Abstract
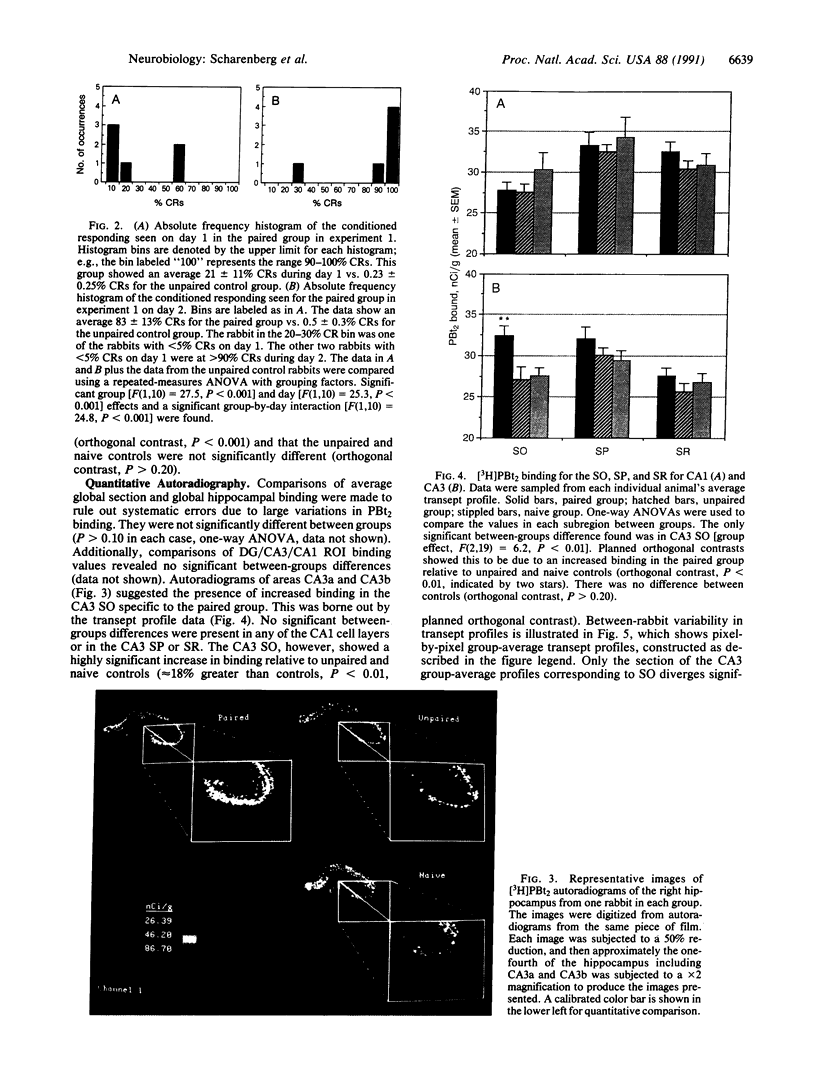
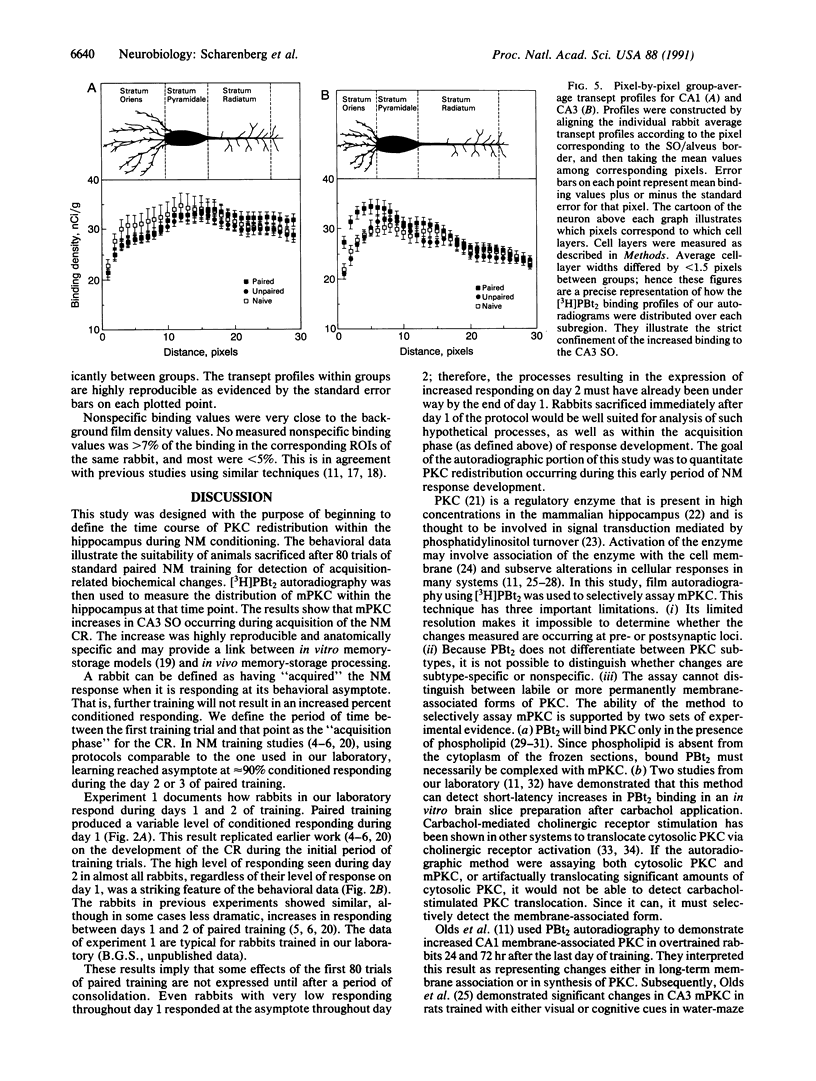
This manuscript describes experiments designed to investigate protein kinase C redistribution occurring during acquisition of the rabbit nictitating membrane (NM) conditioned response (CR). The first experiment defined the acquisition phase of the NM response for our laboratory. A group of rabbits (n = 6) was given 2 days of paired NM training; a second group (n = 6) was given 2 days of unpaired NM training. The data document a variable level of responding on day 1 for rabbits given paired training (mean +/- SEM, 21 +/- 11% CRs) but show that on day 2 most rabbits reached the behavioral asymptote (five of six rabbits responding with greater than 85% CRs). Rabbits responding at the behavioral asymptote were defined as having acquired the NM conditioned response. These data were interpreted to indicate that 1 day of training initiated processes necessary for behavioral acquisition (i.e., responding at the behavioral asymptote). A quantitative film autoradiographic study of [3H]phorbol 12,13-dibutyrate binding was then used to determine the distribution of hippocampal protein kinase C in rabbits sacrificed after receiving either 1 day of paired stimuli (n = 10), 1 day of unpaired stimuli (n = 6), or no stimuli (n = 6). Autoradiograms were analyzed by measuring binding in strictly defined regions of interest and from transept profiles. A significant increase in binding of the phorbol ester was found in the CA3 stratum oriens in the paired group relative to unpaired and naive controls. No other significant differences were found.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akase E., Alkon D. L., Disterhoft J. F. Hippocampal lesions impair memory of short-delay conditioned eye blink in rabbits. Behav Neurosci. 1989 Oct;103(5):935–943. doi: 10.1037//0735-7044.103.5.935. [DOI] [PubMed] [Google Scholar]
- Akers R. F., Lovinger D. M., Colley P. A., Linden D. J., Routtenberg A. Translocation of protein kinase C activity may mediate hippocampal long-term potentiation. Science. 1986 Feb 7;231(4738):587–589. doi: 10.1126/science.3003904. [DOI] [PubMed] [Google Scholar]
- Bank B., DeWeer A., Kuzirian A. M., Rasmussen H., Alkon D. L. Classical conditioning induces long-term translocation of protein kinase C in rabbit hippocampal CA1 cells. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1988–1992. doi: 10.1073/pnas.85.6.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger T. W., Rinaldi P. C., Weisz D. J., Thompson R. F. Single-unit analysis of different hippocampal cell types during classical conditioning of rabbit nictitating membrane response. J Neurophysiol. 1983 Nov;50(5):1197–1219. doi: 10.1152/jn.1983.50.5.1197. [DOI] [PubMed] [Google Scholar]
