 3.98 ± 0.06 |
| Serum Chloride, mmol·L−1 | 101.1 ± 0.5 | 104.0 ± 0.4† | 102.6 ± 0.3* | 106.4 ± 0.4*† |
| Hemoglobin, g·dL−1 | 16.0 ± 0.9 | 15.3 ± 0.8† | 13.0 ± 0.2* | 11.8 ± 0.2*†‡ |
| Hematocrit, % | 43 ± 1 | 42 ± 1 | 39 ± 1* | 37 ± 1*†‡ |
| Nocturnal DBP Dipping, % | 14.7 ± 1.4 | 13.8 ± 1.8 | 15.8 ± 1.3 | 14.9 ± 1.0 |
| Nocturnal MAP Dipping, % | 12.9 ± 1.2 | 11.4 ± 1.4 | 12.8 ± 1.0 | 12.5 ± 0.8 |
Values are means ± SE. DBP, Diastolic blood pressure; MAP, Mean arterial pressure; SBP, Systolic blood pressure.
p <0.05 (men vs. women);
p <0.05 (low sodium vs high sodium);
p <0.05 (sex x diet).
Figure 4.
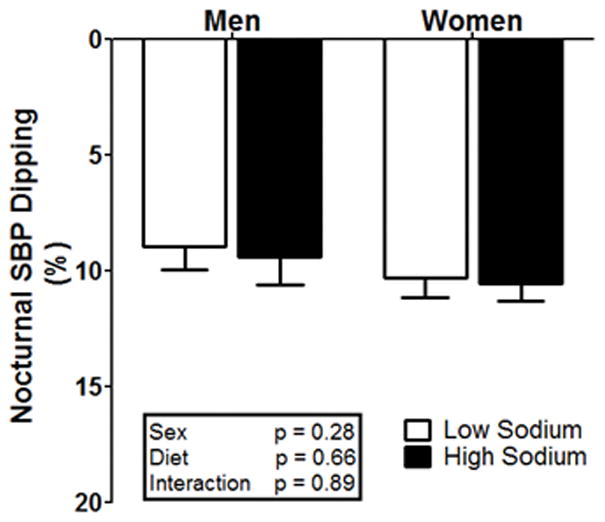
Nocturnal systolic blood pressure dipping (%) in men and women under controlled LS (white bars) and HS (black bars) diets.
A secondary analysis was performed to examine nocturnal dipping during LS and HS diets in those with <10% nocturnal BP decline prior to randomization. The secondary analysis was performed where participants were divided based on whether they exhibited a dipping pattern (>10% dip in systolic BP; n=48) or non-dipping pattern (<10% dip in systolic BP; n=32) during the standardized run-in diet. The subsequent BP dipping responses of these two groups were compared after randomization to the LS and HS diets. No differences were observed in the dipping group (LS BP dipping= 11.2±0.8%, HS BP dipping= 12.0±1.0%, p>0.05) or the non-dipping group (LS BP dipping= 7.2±1.1%, HS BP dipping= 6.9±0.9%, p>0.05). Further, no differences were observed between men and women when comparing dippers and non-dippers during the LS and HS diets (p>0.05). Another secondary analysis was performed to determine whether individuals classified as “salt-sensitive” had impaired nocturnal dipping during the HS diet. Salt-sensitivity was defined as a 5 mmHg or more change in 24h mean arterial pressure from LS to HS, as previously described (22). Ten participants were classified as salt-sensitive (Δ mean arterial pressure = 9±1 mmHg). Among those classified as salt-sensitive, nocturnal dipping response was not different from a LS to HS diet (LS= 8.7±2.3% vs. HS= 9.1±1.5%, p>0.05).
DISCUSSION
The main findings of the current study are that (1) men and women have similar nocturnal dipping responses during a standardized run-in 100 mmol sodium·day−1 diet, and (2) a HS diet does not blunt nocturnal dipping in normotensive men and women. Nocturnal dipping is the degree to which pressure declines during sleep periods. Impairments in circadian dipping patterns have been shown to increase cardiovascular disease risks in hypertension, diabetes, chronic kidney disease, and aldosteronism (2,5,10,13,23). Among normotensives, impaired nocturnal dipping is associated with increased cardiovascular mortality (3). However, our findings demonstrate that one week of HS loading does not impair nocturnal dipping in normotensive adults.
Impaired nocturnal dipping and high dietary sodium can have a detrimental impact on the cardiovascular system. A favorable reduction in BP during nocturnal periods reduces hemodynamic load placed on the heart, arteries, and kidneys for prolonged periods. Understanding the effects of dietary sodium on nocturnal BP decline is important because even with a normal BP, there is evidence that impaired dipping increases cardiac remodeling, left ventricular diastolic dysfunction (2), carotid-intima thickness, and the presence of carotid artery plaque formation (24). These subclinical measurements are all associated with increased future cardiovascular events and hypertension (25). Further, disturbed circadian BP patterns with no decline in nocturnal BP are associated with increased microalbuminuria and future risk of renal damage (7). Similarly, excess dietary sodium has previously been shown to have adverse cardiovascular effects leading to target organ damage (26), vascular dysfunction (27–29), and sodium dependent hypertension (30) in individuals with hypertensive and normotensive BP. Despite the increase cardiovascular risks with excess dietary sodium consumption, we observed no significant differences in nocturnal dipping during a HS diet. These findings suggest that dietary sodium does not adversely influence circadian BP patterns in normotensive adults.
Among healthy men and women, there are observed differences in BP regulation (31). Studies measuring ambulatory 24h BPs have shown men to have higher average BP than age-matched women (19), as documented during the run-in diet in the current study. The differences in 24h average BP are thought to be influenced by both the endocrine and autonomic nervous systems. In men, elevation in testosterone after puberty has been shown to increase BP and blunt dipping compared to prepubescent testosterone levels (32). While in premenopausal women, estrogen has a direct vasodilator effect on blood vessels and potentially blunts sympathetic mediated vasoconstriction (33). In healthy women, the menstrual cycle does not influence ambulatory 24h average BP during low (follicular) or high (luteal) estrogen phases of the menstrual cycle during LS and HS diets (34). In our study, average 24h systolic BP was only modestly increased from the LS to HS diet in women, with no change in systolic BP in men. Despite known sex differences in BP regulation and differences in mean 24h SBP, nocturnal dipping was not significantly different between sexes on LS and HS diets.
While our primary focus was to determine if dietary sodium altered BP dipping in normotensive men and women, we also performed a secondary analysis where we divided participants based on whether they exhibited a dipping or non-dipping response during the run-in diet. We then compared the responses between these two groups during the LS and HS diets, and found that the HS diet did not have an effect on BP dipping in adults initially classified as dippers or non-dippers. This secondary analysis is consistent with our primary conclusion that dietary sodium does not influence BP dipping in normotensive adults.
Several methodological aspects of this study strengthen the major findings. First, we utilized a controlled feeding design where a registered dietitian prepared all food. This approach is stronger and overcomes the limitations associated with self-reported dietary intake. Second, following the initial standardized run-in diet, we used a randomized cross-over design to measure nocturnal dipping under LS and HS diets. The findings of the current study builds upon previous studies examining nocturnal dipping under habitual and non-randomized sodium diets (14,17). Third, a large cohort consisting of 80 normotensive adults completed the controlled feeding study with an adequate number of BPs during daytime and nocturnal periods. This was done by utilizing strict criteria of previously established guidelines set forth by O’Brien et al (1), to remove data bias and reduce the possibility of a type 1 error. Fourth, we assessed 24h urinary sodium excretion (35) to confirm compliance to the diets. Thus, this comprehensive assessment of nocturnal dipping clearly demonstrates that HS does not disrupt nocturnal dipping in healthy normotensive men and women.
A limitation to our study is that estrogen levels and menopausal status were not assessed. Nonetheless, a previous study reported that varying phases of the menstrual cycle (Iuteal & follicular phase) do not affect ambulatory 24h average BP under LS and HS diets in healthy normotensive women (34). Additionally, we excluded 21 participants from analysis for an inadequate number of BP measurements. Methods for assessing nocturnal dipping and ambulatory BP monitoring vary widely in the literature, therefore we utilized previously established criteria to reduce analysis bias (1). It is important to note that the study findings are the same when including all participants. Thus, excluding participants based on the criteria of O’ Brian et al (1) did not alter the main study conclusions. Although not the focus of the current study, individuals with salt-sensitive hypertension have been shown to have impaired nocturnal dipping, and sodium restriction has been observed to improve nocturnal dipping (9). The findings of the current study found that nocturnal dipping was not altered during the HS condition among the 10 normotensive participants individually determined to be salt-sensitive.