- Berger T. W., Thompson R. F. Neuronal plasticity in the limbic system during classical conditioning of the rabbit nictitating membrane response. I. The hippocampus. Brain Res. 1978 Apr 28;145(2):323–346. doi: 10.1016/0006-8993(78)90866-1. [DOI] [PubMed] [Google Scholar]
- Bland B. H., Seto M. G., Sinclair B. R., Fraser S. M. The pharmacology of hippocampal theta cells: evidence that the sensory processing correlate is cholinergic. Brain Res. 1984 May 7;299(1):121–131. doi: 10.1016/0006-8993(84)90794-7. [DOI] [PubMed] [Google Scholar]
- Bland B. H. The physiology and pharmacology of hippocampal formation theta rhythms. Prog Neurobiol. 1986;26(1):1–54. doi: 10.1016/0301-0082(86)90019-5. [DOI] [PubMed] [Google Scholar]
- Bland S. K., Bland B. H. Medial septal modulation of hippocampal theta cell discharges. Brain Res. 1986 Jun 4;375(1):102–116. doi: 10.1016/0006-8993(86)90963-7. [DOI] [PubMed] [Google Scholar]
- Coulter D. A., Lo Turco J. J., Kubota M., Disterhoft J. F., Moore J. W., Alkon D. L. Classical conditioning reduces amplitude and duration of calcium-dependent afterhyperpolarization in rabbit hippocampal pyramidal cells. J Neurophysiol. 1989 May;61(5):971–981. doi: 10.1152/jn.1989.61.5.971. [DOI] [PubMed] [Google Scholar]
- Crutcher K. A., Madison R., Davis J. N. A study of the rat septohippocampal pathway using anterograde transport of horseradish peroxidase. Neuroscience. 1981;6(10):1961–1973. doi: 10.1016/0306-4522(81)90036-1. [DOI] [PubMed] [Google Scholar]
- Disterhoft J. F., Coulter D. A., Alkon D. L. Conditioning-specific membrane changes of rabbit hippocampal neurons measured in vitro. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2733–2737. doi: 10.1073/pnas.83.8.2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disterhoft J. F., Golden D. T., Read H. L., Coulter D. A., Alkon D. L. AHP reductions in rabbit hippocampal neurons during conditioning correlate with acquisition of the learned response. Brain Res. 1988 Oct 11;462(1):118–125. doi: 10.1016/0006-8993(88)90593-8. [DOI] [PubMed] [Google Scholar]
- Disterhoft J. F., Kwan H. H., Lo W. D. Nictitating membrane conditioning to tone in the immobilized albino rabbit. Brain Res. 1977 Nov 25;137(1):127–143. doi: 10.1016/0006-8993(77)91016-2. [DOI] [PubMed] [Google Scholar]
- Dunphy W. G., Kochenburger R. J., Castagna M., Blumberg P. M. Kinetics and subcellular localization of specific [3H]phorbol 12, 13-dibutyrate binding by mouse brain. Cancer Res. 1981 Jul;41(7):2640–2647. [PubMed] [Google Scholar]