In summary, among adults with normotensive BP, nocturnal dipping was not different between LS and HS diets. Despite known sex differences in BP regulation, dietary sodium did not influence nocturnal dipping in normotensive men and women. Thus, dietary sodium likely does not increase CVD risks through impaired nocturnal dipping in normotensive men and women. Future studies are needed to determine if dietary sodium alters other aspects of the circadian BP pattern.
SUMMARY TABLE.
What is known about topic
High dietary sodium and impaired nocturnal blood pressure dipping have been shown to have adverse cardiovascular effects.
Several clinical populations, including hypertensives, have impaired blood pressure dipping when consuming a high sodium diet, which is restored during sodium restriction.
What this study adds
Few studies have examined nocturnal blood pressure dipping in normotensive men and women while precisely controlling dietary sodium levels.
The findings of the current study provide evidence that nocturnal blood pressure dipping is not different between normotensive men and women during well-controlled high and low sodium diets.
Acknowledgments
The authors would like to thank Ryan Polig PhD, Paul Kolm PhD, Allen Prettyman PhD FNP-BC, Katherine Masso BS, Meghan Ramick BS, Bryce Muth, MS, Alyssa Vogel, BS, and the dietitians at the Eugene du Pont Preventive Medicine & Rehabilitation Institute for their assistance with this study.
GRANTS
This research was funded by NIH grant: R01-HL104106.
Footnotes
DISCLOSURE
No conflicts of interest were declared by the authors.
References
- 1.O’Brien E, Coats A, Owens P, Petrie J, Padfield PL, Littler WA, et al. Use and interpretation of ambulatory blood pressure monitoring: recommendations of the British hypertension society. BMJ. 2000 Apr 22;320(7242):1128–1134. doi: 10.1136/bmj.320.7242.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Soylu A, Duzenli MA, Yazici M, Ozdemir K, Tokac M, Gok H. The effect of nondipping blood pressure patterns on cardiac structural changes and left ventricular diastolic functions in normotensives. Echocardiography. 2009;26(4):378–387. doi: 10.1111/j.1540-8175.2008.00821.x. [DOI] [PubMed] [Google Scholar]
- 3.Ohkubo T, Hozawa A, Yamaguchi J, Kikuya M, Ohmori K, Michimata M, et al. Prognostic significance of the nocturnal decline in blood pressure in individuals with and without high 24-h blood pressure: the Ohasama study. J Hypertens. 2002;20(11):2183–2189. doi: 10.1097/00004872-200211000-00017. [DOI] [PubMed] [Google Scholar]
- 4.Verdecchia P, Schillaci G, Guerrieri M, Gatteschi C, Benemio G, Boldrini F, et al. Circadian blood pressure changes and left ventricular hypertrophy in essential hypertension. Circulation. 1990 Feb;81(2):528–536. doi: 10.1161/01.cir.81.2.528. [DOI] [PubMed] [Google Scholar]
- 5.Kario K, Matsuo T, Kobayashi H, Imiya M, Matsuo M, Shimada K. Nocturnal fall of blood pressure and silent cerebrovascular damage in elderly hypertensive patients. Advanced silent cerebrovascular damage in extreme dippers. Hypertension. 1996 Jan;27(1):130–135. doi: 10.1161/01.hyp.27.1.130. [DOI] [PubMed] [Google Scholar]
- 6.Shimada K, Kawamoto A, Matsubayashi K, Nishinaga M, Kimura S, Ozawa T. Diurnal blood pressure variations and silent cerebrovascular damage in elderly patients with hypertension. J Hypertens. 1992 Aug;10(8):875–878. [PubMed] [Google Scholar]
- 7.Bianchi S, Bigazzi R, Baldari G, Sgherri G, Campese VM. Diurnal variations of blood pressure and microalbuminuria in essential hypertension. Am J Hypertens. 1994 Jan;7(1):23–29. doi: 10.1093/ajh/7.1.23. [DOI] [PubMed] [Google Scholar]
- 8.Uzu T, Nishimura M, Fujii T, Takeji M, Kuroda S, Nakamura S, et al. Changes in the circadian rhythm of blood pressure in primary aldosteronism in response to dietary sodium restriction and adrenalectomy. J Hypertens. 1998;16(12):1745–1748. doi: 10.1097/00004872-199816120-00006. [DOI] [PubMed] [Google Scholar]
- 9.Uzu T, Ishikawa K, Fujii T, Nakamura S, Inenaga T, Kimura G. Sodium restriction shifts circadian rhythm of blood pressure from nondipper to dipper in essential hypertension. Circulation. 1997 Sep 16;96(6):1859–1862. doi: 10.1161/01.cir.96.6.1859. [DOI] [PubMed] [Google Scholar]
- 10.Fukuda M, Munemura M, Usami T, Nakao N, Takeuchi O, Kamiya Y, et al. Nocturnal blood pressure is elevated with natriuresis and proteinuria as renal function deteriorates in nephropathy. Kidney Int. 2004;65(2):621–625. doi: 10.1111/j.1523-1755.2004.00419.x. [DOI] [PubMed] [Google Scholar]
- 11.Mancia G. Short- and long-term blood pressure variability: present and future. Hypertension. 2012 Aug;60(2):512–517. doi: 10.1161/HYPERTENSIONAHA.112.194340. [DOI] [PubMed] [Google Scholar]
- 12.Smolensky MH, Haus E. Circadian rhythms and clinical medicine with applications to hypertension. American journal of hypertension. 2001;14(9):S280–S290. doi: 10.1016/s0895-7061(01)02175-6. [DOI] [PubMed] [Google Scholar]
- 13.Okamoto LE, Gamboa A, Shibao C, Black BK, Diedrich A, Raj SR, et al. Nocturnal blood pressure dipping in the hypertension of autonomic failure. Hypertension. 2009 Feb;53(2):363–369. doi: 10.1161/HYPERTENSIONAHA.108.124552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bankir L, Bochud M, Maillard M, Bovet P, Gabriel A, Burnier M. Nighttime blood pressure and nocturnal dipping are associated with daytime urinary sodium excretion in African subjects. Hypertension. 2008 Apr;51(4):891–898. doi: 10.1161/HYPERTENSIONAHA.107.105510. [DOI] [PubMed] [Google Scholar]
- 15.Sachdeva A, Weder AB. Nocturnal sodium excretion, blood pressure dipping, and sodium sensitivity. Hypertension. 2006 Oct;48(4):527–533. doi: 10.1161/01.HYP.0000240268.37379.7c. [DOI] [PubMed] [Google Scholar]
- 16.Uzu T, Kimura G. Diuretics shift circadian rhythm of blood pressure from nondipper to dipper in essential hypertension. Circulation. 1999 Oct 12;100(15):1635–1638. doi: 10.1161/01.cir.100.15.1635. [DOI] [PubMed] [Google Scholar]
- 17.Simonetti GD, Farese S, Aregger F, Uehlinger D, Frey FJ, Mohaupt MG. Nocturnal dipping behaviour in normotensive white children and young adults in response to changes in salt intake. J Hypertens. 2010 May;28(5):1027–1033. doi: 10.1097/HJH.0b013e328337854d. [DOI] [PubMed] [Google Scholar]
- 18.Mente A, O’Donnell MJ, Rangarajan S, McQueen MJ, Poirier P, Wielgosz A, et al. Association of urinary sodium and potassium excretion with blood pressure. N Engl J Med. 2014;371(7):601–611. doi: 10.1056/NEJMoa1311989. [DOI] [PubMed] [Google Scholar]
- 19.Reckelhoff JF. Gender differences in the regulation of blood pressure. Hypertension. 2001 May;37(5):1199–1208. doi: 10.1161/01.hyp.37.5.1199. [DOI] [PubMed] [Google Scholar]
- 20.Frankenfield D, Roth-Yousey L, Compher C Evidence Analysis Working Group. Comparison of predictive equations for resting metabolic rate in healthy nonobese and obese adults: a systematic review. J Am Diet Assoc. 2005;105(5):775–789. doi: 10.1016/j.jada.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 21.Fotherby MD, Potter JF. Reproducibility of ambulatory and clinic blood pressure measurements in elderly hypertensive subjects. J Hypertens. 1993;11(5):573–580. doi: 10.1097/00004872-199305000-00014. [DOI] [PubMed] [Google Scholar]