- Farrar W. L., Anderson W. B. Interleukin-2 stimulates association of protein kinase C with plasma membrane. Nature. 1985 May 16;315(6016):233–235. doi: 10.1038/315233a0. [DOI] [PubMed] [Google Scholar]
- Gormezano I., Coleman S. R. The law of effect and CR contingent modification of the UCS. Cond Reflex. 1973 Jan-Mar;8(1):41–56. doi: 10.1007/BF03000282. [DOI] [PubMed] [Google Scholar]
- Harris E. W., Cotman C. W. Long-term potentiation of guinea pig mossy fiber responses is not blocked by N-methyl D-aspartate antagonists. Neurosci Lett. 1986 Sep 25;70(1):132–137. doi: 10.1016/0304-3940(86)90451-9. [DOI] [PubMed] [Google Scholar]
- Huang F. L., Yoshida Y., Nakabayashi H., Huang K. P. Differential distribution of protein kinase C isozymes in the various regions of brain. J Biol Chem. 1987 Nov 15;262(32):15714–15720. [PubMed] [Google Scholar]
- Konopacki J., MacIver M. B., Bland B. H., Roth S. H. Carbachol-induced EEG 'theta' activity in hippocampal brain slices. Brain Res. 1987 Mar 3;405(1):196–198. doi: 10.1016/0006-8993(87)91009-2. [DOI] [PubMed] [Google Scholar]
- Kramis R., Vanderwolf C. H., Bland B. H. Two types of hippocampal rhythmical slow activity in both the rabbit and the rat: relations to behavior and effects of atropine, diethyl ether, urethane, and pentobarbital. Exp Neurol. 1975 Oct;49(1 Pt 1):58–85. doi: 10.1016/0014-4886(75)90195-8. [DOI] [PubMed] [Google Scholar]
- Leach K. L., James M. L., Blumberg P. M. Characterization of a specific phorbol ester aporeceptor in mouse brain cytosol. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4208–4212. doi: 10.1073/pnas.80.14.4208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liles W. C., Hunter D. D., Meier K. E., Nathanson N. M. Activation of protein kinase C induces rapid internalization and subsequent degradation of muscarinic acetylcholine receptors in neuroblastoma cells. J Biol Chem. 1986 Apr 25;261(12):5307–5313. [PubMed] [Google Scholar]
- LoTurco J. L., Coulter D. A., Alkon D. L. Enhancement of synaptic potentials in rabbit CA1 pyramidal neurons following classical conditioning. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1672–1676. doi: 10.1073/pnas.85.5.1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch G., Rose G., Gall C. Anatomical and functional aspects of the septo-hippocampal projections. Ciba Found Symp. 1977;(58):5–24. doi: 10.1002/9780470720394.ch3. [DOI] [PubMed] [Google Scholar]