- 22.Matthews EL, Brian MS, Ramick MG, Lennon-Edwards SL, Edwards DG, Farquhar WB. High Dietary Sodium Reduces Brachial Artery Flow Mediated Dilation in Humans with Salt Sensitive and Salt Resistant Blood Pressure. J Appl Physiol. 2015 doi: 10.1152/japplphysiol.00023.2015. jap. 00023.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kario K, Pickering TG, Matsuo T, Hoshide S, Schwartz JE, Shimada K. Stroke prognosis and abnormal nocturnal blood pressure falls in older hypertensives. Hypertension. 2001 Oct;38(4):852–857. doi: 10.1161/hy1001.092640. [DOI] [PubMed] [Google Scholar]
- 24.Routledge FS, McFetridge-Durdle JA, Dean C. Night-time blood pressure patterns and target organ damage: a review. Can J Cardiol. 2007;23(2):132–138. doi: 10.1016/s0828-282x(07)70733-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Touboul PJ, Labreuche J, Vicaut E, Amarenco P GENIC Investigators. Carotid intima-media thickness, plaques, and Framingham risk score as independent determinants of stroke risk. Stroke. 2005 Aug;36(8):1741–1745. doi: 10.1161/01.STR.0000174490.23495.57. [DOI] [PubMed] [Google Scholar]
- 26.du Cailar G, Ribstein J, Mimran A. Dietary sodium and target organ damage in essential hypertension. Am J Hypertens. 2002 Mar;15(3):222–229. doi: 10.1016/s0895-7061(01)02287-7. [DOI] [PubMed] [Google Scholar]
- 27.DuPont JJ, Greaney JL, Wenner MM, Lennon-Edwards SL, Sanders PW, Farquhar WB, et al. High dietary sodium intake impairs endothelium-dependent dilation in healthy salt-resistant humans. J Hypertens. 2013 Mar;31(3):530–536. doi: 10.1097/HJH.0b013e32835c6ca8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greaney JL, DuPont JJ, Lennon-Edwards SL, Sanders PW, Edwards DG, Farquhar WB. Dietary sodium loading impairs microvascular function independent of blood pressure in humans: role of oxidative stress. J Physiol. 2012 Nov 1;590(Pt 21):5519–5528. doi: 10.1113/jphysiol.2012.236992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dickinson KM, Clifton PM, Burrell LM, Barrett PHR, Keogh JB. Postprandial effects of a high salt meal on serum sodium, arterial stiffness, markers of nitric oxide production and markers of endothelial function. Atherosclerosis. 2014;232(1):211–216. doi: 10.1016/j.atherosclerosis.2013.10.032. [DOI] [PubMed] [Google Scholar]
- 30.Weinberger MH, Fineberg NS, Fineberg SE, Weinberger M. Salt sensitivity, pulse pressure, and death in normal and hypertensive humans. Hypertension. 2001 Feb;37(2 Pt 2):429–432. doi: 10.1161/01.hyp.37.2.429. [DOI] [PubMed] [Google Scholar]
- 31.Hart EC, Charkoudian N, Wallin BG, Curry TB, Eisenach JH, Joyner MJ. Sex differences in sympathetic neural-hemodynamic balance: implications for human blood pressure regulation. Hypertension. 2009 Mar;53(3):571–576. doi: 10.1161/HYPERTENSIONAHA.108.126391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harshfield GA, Alpert BS, Pulliam DA, Somes GW, Wilson DK. Ambulatory blood pressure recordings in children and adolescents. Pediatrics. 1994 Aug;94(2 Pt 1):180–184. [PubMed] [Google Scholar]
- 33.Gilligan DM, Badar DM, Panza JA, Quyyumi AA, Cannon RO., 3rd Acute vascular effects of estrogen in postmenopausal women. Circulation. 1994 Aug;90(2):786–791. doi: 10.1161/01.cir.90.2.786. [DOI] [PubMed] [Google Scholar]
- 34.Pechere-Bertschi A, Maillard M, Stalder H, Brunner HR, Burnier M. Blood pressure and renal haemodynamic response to salt during the normal menstrual cycle. Clin Sci (Lond) 2000 Jun;98(6):697–702. [PubMed] [Google Scholar]
- 35.McLean RM. Measuring population sodium intake: a review of methods. Nutrients. 2014;6(11):4651–4662. doi: 10.3390/nu6114651. [DOI] [PMC free article] [PubMed] [Google Scholar]