- Mamounas L. A., Thompson R. F., Lynch G., Baudry M. Classical conditioning of the rabbit eyelid response increases glutamate receptor binding in hippocampal synaptic membranes. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2548–2552. doi: 10.1073/pnas.81.8.2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing R. O., Stevens A. M., Kiyasu E., Sneade A. B. Nicotinic and muscarinic agonists stimulate rapid protein kinase C translocation in PC12 cells. J Neurosci. 1989 Feb;9(2):507–512. doi: 10.1523/JNEUROSCI.09-02-00507.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizumori S. J., McNaughton B. L., Barnes C. A., Fox K. B. Preserved spatial coding in hippocampal CA1 pyramidal cells during reversible suppression of CA3c output: evidence for pattern completion in hippocampus. J Neurosci. 1989 Nov;9(11):3915–3928. doi: 10.1523/JNEUROSCI.09-11-03915.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaghan D. T., Holets V. R., Toy D. W., Cotman C. W. Anatomical distributions of four pharmacologically distinct 3H-L-glutamate binding sites. Nature. 1983 Nov 10;306(5939):176–179. doi: 10.1038/306176a0. [DOI] [PubMed] [Google Scholar]
- Monaghan D. T., Yao D., Cotman C. W. Distribution of [3H]AMPA binding sites in rat brain as determined by quantitative autoradiography. Brain Res. 1984 Dec 17;324(1):160–164. doi: 10.1016/0006-8993(84)90636-x. [DOI] [PubMed] [Google Scholar]
- Murphy K. M., Gould R. J., Oster-Granite M. L., Gearhart J. D., Snyder S. H. Phorbol ester receptors: autoradiographic identification in the developing rat. Science. 1983 Dec 2;222(4627):1036–1038. doi: 10.1126/science.6316499. [DOI] [PubMed] [Google Scholar]
- Nicoll R. A., Kauer J. A., Malenka R. C. The current excitement in long-term potentiation. Neuron. 1988 Apr;1(2):97–103. doi: 10.1016/0896-6273(88)90193-6. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Studies and perspectives of protein kinase C. Science. 1986 Jul 18;233(4761):305–312. doi: 10.1126/science.3014651. [DOI] [PubMed] [Google Scholar]
- Nyakas C., Luiten P. G., Spencer D. G., Traber J. Detailed projection patterns of septal and diagonal band efferents to the hippocampus in the rat with emphasis on innervation of CA1 and dentate gyrus. Brain Res Bull. 1987 Apr;18(4):533–545. doi: 10.1016/0361-9230(87)90117-1. [DOI] [PubMed] [Google Scholar]
- Olds J. L., Anderson M. L., McPhie D. L., Staten L. D., Alkon D. L. Imaging of memory-specific changes in the distribution of protein kinase C in the hippocampus. Science. 1989 Aug 25;245(4920):866–869. doi: 10.1126/science.2772638. [DOI] [PubMed] [Google Scholar]
- Olds J. L., Golski S., McPhie D. L., Olton D., Mishkin M., Alkon D. L. Discrimination learning alters the distribution of protein kinase C in the hippocampus of rats. J Neurosci. 1990 Nov;10(11):3707–3713. doi: 10.1523/JNEUROSCI.10-11-03707.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan H. S., Frey K. A., Young A. B., Penney J. B., Jr Changes in [3H]muscimol binding in substantia nigra, entopeduncular nucleus, globus pallidus, and thalamus after striatal lesions as demonstrated by quantitative receptor autoradiography. J Neurosci. 1983 Jun;3(6):1189–1198. doi: 10.1523/JNEUROSCI.03-06-01189.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowntree C. I., Bland B. H. An analysis of cholinoceptive neurons in the hippocampal formation by direct microinfusion. Brain Res. 1986 Jan 1;362(1):98–113. doi: 10.1016/0006-8993(86)91403-4. [DOI] [PubMed] [Google Scholar]
- Shoyab M., Todaro G. J. Specific high affinity cell membrane receptors for biologically active phorbol and ingenol esters. Nature. 1980 Dec 4;288(5790):451–455. doi: 10.1038/288451a0. [DOI] [PubMed] [Google Scholar]
- Spencer D. G., Jr, Horváth E., Traber J. Direct autoradiographic determination of M1 and M2 muscarinic acetylcholine receptor distribution in the rat brain: relation to cholinergic nuclei and projections. Brain Res. 1986 Aug 13;380(1):59–68. doi: 10.1016/0006-8993(86)91429-0. [DOI] [PubMed] [Google Scholar]
- Swanson L. W., Cowan W. M. The connections of the septal region in the rat. J Comp Neurol. 1979 Aug 15;186(4):621–655. doi: 10.1002/cne.901860408. [DOI] [PubMed] [Google Scholar]
- Takai Y., Kishimoto A., Iwasa Y., Kawahara Y., Mori T., Nishizuka Y. Calcium-dependent activation of a multifunctional protein kinase by membrane phospholipids. J Biol Chem. 1979 May 25;254(10):3692–3695. [PubMed] [Google Scholar]
- Takai Y., Kishimoto A., Iwasa Y., Kawahara Y., Mori T., Nishizuka Y., Tamura A., Fujii T. A role of membranes in the activation of a new multifunctional protein kinase system. J Biochem. 1979 Aug;86(2):575–578. doi: 10.1093/oxfordjournals.jbchem.a132557. [DOI] [PubMed] [Google Scholar]
- TerBush D. R., Holz R. W. Effects of phorbol esters, diglyceride, and cholinergic agonists on the subcellular distribution of protein kinase C in intact or digitonin-permeabilized adrenal chromaffin cells. J Biol Chem. 1986 Dec 25;261(36):17099–17106. [PubMed] [Google Scholar]
- Vinogradova O. S., Brazhnik E. S. Neuronal aspects of septo-hippocampal relations. Ciba Found Symp. 1977;(58):145–177. doi: 10.1002/9780470720394.ch8. [DOI] [PubMed] [Google Scholar]
- Whishaw I. Q., Dyck R. Comparative potency of tactile, auditory, and visual stimulus repetition in eliciting activated forebrain EEG in the rabbit. Behav Neurosci. 1984 Apr;98(2):333–344. doi: 10.1037//0735-7044.98.2.333. [DOI] [PubMed] [Google Scholar]
- Worley P. F., Baraban J. M., Snyder S. H. Heterogeneous localization of protein kinase C in rat brain: autoradiographic analysis of phorbol ester receptor binding. J Neurosci. 1986 Jan;6(1):199–207. doi: 10.1523/JNEUROSCI.06-01-00199.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]